Chapter 6 Understanding Organic Reactions Organic Chemistry Second

![Chemical Kinetics A B D[A] rate = Dt D[B] rate = Dt 64 Chemical Kinetics A B D[A] rate = Dt D[B] rate = Dt 64](https://slidetodoc.com/presentation_image_h/fb1e9261201d0901a1e54ec3ce050953/image-64.jpg)
![Chemical Kinetics : First-Order Reactions A k= product D[A] rate = Dt rate M/s Chemical Kinetics : First-Order Reactions A k= product D[A] rate = Dt rate M/s](https://slidetodoc.com/presentation_image_h/fb1e9261201d0901a1e54ec3ce050953/image-66.jpg)
![Chemical Kinetics : Second-Order Reactions A product D[A] rate = Dt rate M/s = Chemical Kinetics : Second-Order Reactions A product D[A] rate = Dt rate M/s =](https://slidetodoc.com/presentation_image_h/fb1e9261201d0901a1e54ec3ce050953/image-69.jpg)
![Chemical Kinetics : Zero-Order Reactions A product D[A] rate = Dt D[A] =k Dt Chemical Kinetics : Zero-Order Reactions A product D[A] rate = Dt D[A] =k Dt](https://slidetodoc.com/presentation_image_h/fb1e9261201d0901a1e54ec3ce050953/image-70.jpg)
- Slides: 71

Chapter 6 Understanding Organic Reactions Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai’i Prepared by Rabi Ann Musah State University of New York at Albany Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. 1

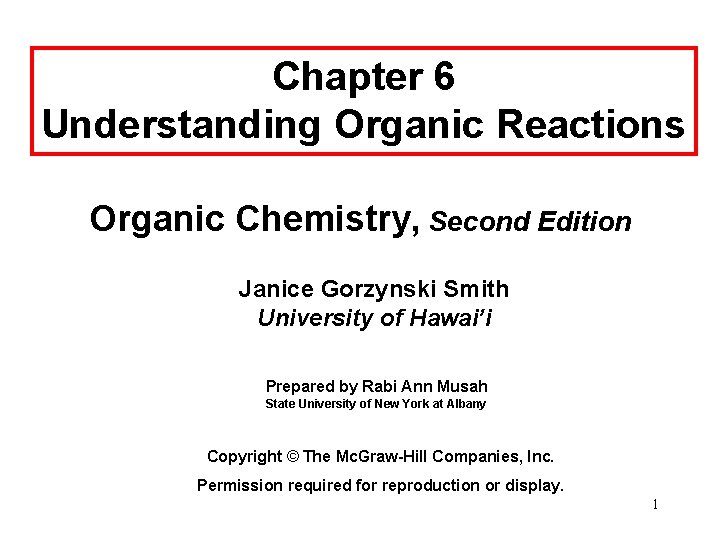
Writing Equations for Organic Reactions • Equations for organic reactions are usually drawn with a single reaction arrow ( ) between the starting material and product. • The reagent, the chemical substance with which an organic compound reacts, is sometimes drawn on the left side of the equation with the other reactants. At other times, the reagent is drawn above the arrow itself. • Although the solvent is often omitted from the equation, most organic reactions take place in liquid solvent. • The solvent and temperature of the reaction may be added above or below the arrow. • The symbols “h ” and “ ” are used for reactions that require 2 light and heat respectively.

Writing Equations for Organic Reactions Figure 6. 1 Different ways of writing organic reactions 3

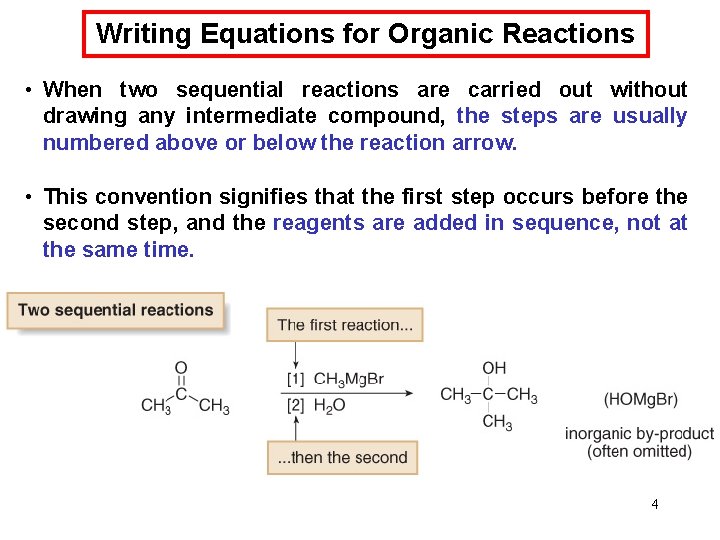
Writing Equations for Organic Reactions • When two sequential reactions are carried out without drawing any intermediate compound, the steps are usually numbered above or below the reaction arrow. • This convention signifies that the first step occurs before the second step, and the reagents are added in sequence, not at the same time. 4

Kinds of Organic Reactions • A substitution is a reaction in which an atom or a group of atoms is replaced by another atom or group of atoms. • In a general substitution, Y replaces Z on a carbon atom. 5

Substitution reactions • Substitution reactions involve bonds: one bond breaks and another forms at the same carbon atom. • The most common examples of substitution occur when Z is a halogen or a heteroatom that is more electronegative than carbon. 6 6

Kinds of Organic Reactions • Elimination is a reaction in which elements of the starting material are “lost” and a bond is formed. 7

Elimination reaction • In an elimination reaction, two groups X and Y are removed from a starting material. • Two bonds are broken, and a bond is formed between adjacent atoms. • The most common examples of elimination occur when X = H and Y is a heteroatom more electronegative than carbon. 8 8

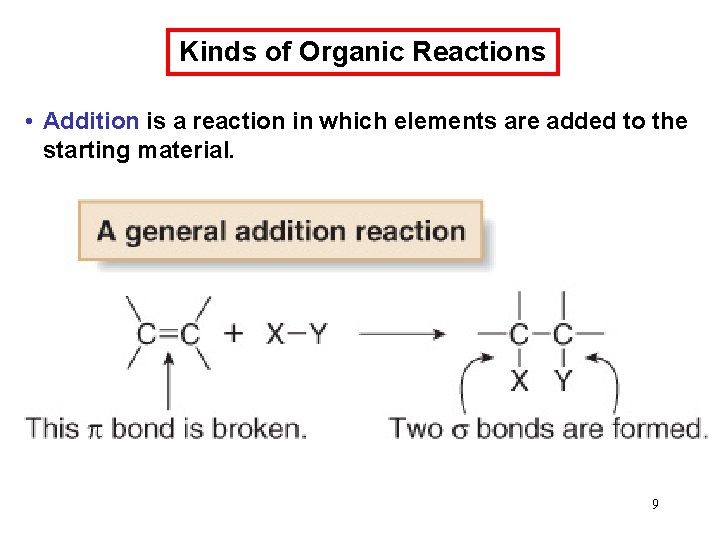
Kinds of Organic Reactions • Addition is a reaction in which elements are added to the starting material. 9

Addition reaction • In an addition reaction, new groups X and Y are added to the starting material. A bond is broken and two bonds are formed. 10 10

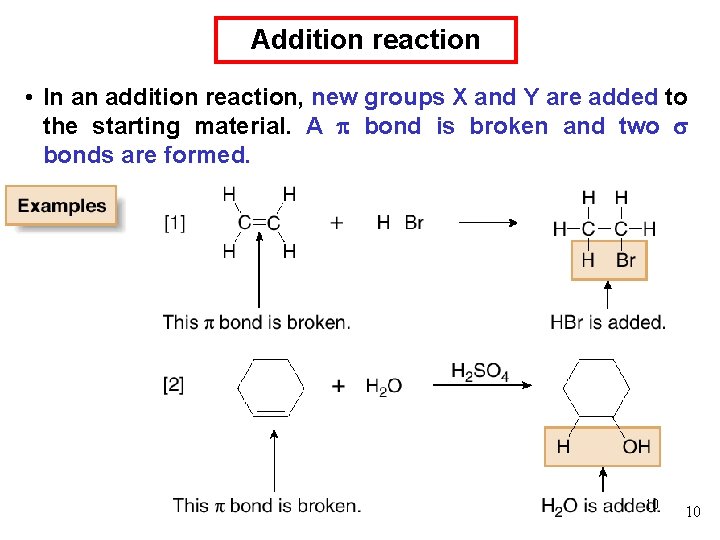
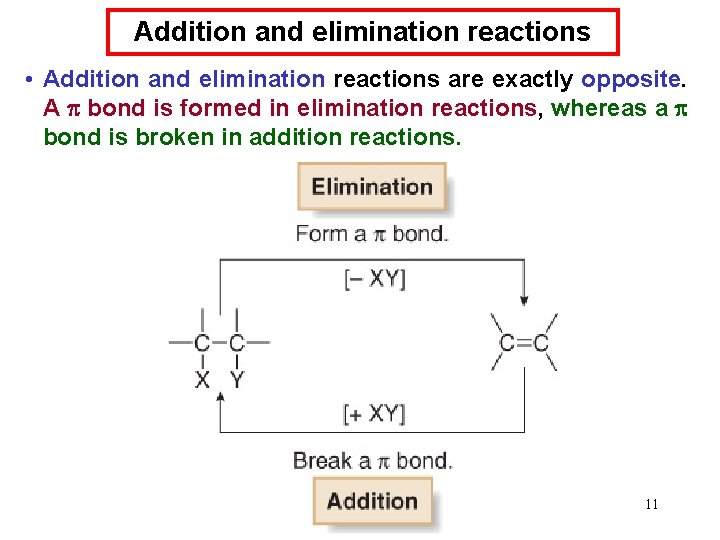
Addition and elimination reactions • Addition and elimination reactions are exactly opposite. A bond is formed in elimination reactions, whereas a bond is broken in addition reactions. 11

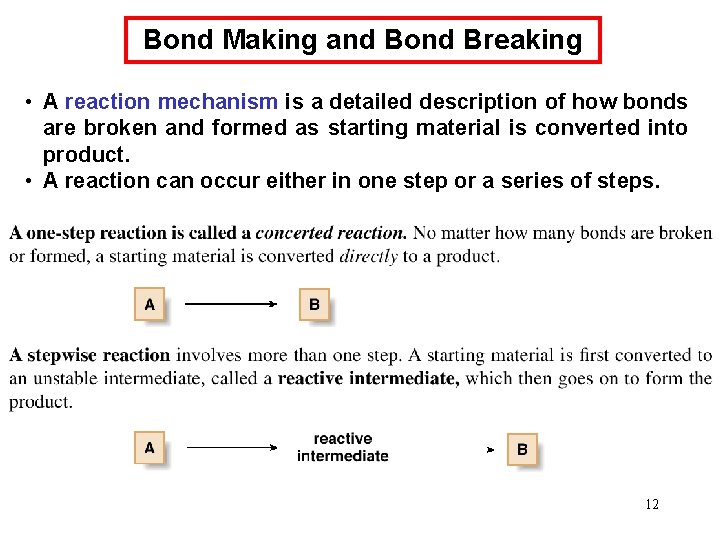
Bond Making and Bond Breaking • A reaction mechanism is a detailed description of how bonds are broken and formed as starting material is converted into product. • A reaction can occur either in one step or a series of steps. 12

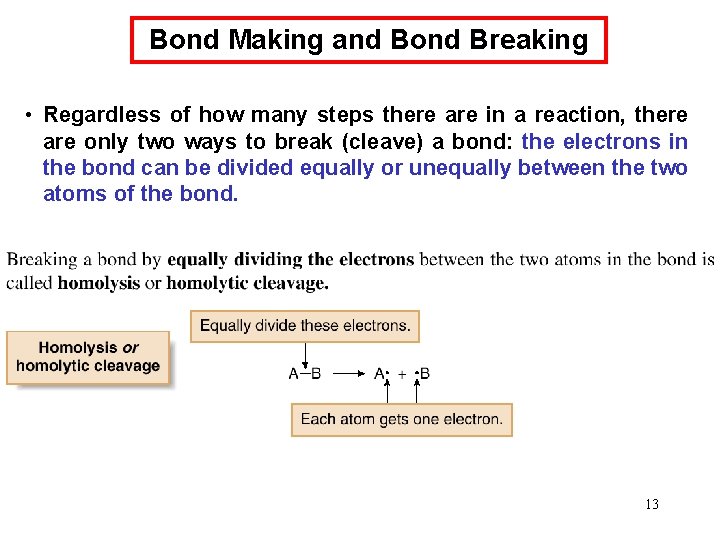
Bond Making and Bond Breaking • Regardless of how many steps there are in a reaction, there are only two ways to break (cleave) a bond: the electrons in the bond can be divided equally or unequally between the two atoms of the bond. 13

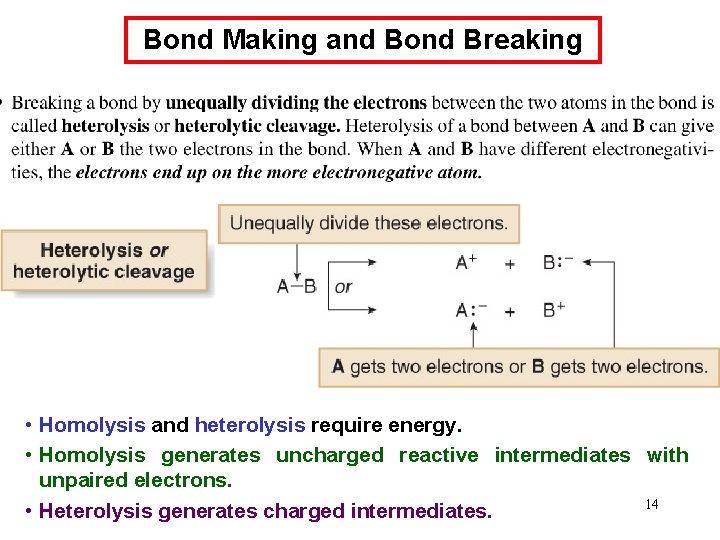
Bond Making and Bond Breaking • Homolysis and heterolysis require energy. • Homolysis generates uncharged reactive intermediates with unpaired electrons. 14 • Heterolysis generates charged intermediates.

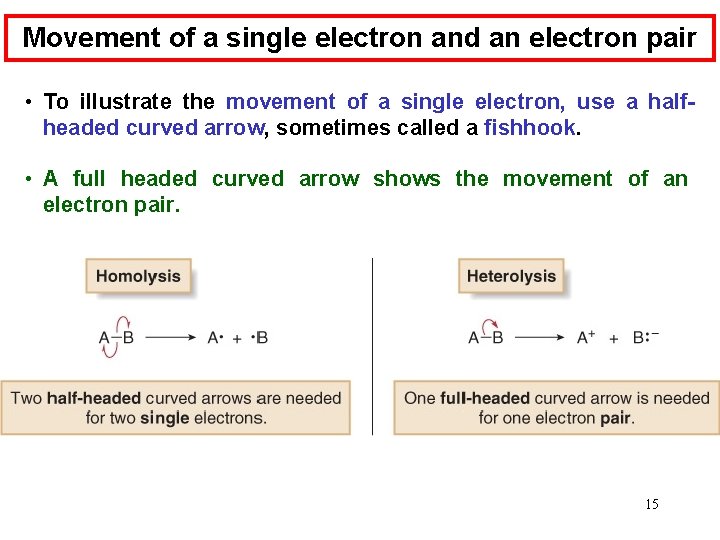
Movement of a single electron and an electron pair • To illustrate the movement of a single electron, use a halfheaded curved arrow, sometimes called a fishhook. • A full headed curved arrow shows the movement of an electron pair. 15

Homolysis and Heterolysis • Homolysis generates two uncharged species with unpaired electrons. • A reactive intermediate with a single unpaired electron is called a radical. • Radicals are highly unstable because they contain an atom that does not have an octet of electrons. • Heterolysis generates a carbocation or a carbanion. • Both carbocations and carbanions are unstable intermediates. A carbocation contains a carbon surrounded by only six electrons, and a carbanion has a negative charge on carbon, which is not a very electronegative atom. 16

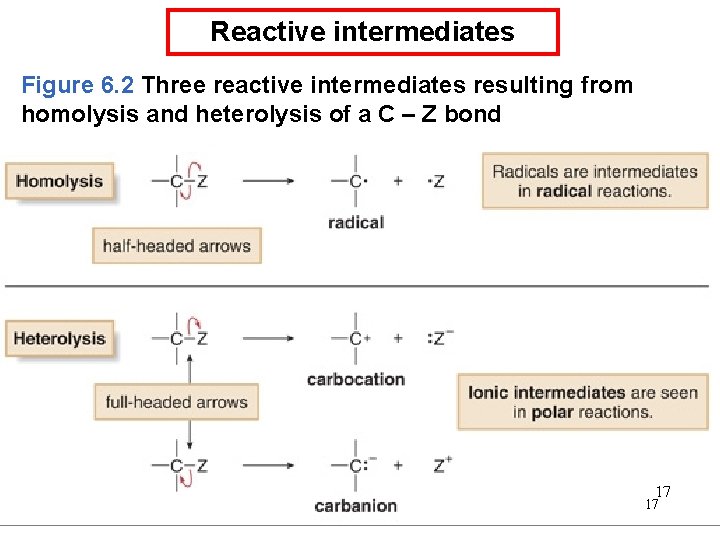
Reactive intermediates Figure 6. 2 Three reactive intermediates resulting from homolysis and heterolysis of a C – Z bond 17 17

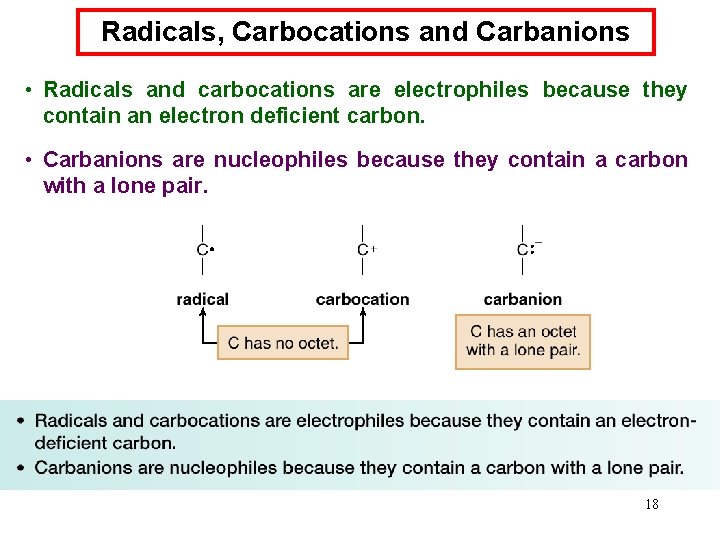
Radicals, Carbocations and Carbanions • Radicals and carbocations are electrophiles because they contain an electron deficient carbon. • Carbanions are nucleophiles because they contain a carbon with a lone pair. 18

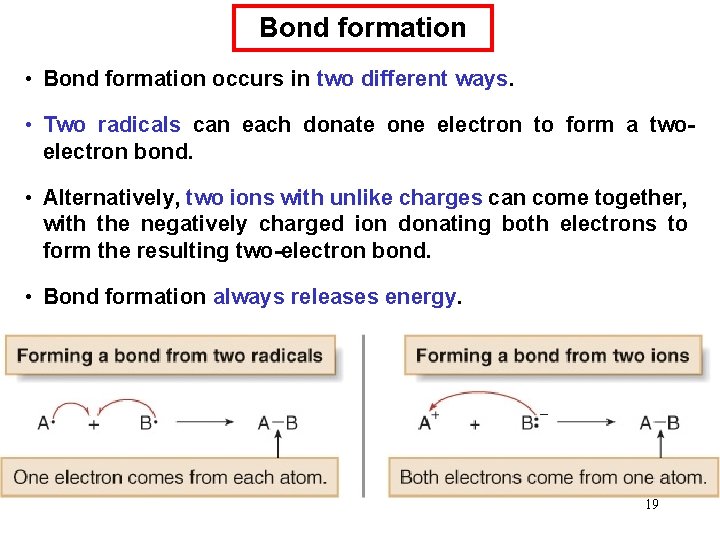
Bond formation • Bond formation occurs in two different ways. • Two radicals can each donate one electron to form a twoelectron bond. • Alternatively, two ions with unlike charges can come together, with the negatively charged ion donating both electrons to form the resulting two-electron bond. • Bond formation always releases energy. 19

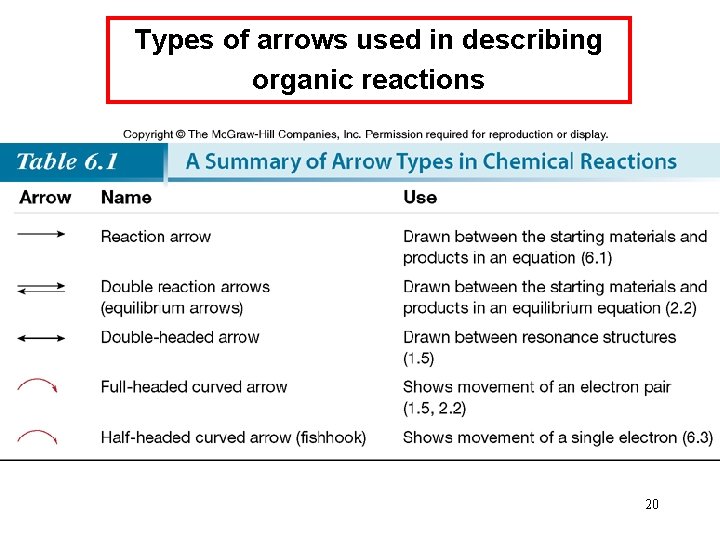
Types of arrows used in describing organic reactions 20

Bond Dissociation Energy • The energy absorbed or released in any reaction, symbolized by H 0, is called the enthalpy change or heat of reaction. • Bond dissociation energy is the H 0 for a specific kind of reaction—the homolysis of a covalent bond to form two 21 radicals.

Bond Dissociation Energy • Because bond breaking requires energy, bond dissociation energies are always positive numbers, and homolysis is always endothermic. • Conversely, bond formation always releases energy, and thus is always exothermic. For example, the H—H bond requires +104 kcal/mol to cleave and releases – 104 kcal/mol when formed. 22

23

Bond Dissociation Energy • Comparing bond dissociation comparing bond strength. energies is equivalent to • The stronger the bond, the higher its bond dissociation energy. • Bond dissociation energies decrease down a column of the periodic table. • Generally, shorter bonds are stronger bonds. 24 24

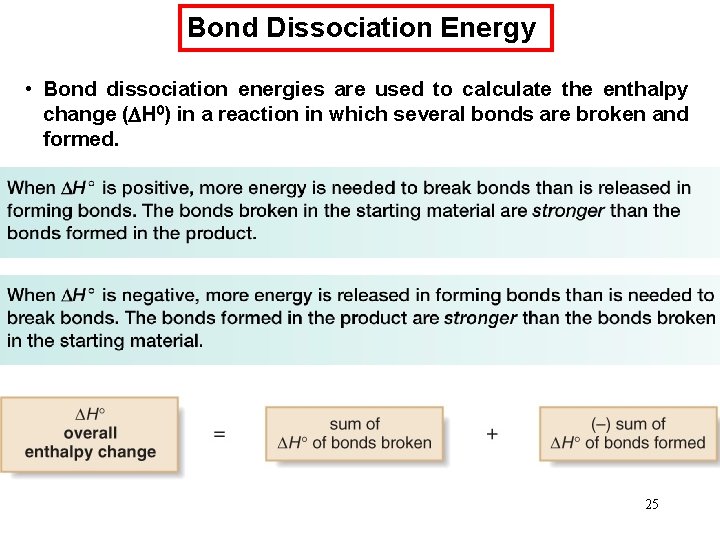
Bond Dissociation Energy • Bond dissociation energies are used to calculate the enthalpy change ( H 0) in a reaction in which several bonds are broken and formed. 25

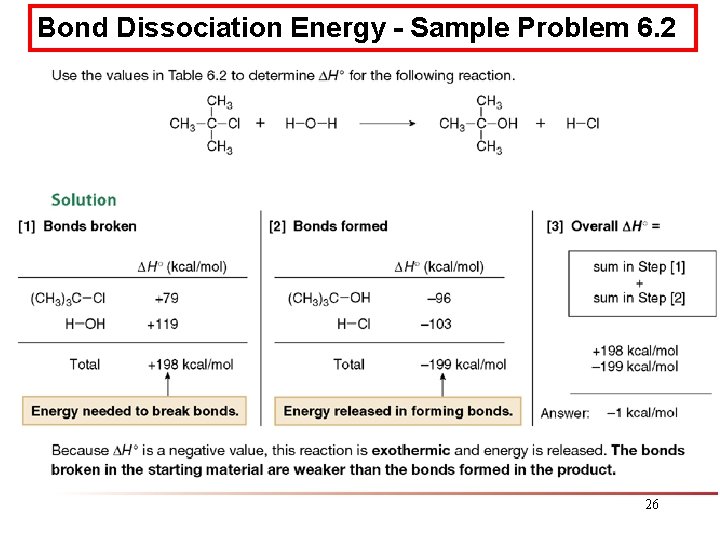
Bond Dissociation Energy - Sample Problem 6. 2 26

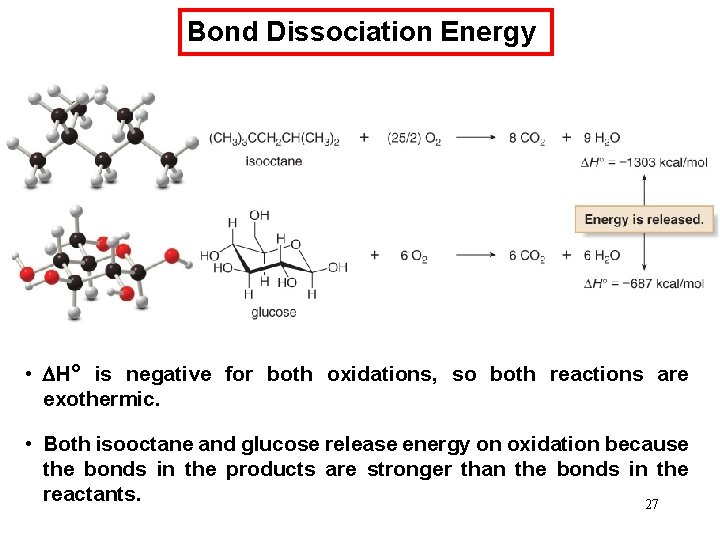
Bond Dissociation Energy • H° is negative for both oxidations, so both reactions are exothermic. • Both isooctane and glucose release energy on oxidation because the bonds in the products are stronger than the bonds in the reactants. 27

Bond dissociation energies - important limitations • Bond dissociation energies present overall energy changes only. They reveal nothing about the reaction mechanism or how fast a reaction proceeds. • Bond dissociation energies are determined for reactions in the gas phase, whereas most organic reactions occur in a liquid solvent where solvation energy contributes to the overall enthalpy of a reaction. • Bond dissociation energies are imperfect indicators of energy changes in a reaction. However, using bond dissociation energies to calculate H° gives a useful approximation of the energy changes that occur when bonds are broken and formed in a reaction. 28

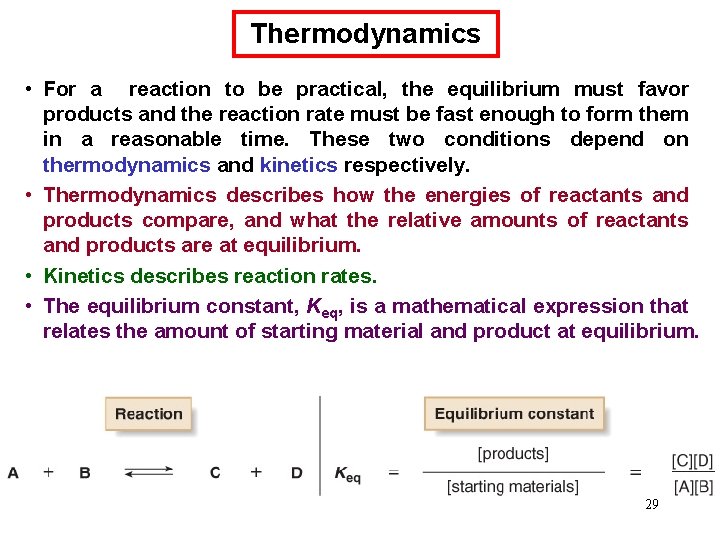
Thermodynamics • For a reaction to be practical, the equilibrium must favor products and the reaction rate must be fast enough to form them in a reasonable time. These two conditions depend on thermodynamics and kinetics respectively. • Thermodynamics describes how the energies of reactants and products compare, and what the relative amounts of reactants and products are at equilibrium. • Kinetics describes reaction rates. • The equilibrium constant, Keq, is a mathematical expression that relates the amount of starting material and product at equilibrium. 29

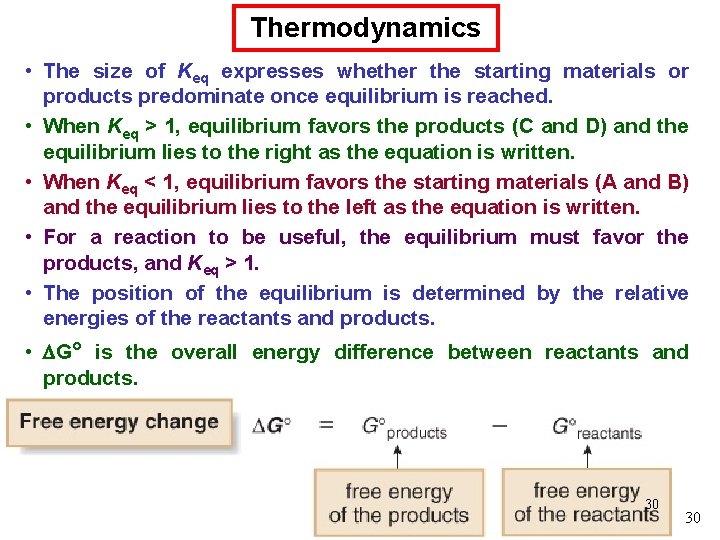
Thermodynamics • The size of Keq expresses whether the starting materials or products predominate once equilibrium is reached. • When Keq > 1, equilibrium favors the products (C and D) and the equilibrium lies to the right as the equation is written. • When Keq < 1, equilibrium favors the starting materials (A and B) and the equilibrium lies to the left as the equation is written. • For a reaction to be useful, the equilibrium must favor the products, and Keq > 1. • The position of the equilibrium is determined by the relative energies of the reactants and products. • G° is the overall energy difference between reactants and products. 30 30

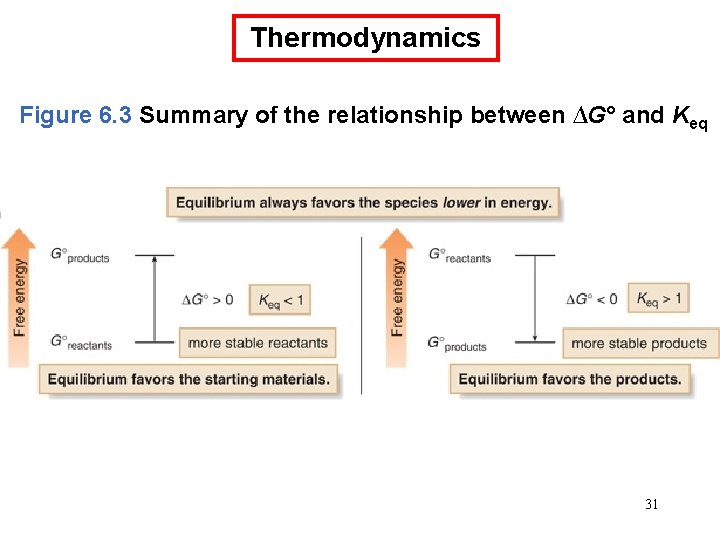
Thermodynamics Figure 6. 3 Summary of the relationship between ∆G° and Keq 31

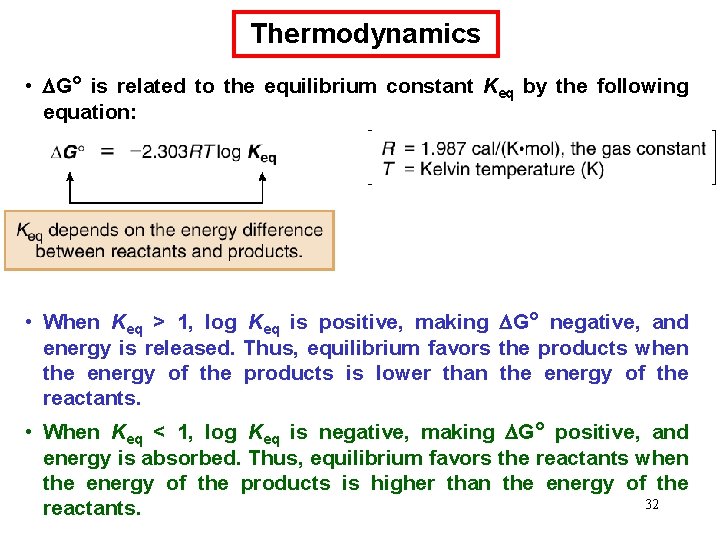
Thermodynamics • G° is related to the equilibrium constant Keq by the following equation: • When Keq > 1, log Keq is positive, making G° negative, and energy is released. Thus, equilibrium favors the products when the energy of the products is lower than the energy of the reactants. • When Keq < 1, log Keq is negative, making G° positive, and energy is absorbed. Thus, equilibrium favors the reactants when the energy of the products is higher than the energy of the 32 reactants.

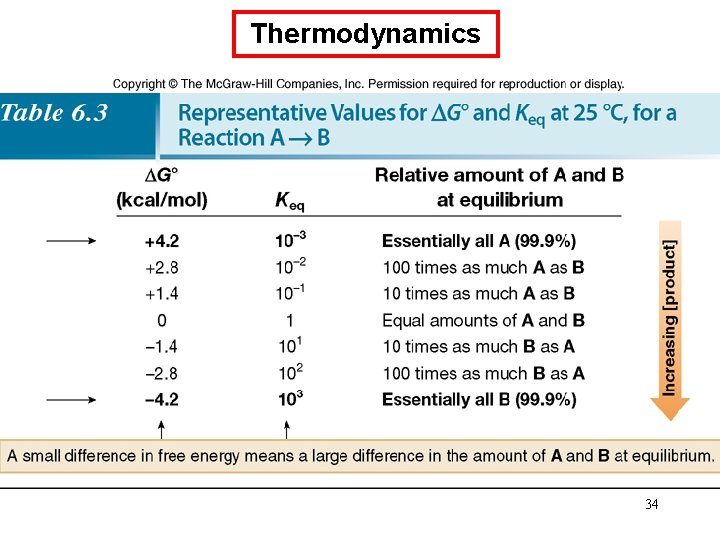
Thermodynamics • Compounds that are lower in energy have increased stability. • The equilibrium favors the products when they are more stable (lower in energy) than the starting materials of a reaction. • Because G° depends on the logarithm of Keq, a small change in energy corresponds to a large difference in the relative amount of starting material and product at equilibrium. (Table 6. 3) 33

Thermodynamics 34

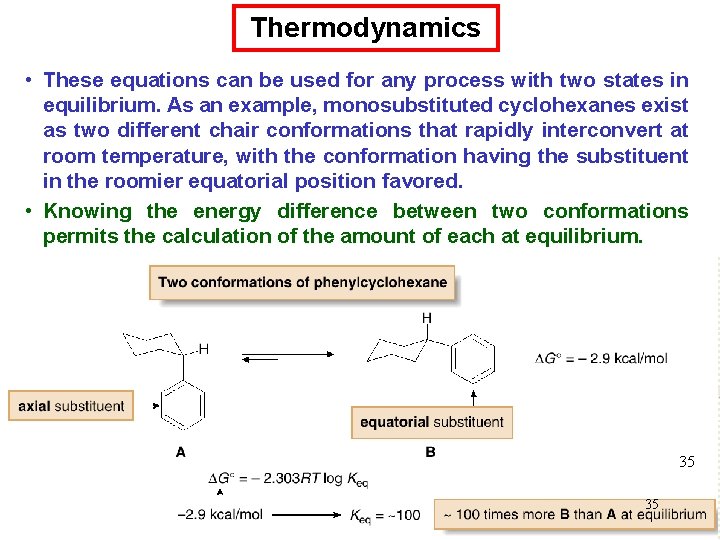
Thermodynamics • These equations can be used for any process with two states in equilibrium. As an example, monosubstituted cyclohexanes exist as two different chair conformations that rapidly interconvert at room temperature, with the conformation having the substituent in the roomier equatorial position favored. • Knowing the energy difference between two conformations permits the calculation of the amount of each at equilibrium. 35 35

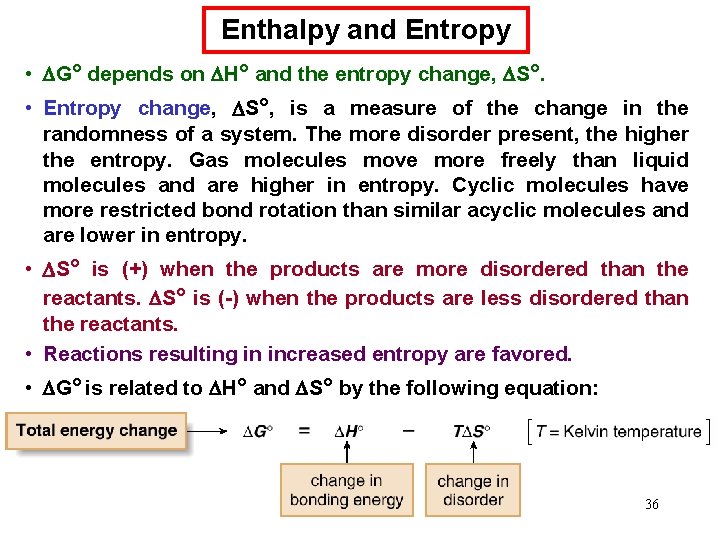
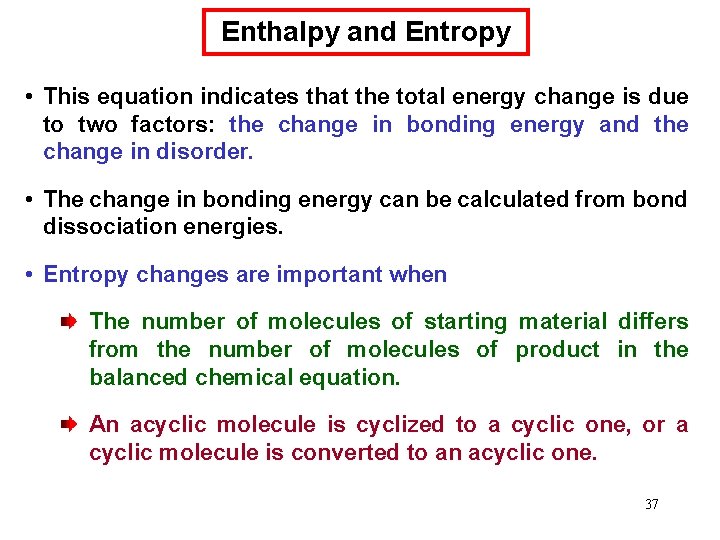
Enthalpy and Entropy • G° depends on H° and the entropy change, S°. • Entropy change, S°, is a measure of the change in the randomness of a system. The more disorder present, the higher the entropy. Gas molecules move more freely than liquid molecules and are higher in entropy. Cyclic molecules have more restricted bond rotation than similar acyclic molecules and are lower in entropy. • S° is (+) when the products are more disordered than the reactants. S° is (-) when the products are less disordered than the reactants. • Reactions resulting in increased entropy are favored. • G° is related to H° and S° by the following equation: 36

Enthalpy and Entropy • This equation indicates that the total energy change is due to two factors: the change in bonding energy and the change in disorder. • The change in bonding energy can be calculated from bond dissociation energies. • Entropy changes are important when The number of molecules of starting material differs from the number of molecules of product in the balanced chemical equation. An acyclic molecule is cyclized to a cyclic one, or a cyclic molecule is converted to an acyclic one. 37

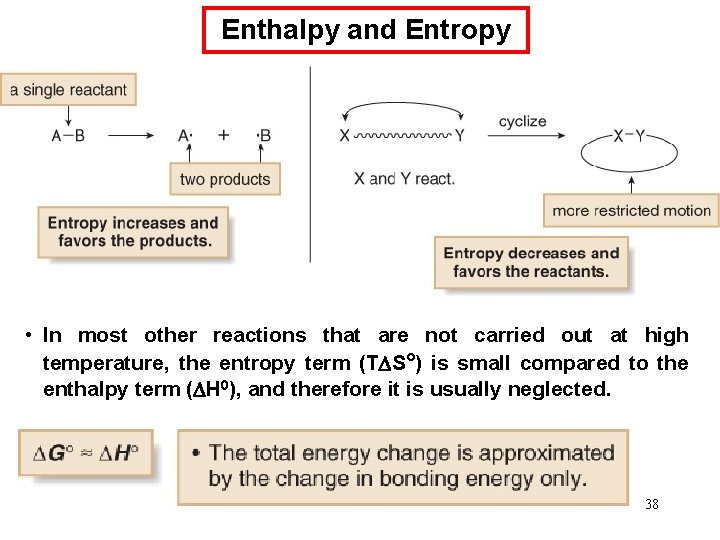
Enthalpy and Entropy • In most other reactions that are not carried out at high temperature, the entropy term (T S°) is small compared to the enthalpy term ( H 0), and therefore it is usually neglected. 38

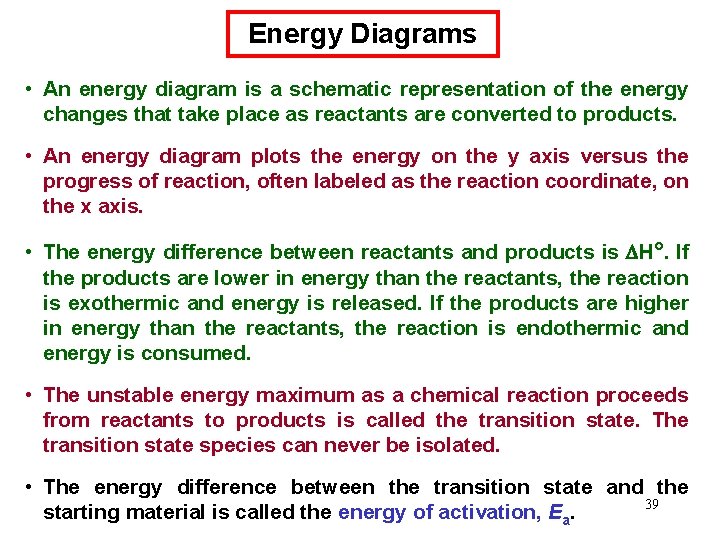
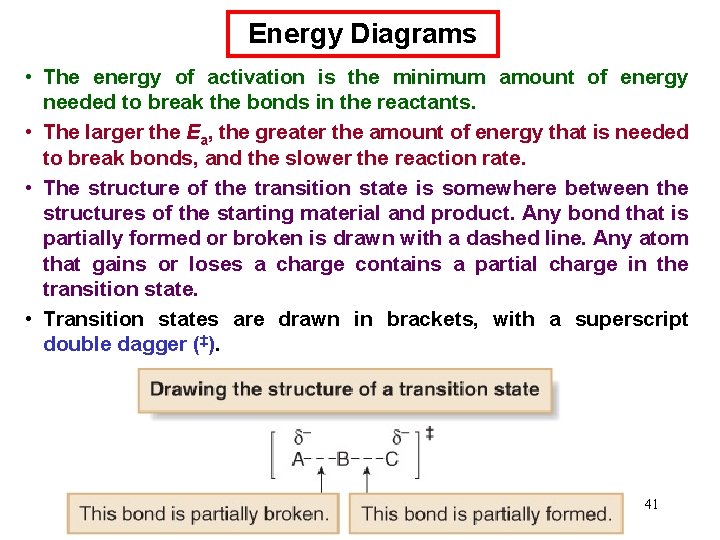
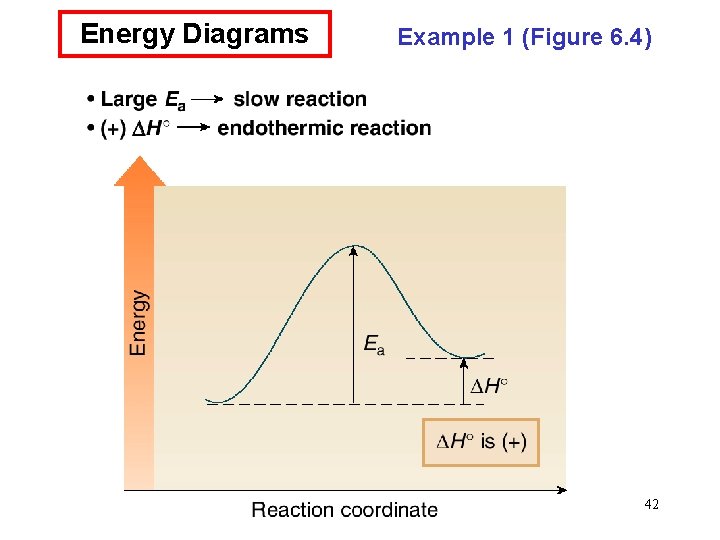
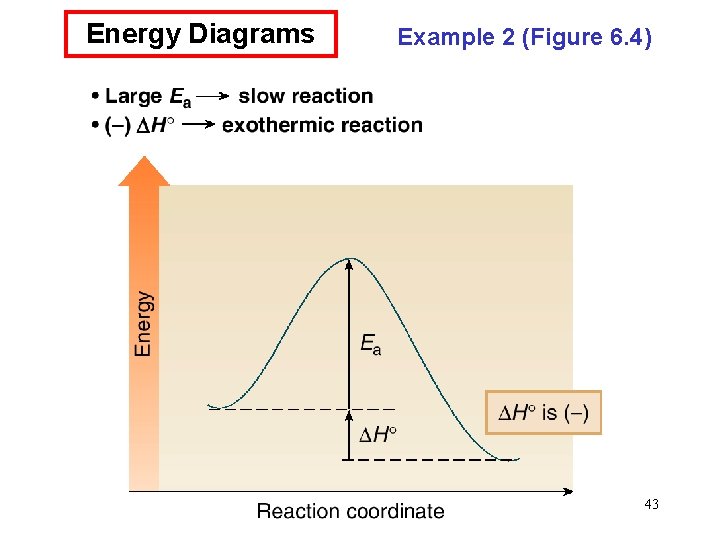
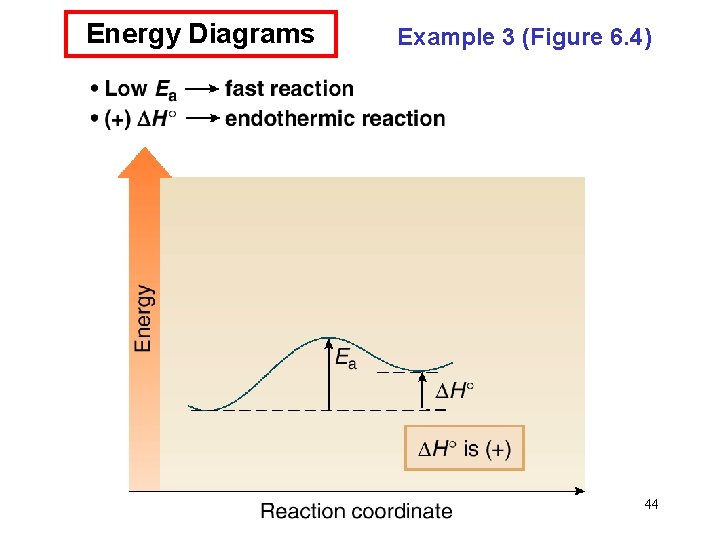
Energy Diagrams • An energy diagram is a schematic representation of the energy changes that take place as reactants are converted to products. • An energy diagram plots the energy on the y axis versus the progress of reaction, often labeled as the reaction coordinate, on the x axis. • The energy difference between reactants and products is H°. If the products are lower in energy than the reactants, the reaction is exothermic and energy is released. If the products are higher in energy than the reactants, the reaction is endothermic and energy is consumed. • The unstable energy maximum as a chemical reaction proceeds from reactants to products is called the transition state. The transition state species can never be isolated. • The energy difference between the transition state and the 39 starting material is called the energy of activation, Ea.

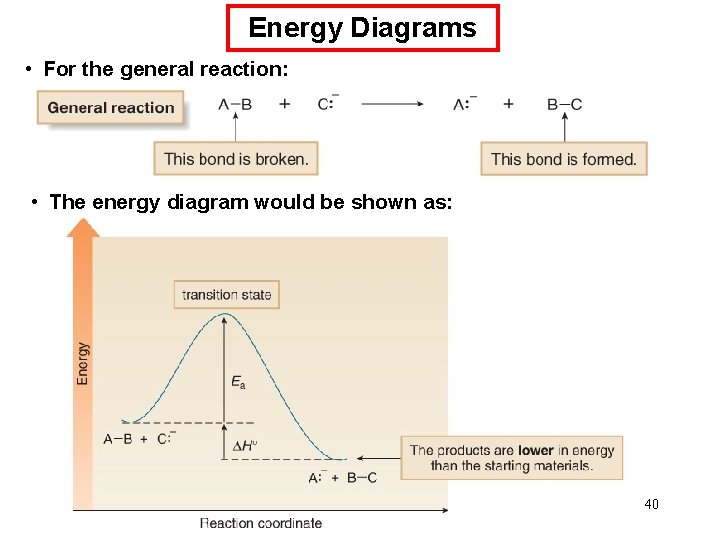
Energy Diagrams • For the general reaction: • The energy diagram would be shown as: 40

Energy Diagrams • The energy of activation is the minimum amount of energy needed to break the bonds in the reactants. • The larger the Ea, the greater the amount of energy that is needed to break bonds, and the slower the reaction rate. • The structure of the transition state is somewhere between the structures of the starting material and product. Any bond that is partially formed or broken is drawn with a dashed line. Any atom that gains or loses a charge contains a partial charge in the transition state. • Transition states are drawn in brackets, with a superscript double dagger (‡). 41

Energy Diagrams Example 1 (Figure 6. 4) 42

Energy Diagrams Example 2 (Figure 6. 4) 43

Energy Diagrams Example 3 (Figure 6. 4) 44

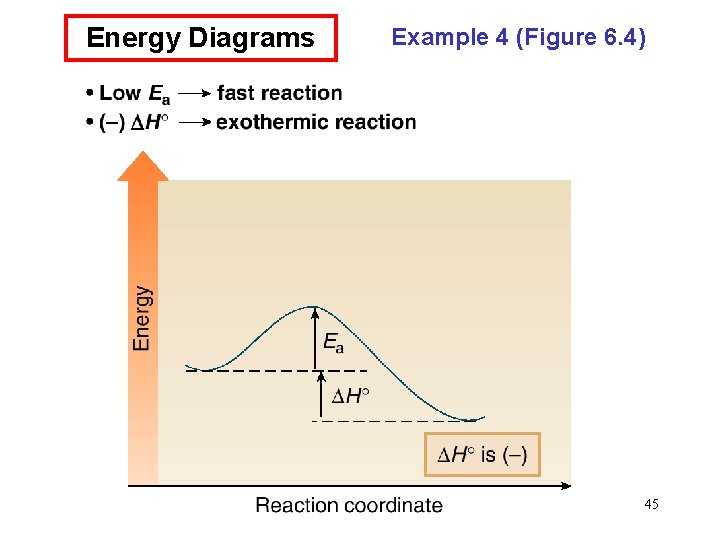
Energy Diagrams Example 4 (Figure 6. 4) 45

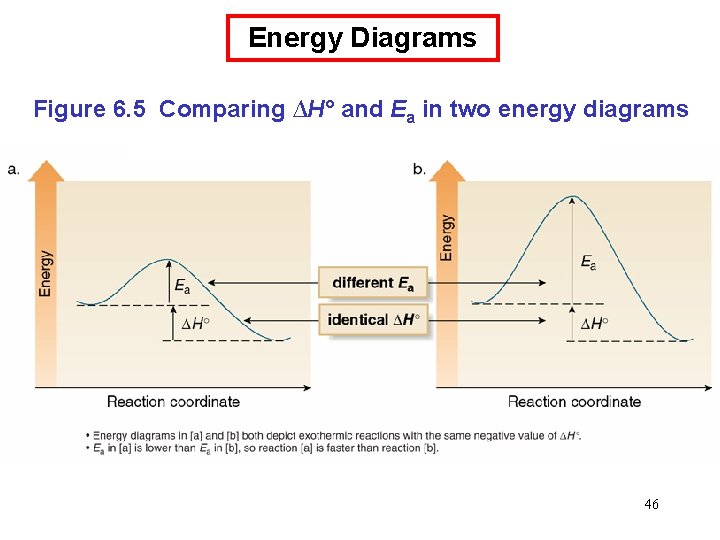
Energy Diagrams Figure 6. 5 Comparing ∆H° and Ea in two energy diagrams 46

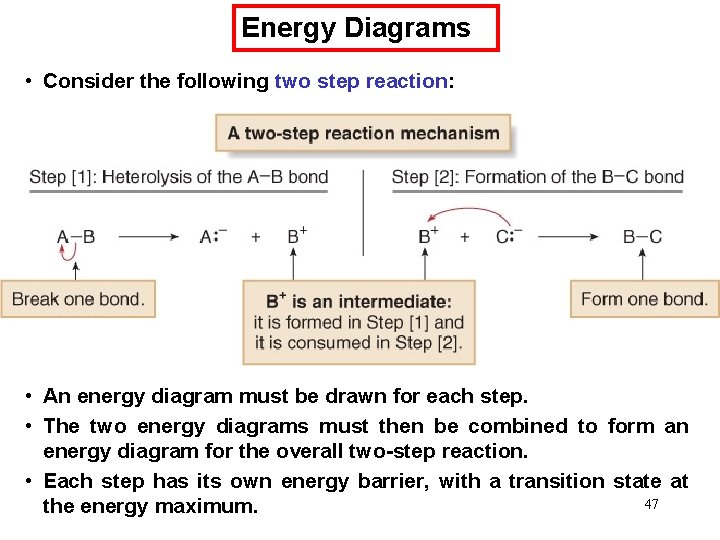
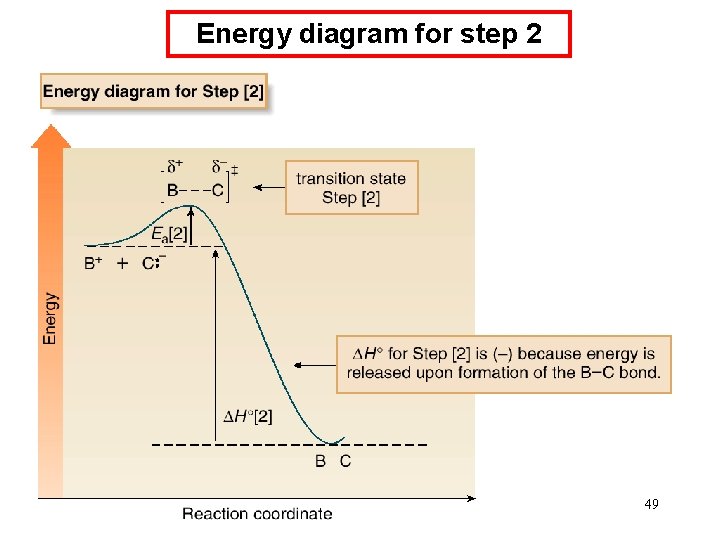
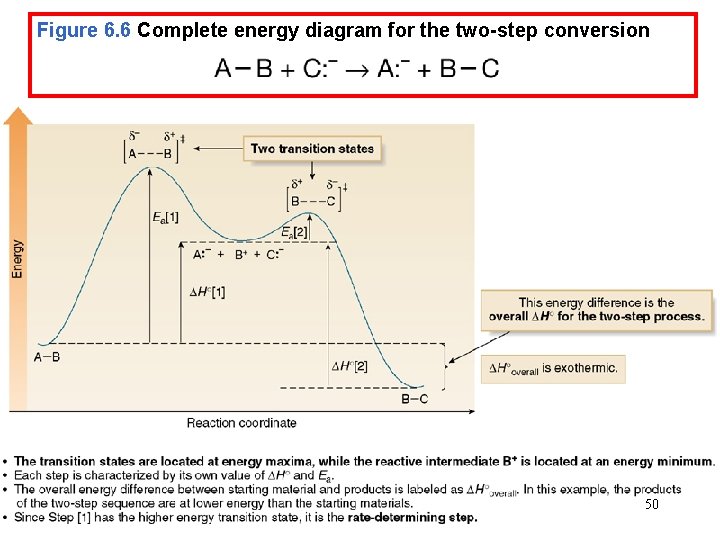
Energy Diagrams • Consider the following two step reaction: • An energy diagram must be drawn for each step. • The two energy diagrams must then be combined to form an energy diagram for the overall two-step reaction. • Each step has its own energy barrier, with a transition state at 47 the energy maximum.

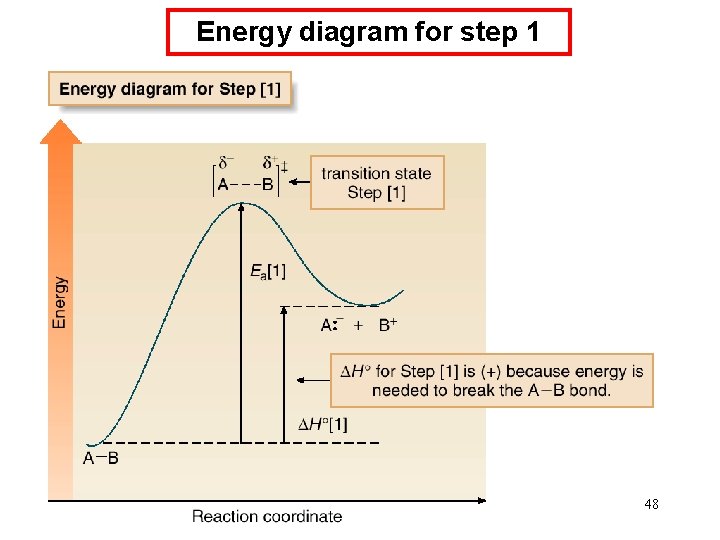
Energy diagram for step 1 48

Energy diagram for step 2 49

Figure 6. 6 Complete energy diagram for the two-step conversion 50

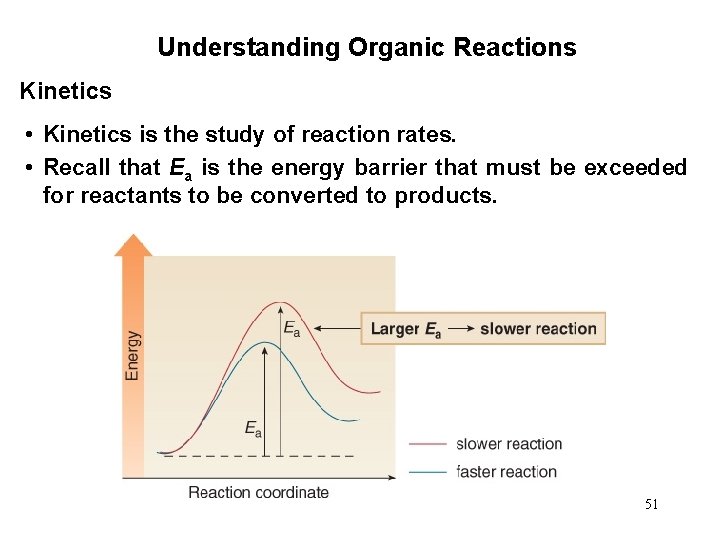
Understanding Organic Reactions Kinetics • Kinetics is the study of reaction rates. • Recall that Ea is the energy barrier that must be exceeded for reactants to be converted to products. 51

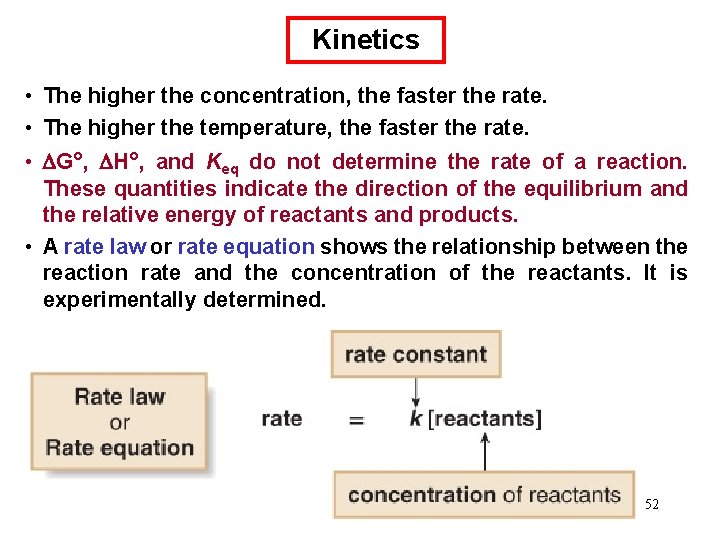
Kinetics • The higher the concentration, the faster the rate. • The higher the temperature, the faster the rate. • G°, H°, and Keq do not determine the rate of a reaction. These quantities indicate the direction of the equilibrium and the relative energy of reactants and products. • A rate law or rate equation shows the relationship between the reaction rate and the concentration of the reactants. It is experimentally determined. 52

Kinetics • Fast reactions have large rate constants. • Slow reactions have small rate constants. • The rate constant k and the energy of activation Ea are inversely related. A high Ea corresponds to a small k. • A rate equation contains concentration terms for all reactants in a one-step mechanism. • A rate equation contains concentration terms for only the reactants involved in the rate-determining step in a multi-step reaction. • The order of a rate equation equals the sum of the exponents of the concentration terms in the rate equation. 53

Kinetics • A two-step reaction has a slow rate-determining step, and a fast step. • In a multi-step mechanism, the reaction can occur no faster than its rate-determining step. • Only the concentration of the reactants in the rate-determining step appears in the rate equation. 54

Catalysts • Some reactions do not proceed at a reasonable rate unless a catalyst is added. • A catalyst is a substance that speeds up the rate of a reaction. It is recovered unchanged in a reaction, and it does not appear in the product. 55

Figure 6. 7 The effect of a catalyst on a reaction 56

Enzymes • Enzymes are biochemical catalysts composed of amino acids held together in a very specific three-dimensional shape. • An enzyme contains a region called its active site which binds an organic reactant, called a substrate. The resulting unit is called the enzyme-substrate complex. • Once bound, the organic substrate undergoes a very specific reaction at an enhanced rate. The products are then released. 57

Applications in Organic Chemistry Laboratory เนอหาหนา 58 -72 ใชในการเรยนภาคปลาย ไมออกขอสอบในเทอมตน 58 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

Heat of Reaction and Hess’s Law The specific heat (s) of a substance is the amount of heat (q) required to raise the temperature of one gram of the substance by one degree Celsius. The heat capacity (C) of a substance is the amount of heat (q) required to raise the temperature of a given quantity (m) of the substance by one degree Celsius. C=mxs Heat (q) absorbed or released: q = m x s x Dt q = C x Dt Dt = tfinal - tinitial 59

Heat of Reaction and Hess’s Law Constant-Pressure Calorimetry No heat enters or leaves! qsys = qwater + qcal + qrxn qsys = 0 qrxn = - (qwater + qcal) qwater = m x s x Dt qcal = Ccal x Dt Reaction at Constant P DH = qrxn 60

Heat of Reaction and Hess’s Law 61

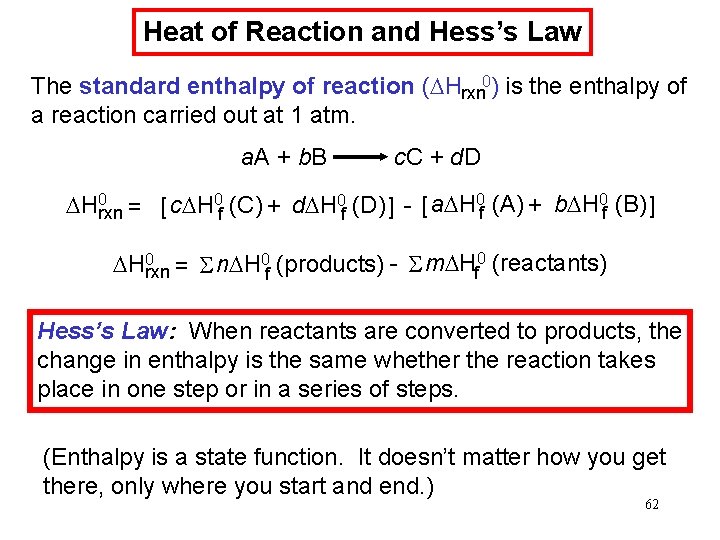
Heat of Reaction and Hess’s Law The standard enthalpy of reaction (DHrxn 0) is the enthalpy of a reaction carried out at 1 atm. a. A + b. B c. C + d. D DH 0 rxn = [ c. DH 0 f (C) + d. DH 0 f (D) ] - [ a. DH 0 f (A) + b. DH 0 f (B) ] DH 0 rxn = S n. DH 0 f (products) - S m. DHf 0 (reactants) Hess’s Law: When reactants are converted to products, the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps. (Enthalpy is a state function. It doesn’t matter how you get there, only where you start and end. ) 62

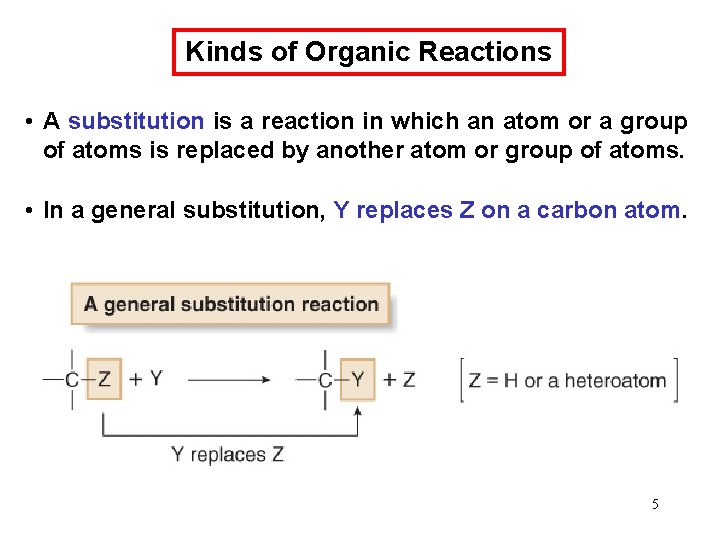
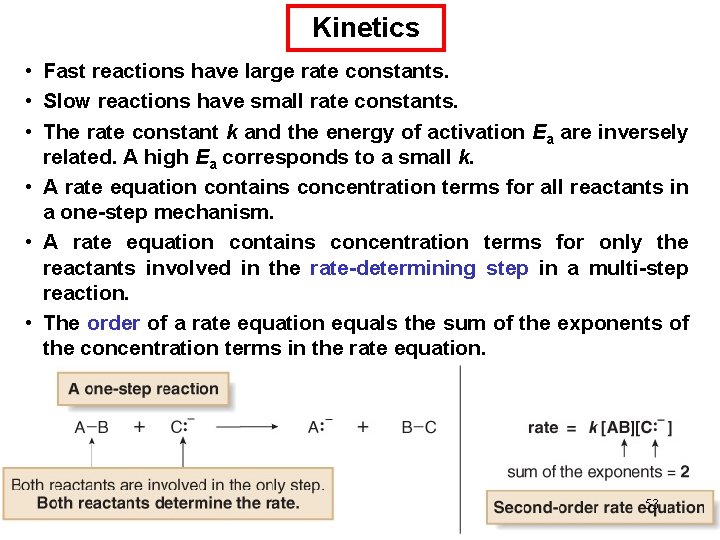
Chemical Kinetics Thermodynamics – does a reaction take place? Kinetics – how fast does a reaction proceed? Reaction rate is the change in the concentration of a reactant or a product with time (M/s). A B D[A] rate = Dt D[A] = change in concentration of A over time period Dt D[B] rate = Dt D[B] = change in concentration of B over time period Dt Because [A] decreases with time, D[A] is negative. 63
![Chemical Kinetics A B DA rate Dt DB rate Dt 64 Chemical Kinetics A B D[A] rate = Dt D[B] rate = Dt 64](https://slidetodoc.com/presentation_image_h/fb1e9261201d0901a1e54ec3ce050953/image-64.jpg)
Chemical Kinetics A B D[A] rate = Dt D[B] rate = Dt 64

Chemical Kinetics : Breath Analyzer 3 CH 2 OH + 2 K 2 Cr 2 O 7 + 8 H 2 SO 4 3 CH 3 COOH + 2 Cr 2(SO 4)3 + 2 K 2 SO 4 + 11 H 2 O [Cr 2 O 72 -] a Absorption 65
![Chemical Kinetics FirstOrder Reactions A k product DA rate Dt rate Ms Chemical Kinetics : First-Order Reactions A k= product D[A] rate = Dt rate M/s](https://slidetodoc.com/presentation_image_h/fb1e9261201d0901a1e54ec3ce050953/image-66.jpg)
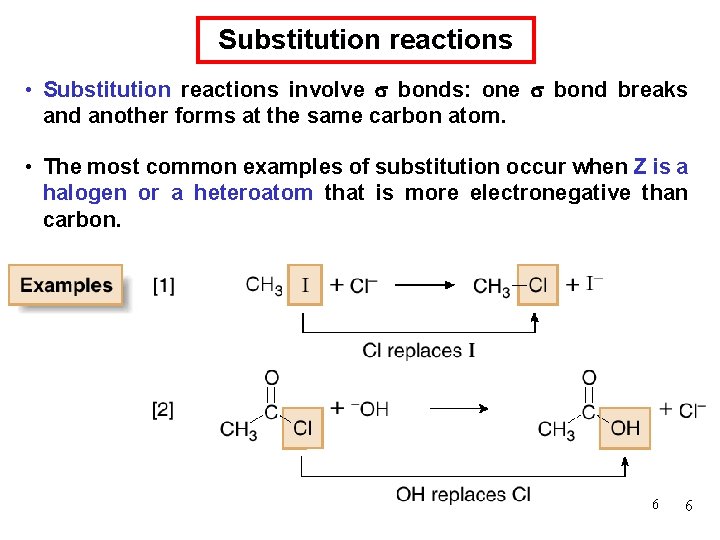
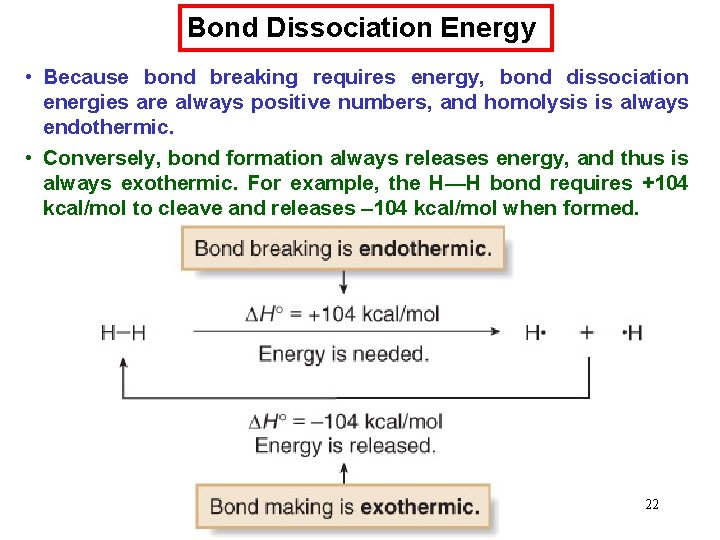
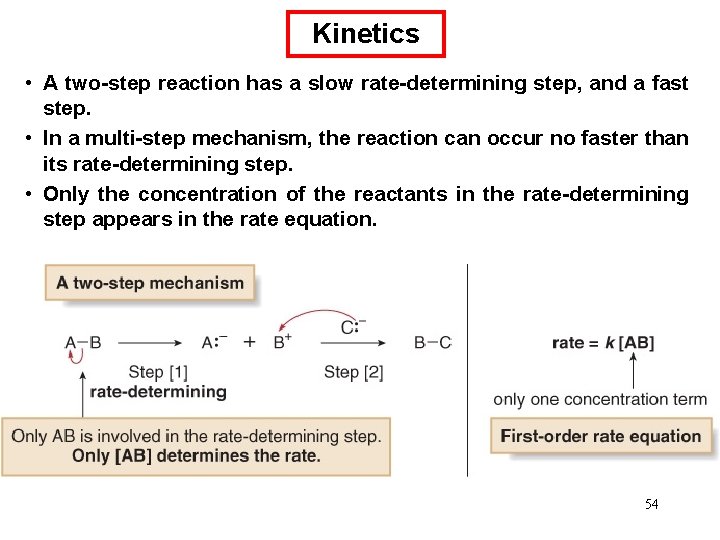
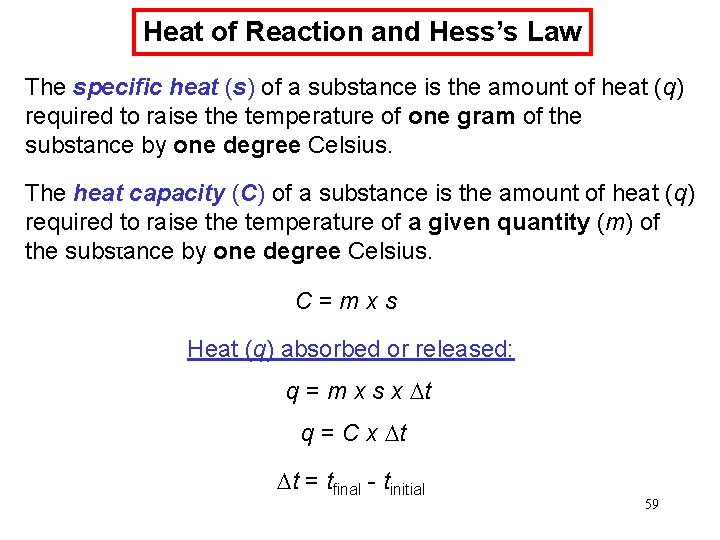
Chemical Kinetics : First-Order Reactions A k= product D[A] rate = Dt rate M/s = = 1/s or s-1 M [A] = [A]0 e−kt rate = k [A] D[A] = k [A] Dt [A] is the concentration of A at any time t [A]0 is the concentration of A at time t=0 ln[A] = ln[A]0 - kt 66

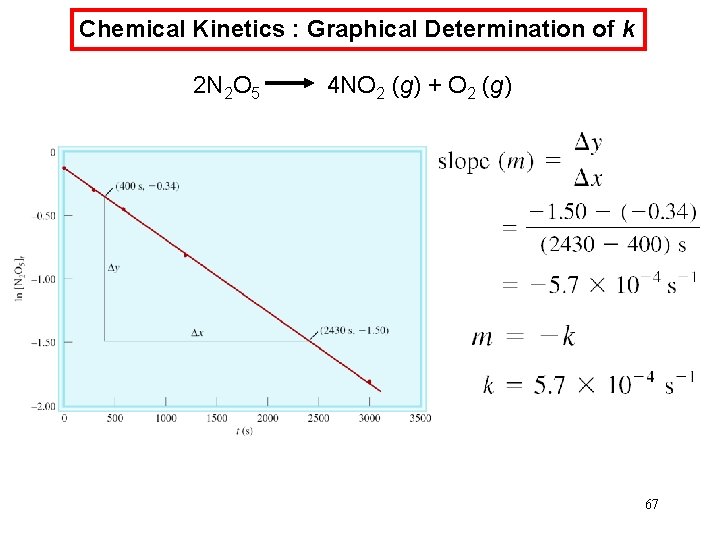
Chemical Kinetics : Graphical Determination of k 2 N 2 O 5 4 NO 2 (g) + O 2 (g) 67

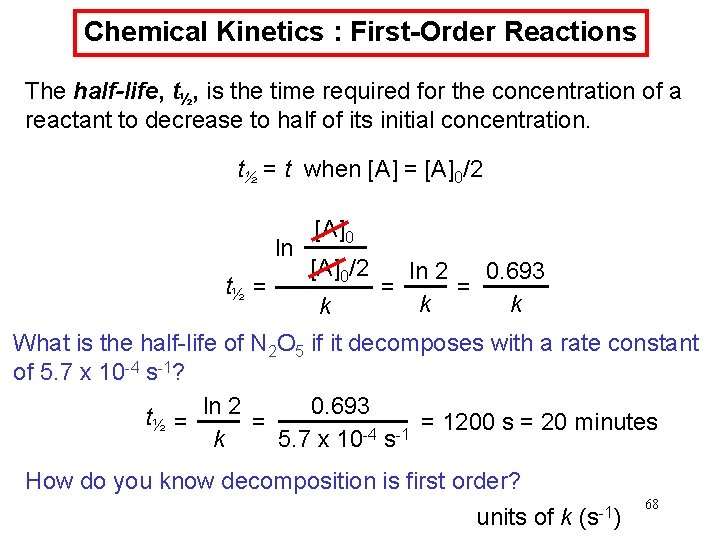
Chemical Kinetics : First-Order Reactions The half-life, t½, is the time required for the concentration of a reactant to decrease to half of its initial concentration. t½ = t when [A] = [A]0/2 ln t½ = [A]0/2 k ln 2 0. 693 = = k k What is the half-life of N 2 O 5 if it decomposes with a rate constant of 5. 7 x 10 -4 s-1? 0. 693 t½ = ln 2 = = 1200 s = 20 minutes -4 -1 k 5. 7 x 10 s How do you know decomposition is first order? units of k (s-1) 68
![Chemical Kinetics SecondOrder Reactions A product DA rate Dt rate Ms Chemical Kinetics : Second-Order Reactions A product D[A] rate = Dt rate M/s =](https://slidetodoc.com/presentation_image_h/fb1e9261201d0901a1e54ec3ce050953/image-69.jpg)
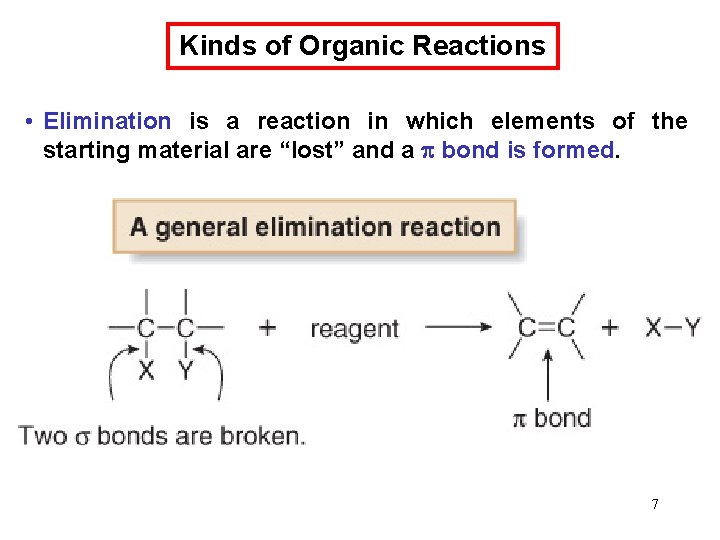
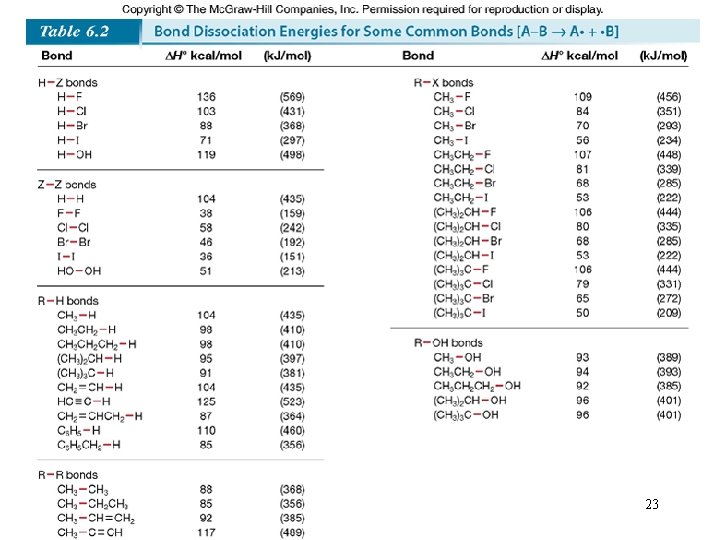
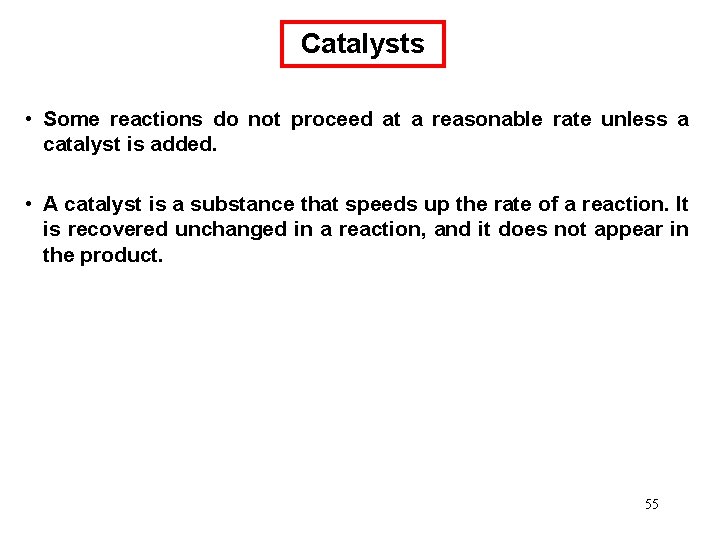
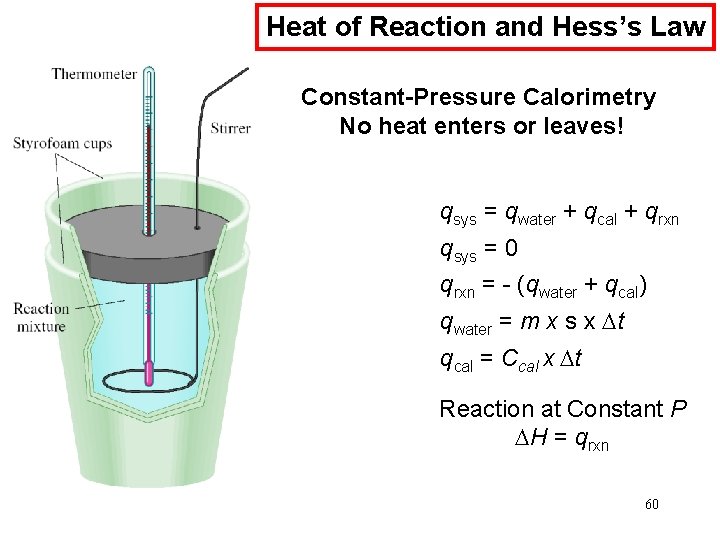
Chemical Kinetics : Second-Order Reactions A product D[A] rate = Dt rate M/s = = 1/M • s k= 2 2 M [A] 1 1 = + kt [A]0 rate = k [A]2 D[A] = k [A]2 Dt [A] is the concentration of A at any time t [A]0 is the concentration of A at time t=0 t½ = t when [A] = [A]0/2 1 t½ = k[A]0 69
![Chemical Kinetics ZeroOrder Reactions A product DA rate Dt DA k Dt Chemical Kinetics : Zero-Order Reactions A product D[A] rate = Dt D[A] =k Dt](https://slidetodoc.com/presentation_image_h/fb1e9261201d0901a1e54ec3ce050953/image-70.jpg)
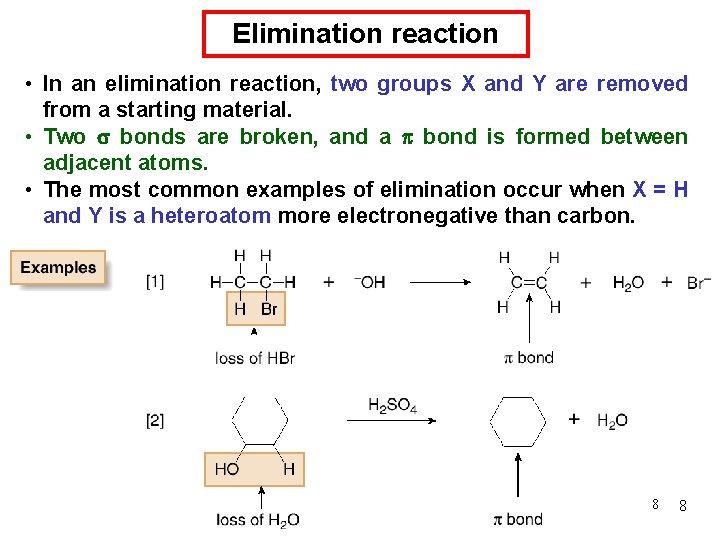
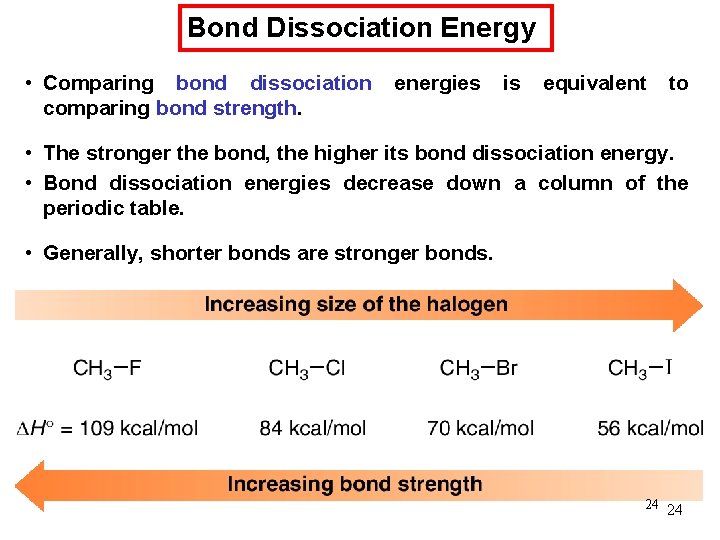
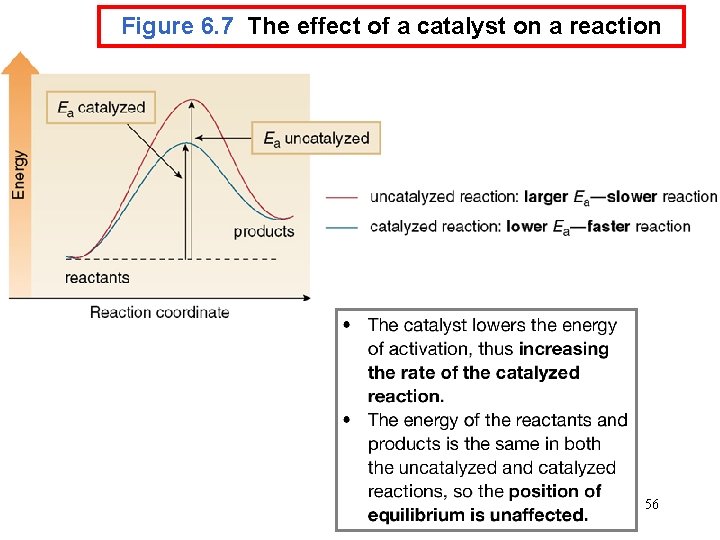
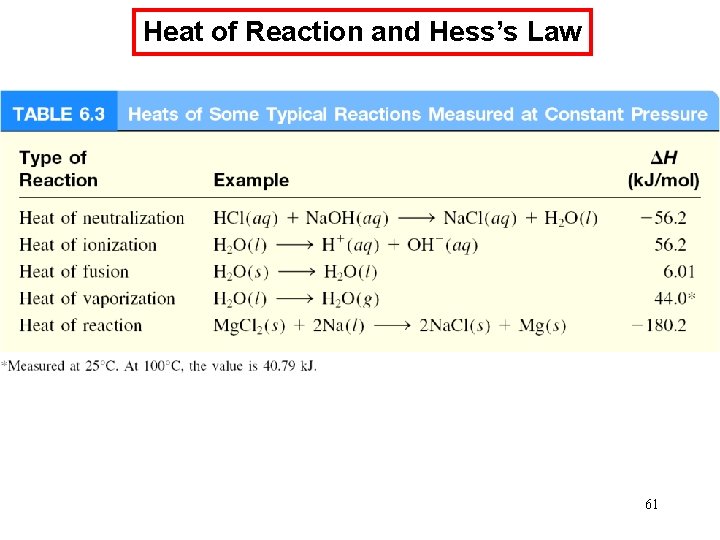
Chemical Kinetics : Zero-Order Reactions A product D[A] rate = Dt D[A] =k Dt rate = M/s k= 0 [A] = [A]0 - kt rate = k [A]0 = k [A] is the concentration of A at any time t [A]0 is the concentration of A at time t = 0 t½ = t when [A] = [A]0/2 [A]0 t½ = 2 k 70

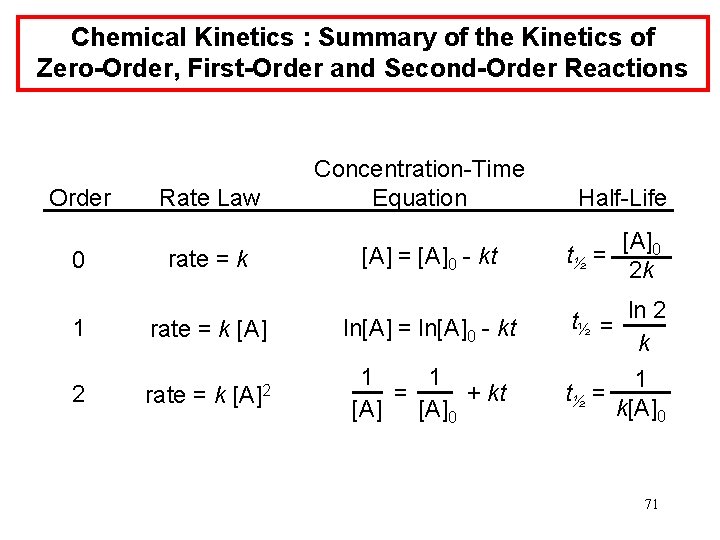
Chemical Kinetics : Summary of the Kinetics of Zero-Order, First-Order and Second-Order Reactions Order 0 Rate Law rate = k 1 rate = k [A] 2 [A]2 rate = k Concentration-Time Equation [A] = [A]0 - kt Half-Life t½ = [A]0 2 k ln[A] = ln[A]0 - kt t½ = ln 2 k 1 1 = + kt [A]0 1 t½ = k[A]0 71