Chapter 6 The structure of matter Chemical Formula






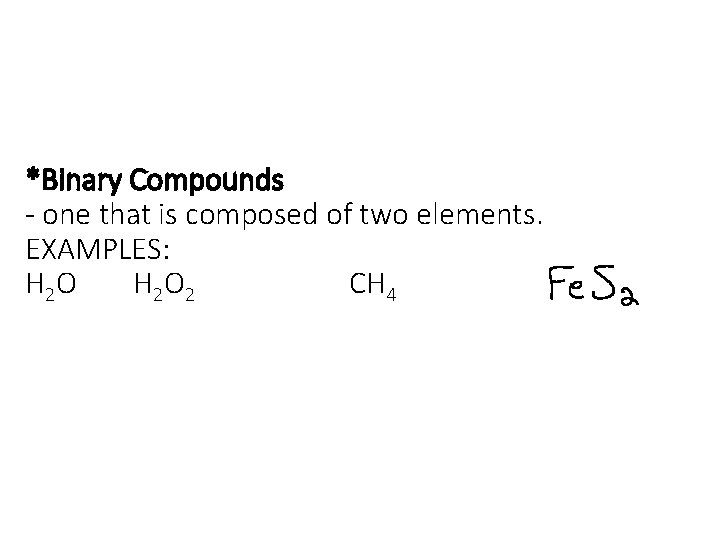
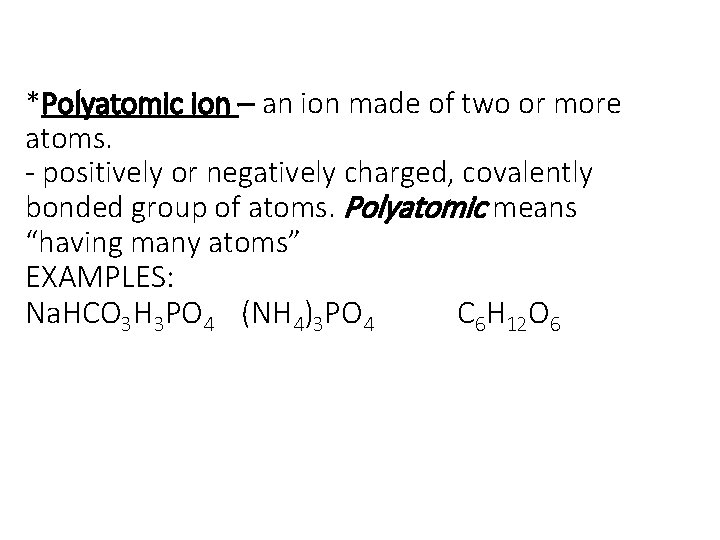




- Slides: 30

Chapter 6 The structure of matter

*Chemical Formula • -tells what elements a compound contains and the exact number of the atoms of each element in a unit of that element • Subscript – means “written below” - tells how many atoms of that element are in a unit of that compound, like the 2 in H 2 O shows there is 2 atoms of Hydrogen.

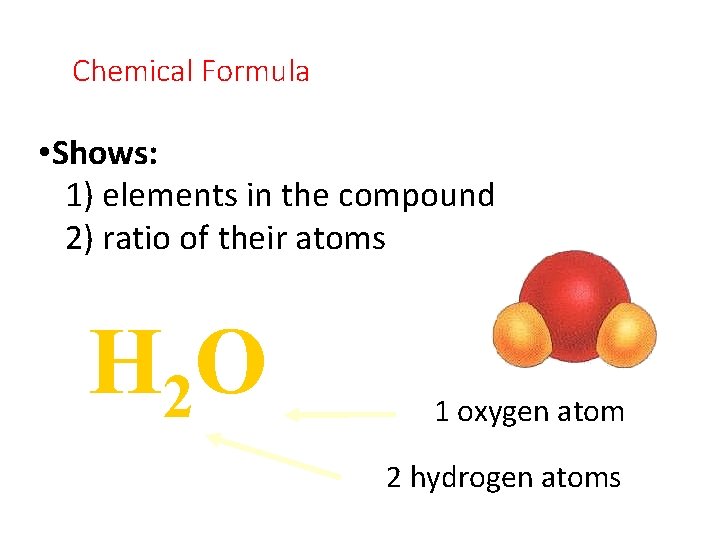
Chemical Formula • Shows: 1) elements in the compound 2) ratio of their atoms H 2 O 1 oxygen atom 2 hydrogen atoms

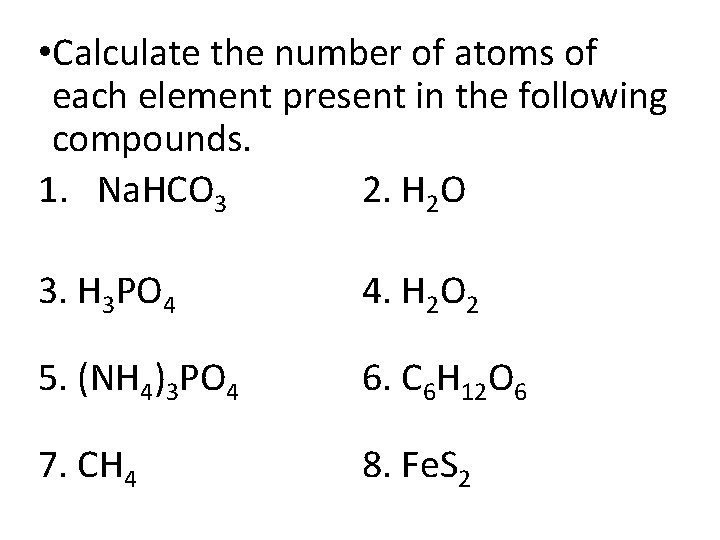
• Calculate the number of atoms of each element present in the following compounds. 1. Na. HCO 3 2. H 2 O 3. H 3 PO 4 4. H 2 O 2 5. (NH 4)3 PO 4 6. C 6 H 12 O 6 7. CH 4 8. Fe. S 2

• Worksheet on Smart Board • On # of atoms

Chemical Bonds • A *compound is made of two or more elements that are chemically combined. • The attractive forces that hold atoms or ions together are called *chemical bonds.

• The structure of chemical compounds can be shown by various models. Different models show different aspects of compounds. • *chemical structure: the arrangement of atoms in a substance

Stability – an atom is *chemically stable when its outer energy level is complete. • Octet Rule • most atoms form bonds in order to have 8 valence e • full outer energy level • like the Noble Gases! Ne • Stability is the driving force behind bond formation!

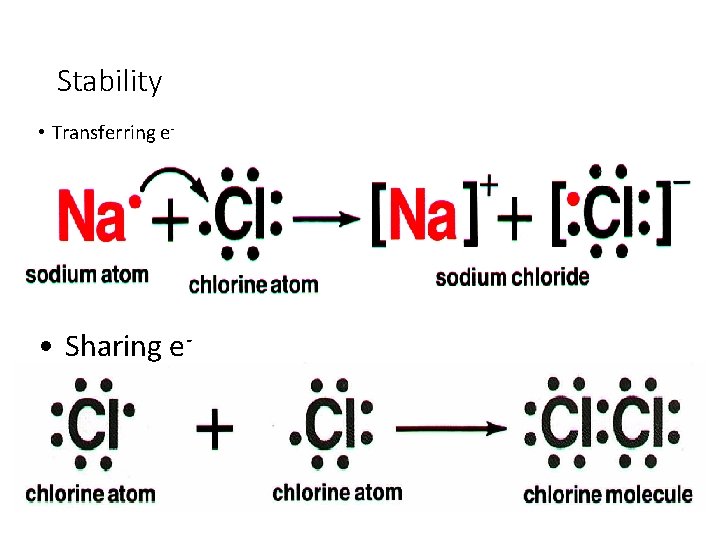
Stability • Transferring e- • Sharing e-

• Worksheet & 2 games on smart board • Choosing whether it’s ionic, covalent, or polyatomic • Then 2 ionic videos…

Ions • - an atom that has lost or gained electrons is called an *ion. • An ion is a charged particle because it has either more or fewer electrons than protons.
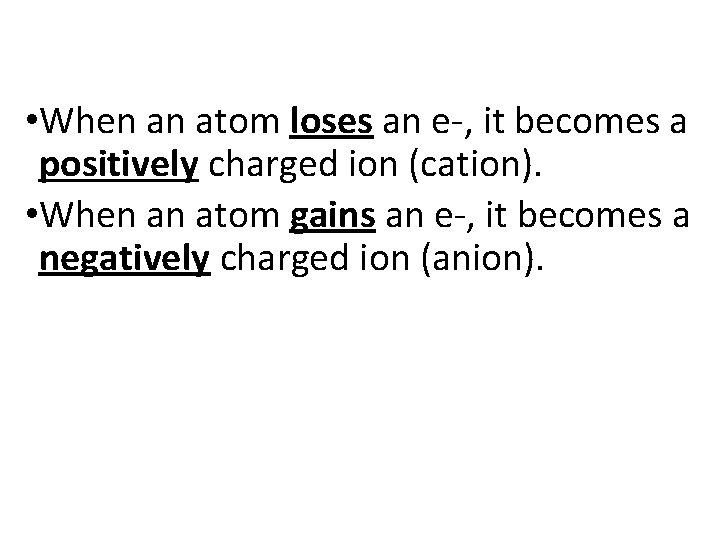
• When an atom loses an e-, it becomes a positively charged ion (cation). • When an atom gains an e-, it becomes a negatively charged ion (anion).

• Smart board game

Ionic Bond • Attraction between 2 oppositely charged ions • Ions - charged atoms • formed by transferring efrom a metal to a nonmetal • Superscript – “written above”

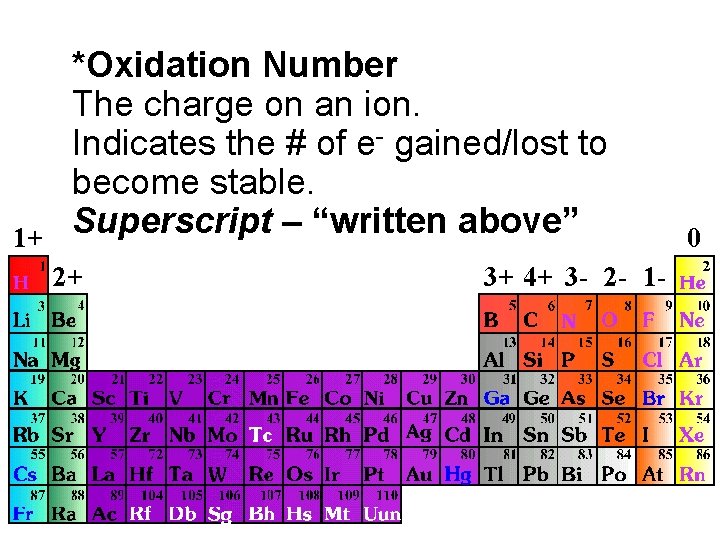
1+ *Oxidation Number The charge on an ion. Indicates the # of e- gained/lost to become stable. Superscript – “written above” 2+ 3+ 4+ 3 - 2 - 1 - 0
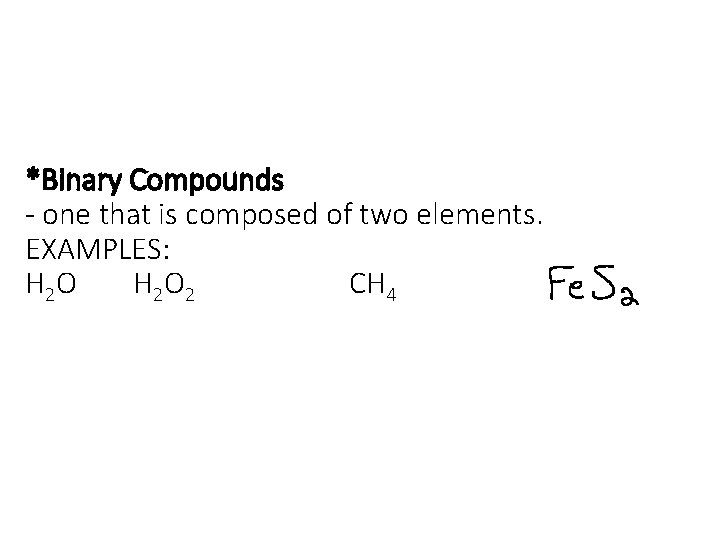
*Binary Compounds - one that is composed of two elements. EXAMPLES: H 2 O 2 CH 4
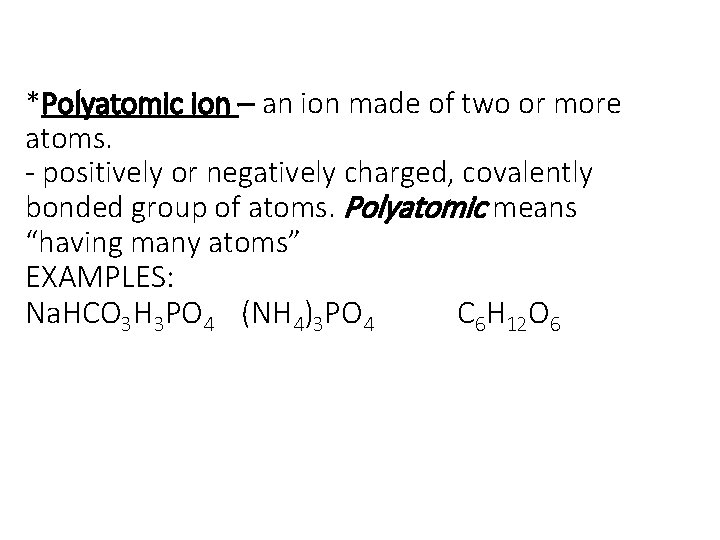
*Polyatomic ion – an ion made of two or more atoms. - positively or negatively charged, covalently bonded group of atoms. Polyatomic means “having many atoms” EXAMPLES: Na. HCO 3 H 3 PO 4 (NH 4)3 PO 4 C 6 H 12 O 6

Ionic Names • Write the names of both elements, cation (positive ion) first. • Change the anion’s (negative ion) ending to -ide. • Overall charge = 0.

Ionic Names • Na. Br – sodium bromide • Li 2 O – Lithium oxide

Ionic Names • Write each ion. Put the cation first. • Overall charge must equal zero. – If charges cancel, just write the symbols. – If not, crisscross the charges to find subscripts.

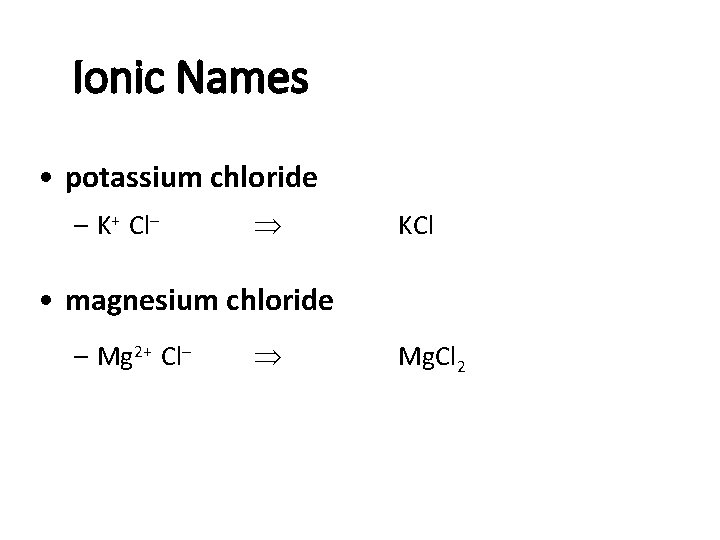
Ionic Names • potassium chloride – K+ Cl- KCl • magnesium chloride – Mg 2+ Cl- Mg. Cl 2

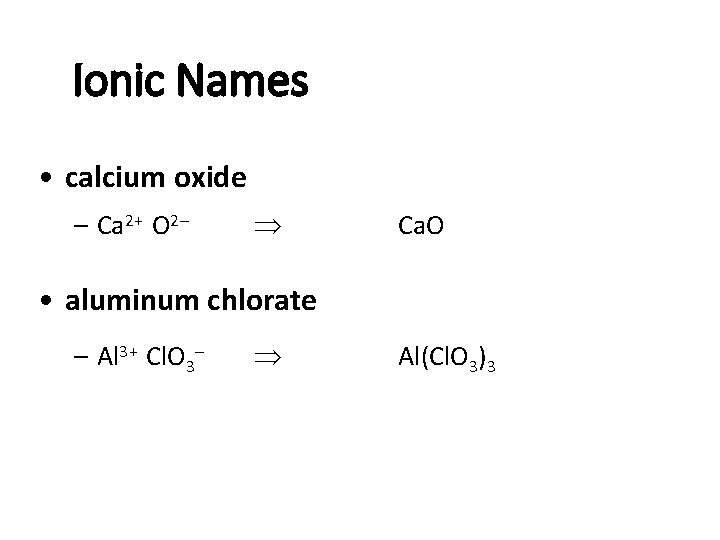
Ionic Names • calcium oxide – Ca 2+ O 2 - Ca. O • aluminum chlorate – Al 3+ Cl. O 3 - Al(Cl. O 3)3

• Smart board game

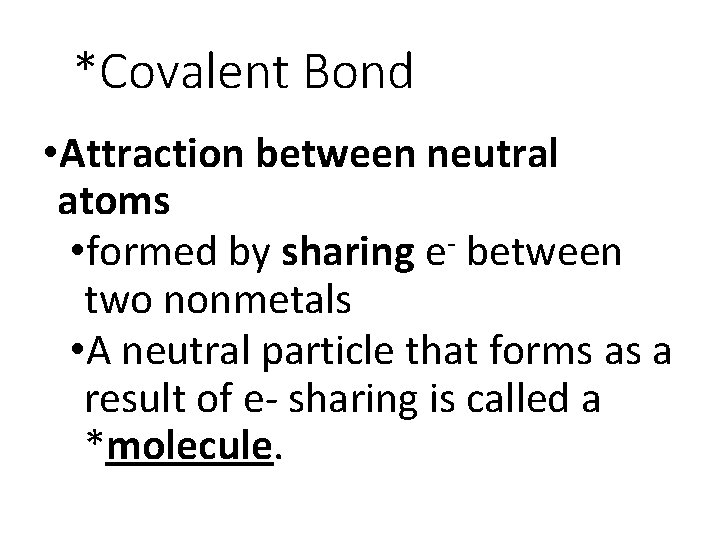
*Covalent Bond • Attraction between neutral atoms • formed by sharing e- between two nonmetals • A neutral particle that forms as a result of e- sharing is called a *molecule.

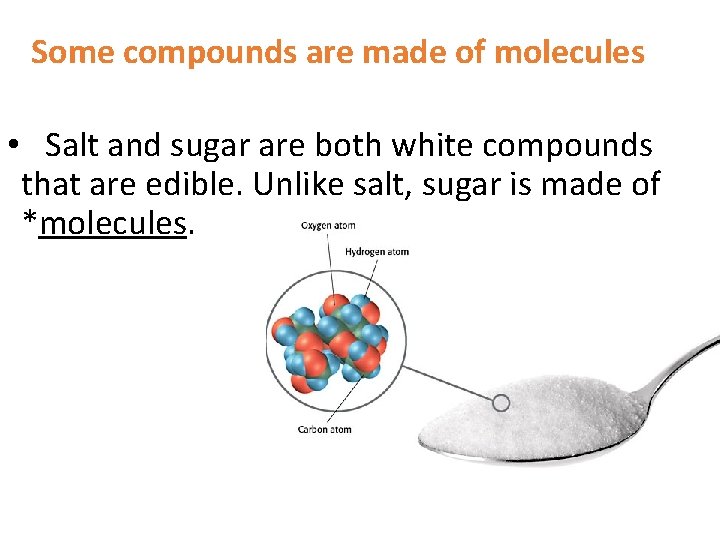
Some compounds are made of molecules • Salt and sugar are both white compounds that are edible. Unlike salt, sugar is made of *molecules.

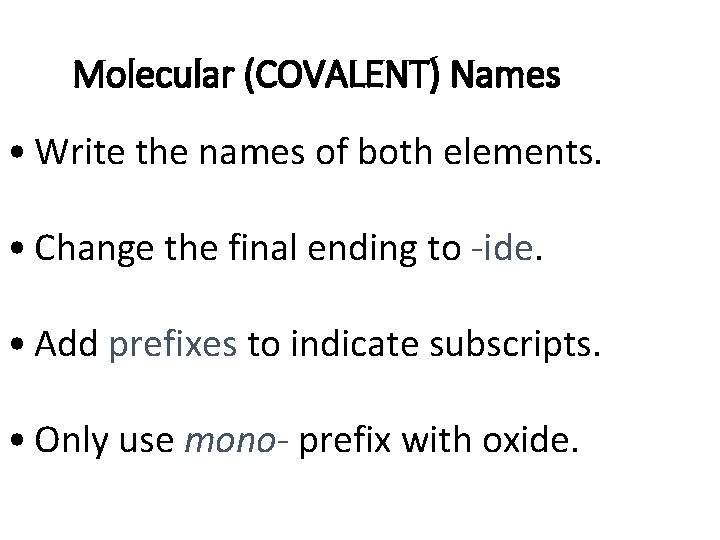
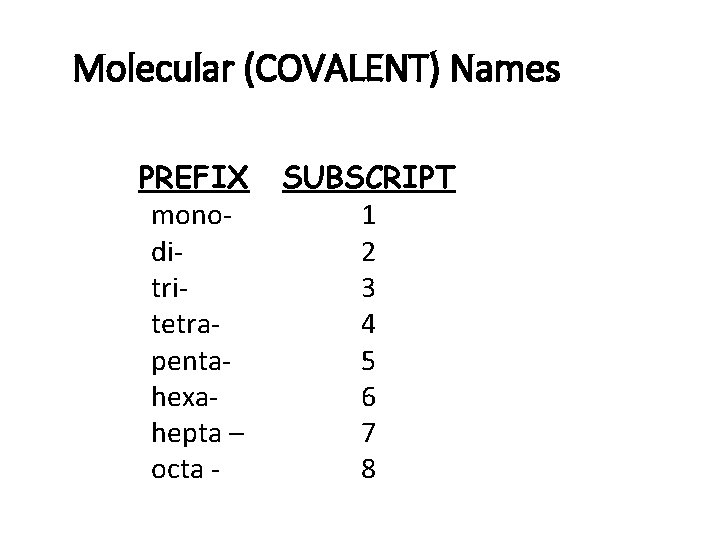
Molecular (COVALENT) Names • Write the names of both elements. • Change the final ending to -ide. • Add prefixes to indicate subscripts. • Only use mono- prefix with oxide.

Molecular (COVALENT) Names PREFIX monoditritetrapentahexahepta – octa - SUBSCRIPT 1 2 3 4 5 6 7 8

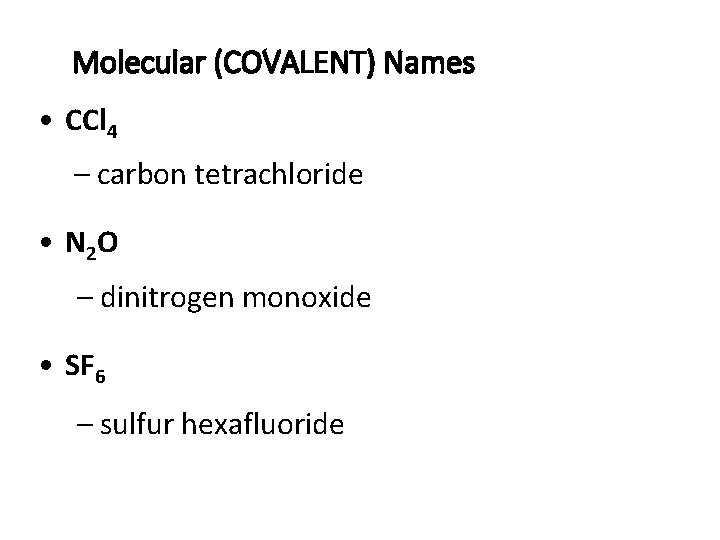
Molecular (COVALENT) Names • CCl 4 – carbon tetrachloride • N 2 O – dinitrogen monoxide • SF 6 – sulfur hexafluoride

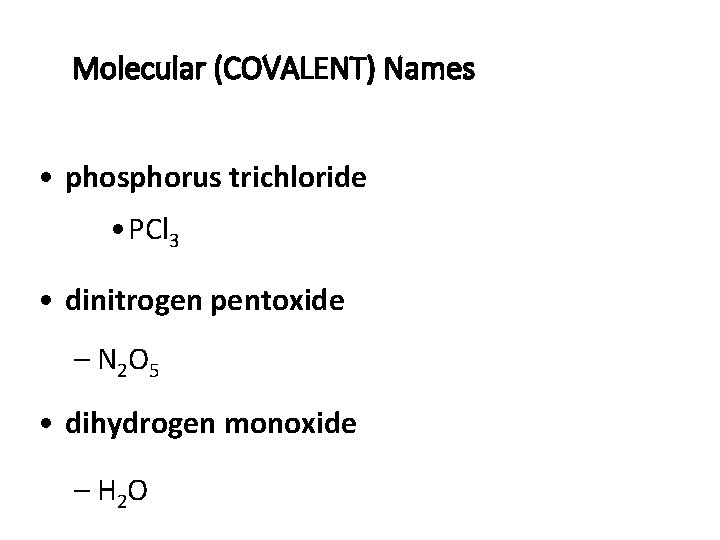
Molecular (COVALENT) Names • phosphorus trichloride • PCl 3 • dinitrogen pentoxide – N 2 O 5 • dihydrogen monoxide – H 2 O

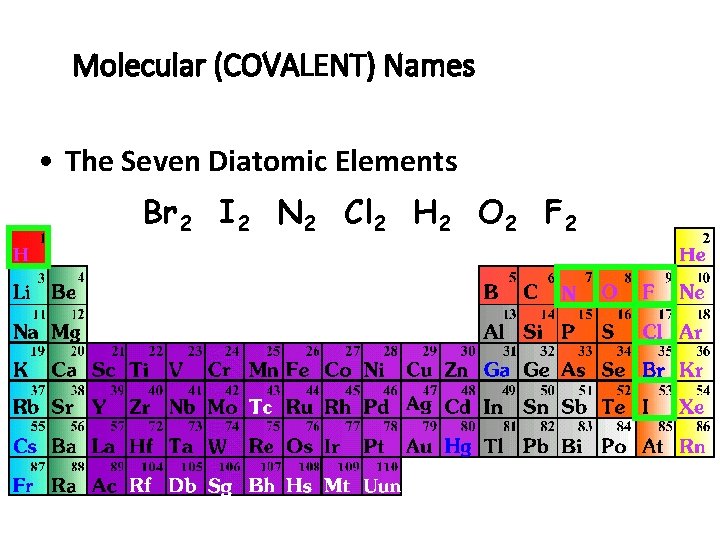
Molecular (COVALENT) Names • The Seven Diatomic Elements Br 2 I 2 N 2 Cl 2 H 2 O 2 F 2