Chapter 6 The Reactions of Alkenes and Alkynes








- Slides: 27

Chapter 6 The Reactions of Alkenes and Alkynes The Stereochemistry of Addition Reactions Paula Yurkanis Bruice University of California, Santa Barbara

Contents of Chapter 6 n Electrophilic Addition Reactions n Carbocations n Skip Carbocation Rearrangements n Various Addition Reactions Chapter 5 2

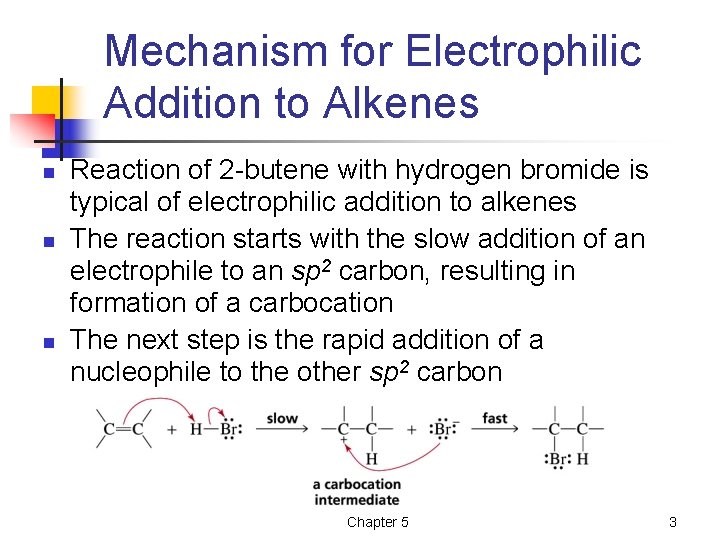
Mechanism for Electrophilic Addition to Alkenes n n n Reaction of 2 -butene with hydrogen bromide is typical of electrophilic addition to alkenes The reaction starts with the slow addition of an electrophile to an sp 2 carbon, resulting in formation of a carbocation The next step is the rapid addition of a nucleophile to the other sp 2 carbon Chapter 5 3

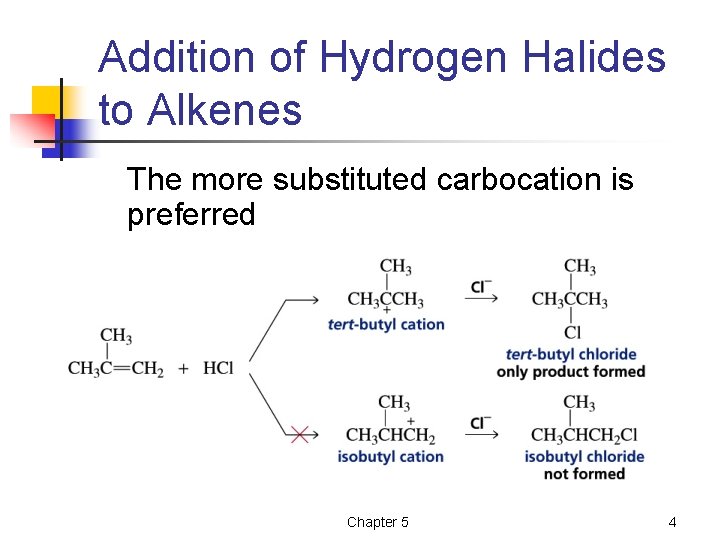
Addition of Hydrogen Halides to Alkenes The more substituted carbocation is preferred Chapter 5 4

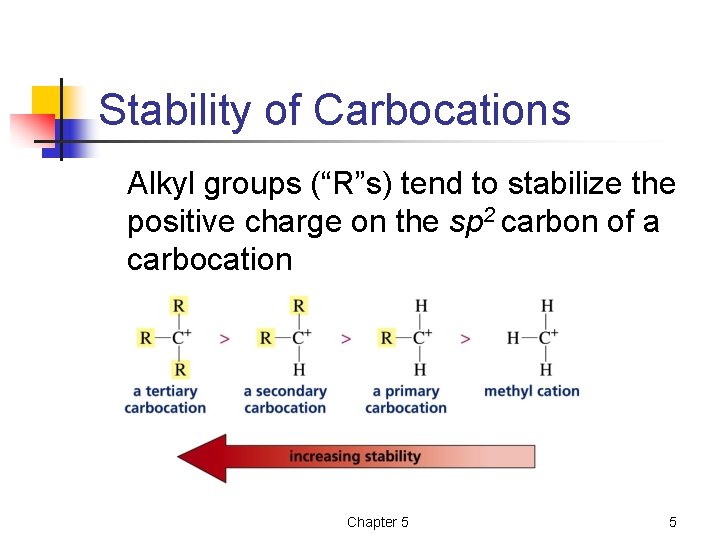
Stability of Carbocations Alkyl groups (“R”s) tend to stabilize the positive charge on the sp 2 carbon of a carbocation Chapter 5 5

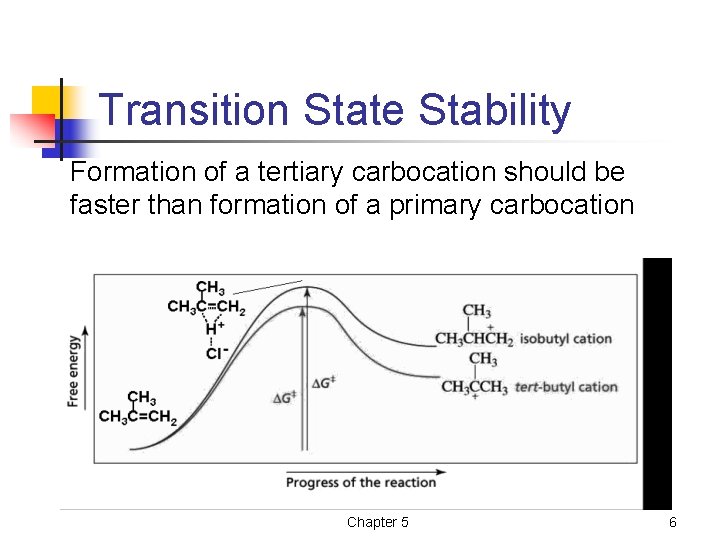
Transition State Stability Formation of a tertiary carbocation should be faster than formation of a primary carbocation Chapter 5 6

Markovnikov’s Rule n n Modern equivalent statement: The electrophile adds to the sp 2 carbon that forms the least stable carbocation The other carbon forms the carbocation intermediate. Predicting product: put + and – signs under carbons and electrophile and nucleophile and swap partners. Chapter 5 7

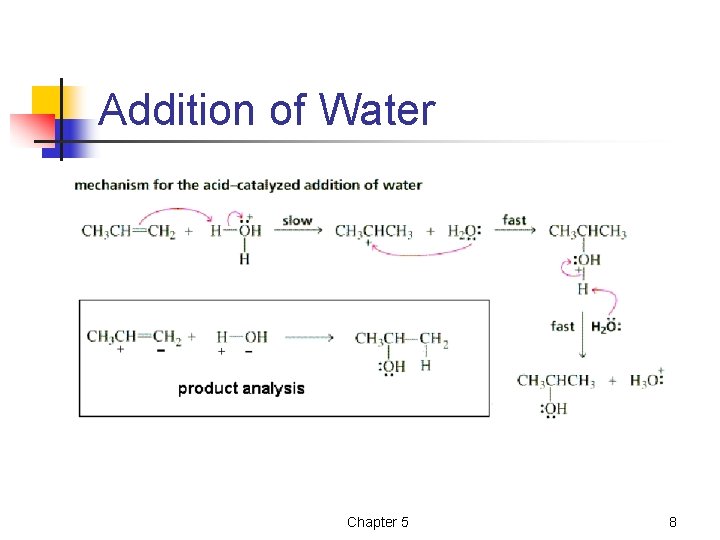
Addition of Water Chapter 5 8

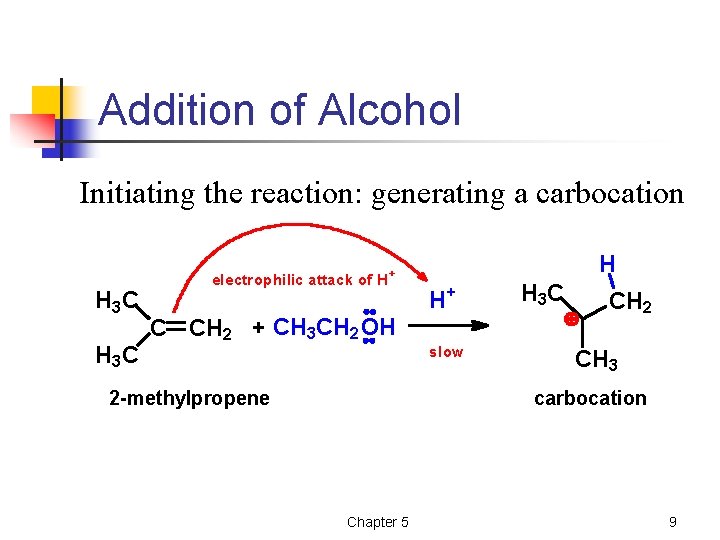
Addition of Alcohol Initiating the reaction: generating a carbocation H 3 C electrophilic attack of H + C CH 2 + CH 3 CH 2 OH 2 -methylpropene H H+ slow H 3 C CH 2 CH 3 carbocation Chapter 5 9

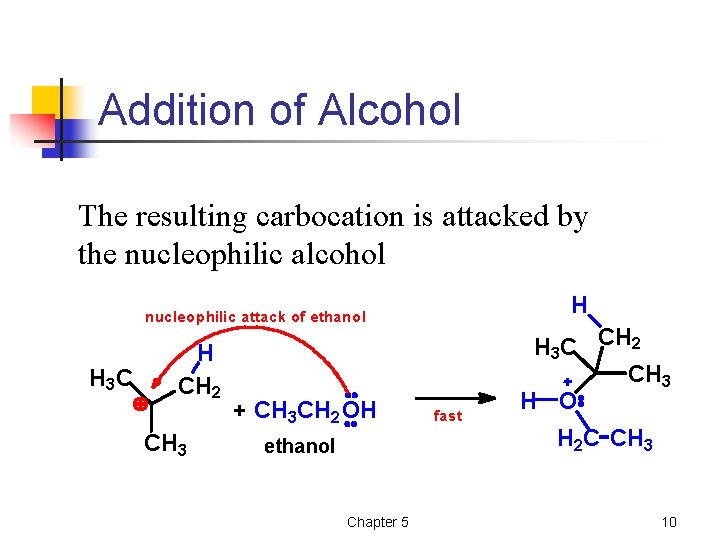
Addition of Alcohol The resulting carbocation is attacked by the nucleophilic alcohol H nucleophilic attack of ethanol H 3 C H CH 2 CH 3 + CH 3 CH 2 OH ethanol Chapter 5 fast H 3 C CH 2 CH 3 H O H 2 C CH 3 10

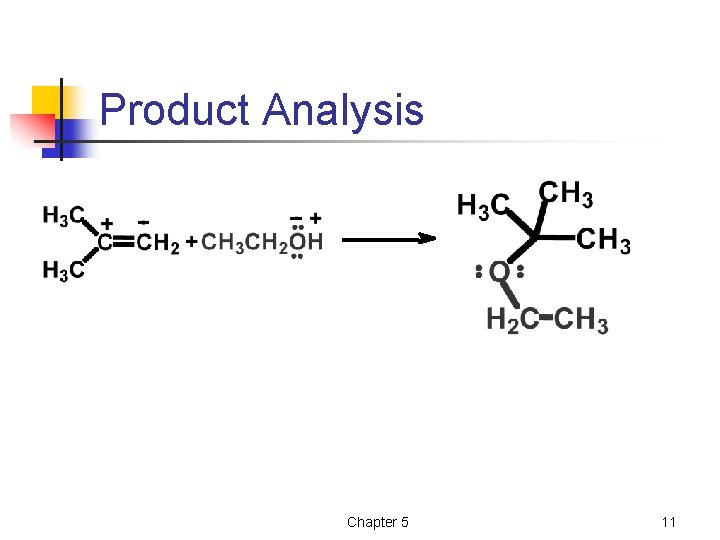
Product Analysis Chapter 5 11

Alkynes Common names of alkynes are based on substitution of the simplest alkyne, acetylene Chapter 5 12

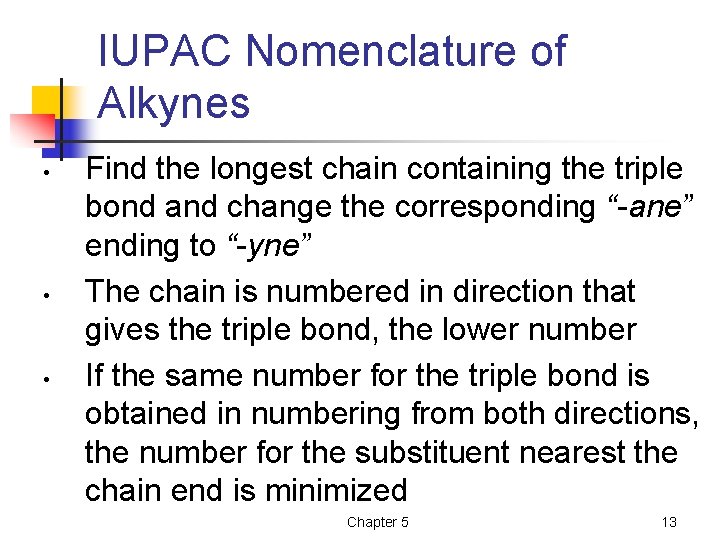
IUPAC Nomenclature of Alkynes • • • Find the longest chain containing the triple bond and change the corresponding “-ane” ending to “-yne” The chain is numbered in direction that gives the triple bond, the lower number If the same number for the triple bond is obtained in numbering from both directions, the number for the substituent nearest the chain end is minimized Chapter 5 13

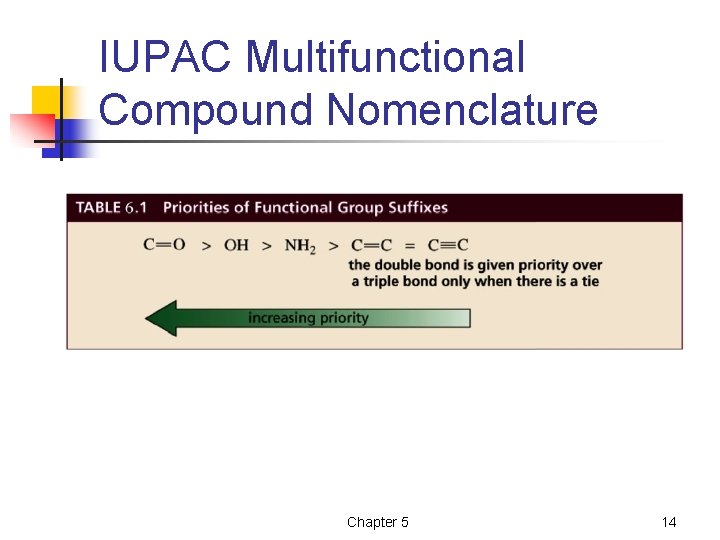
IUPAC Multifunctional Compound Nomenclature Chapter 5 14

IUPAC Multifunctional Compound Nomenclature • • The longest chain has to go past the highest-priority functional group High prio group has lowest possible number If not highest priority NH 2 is amino and OH is hydroxy substituent General form is n-substit-n-alken-n-yn-n-groupsuffix Chapter 5 15

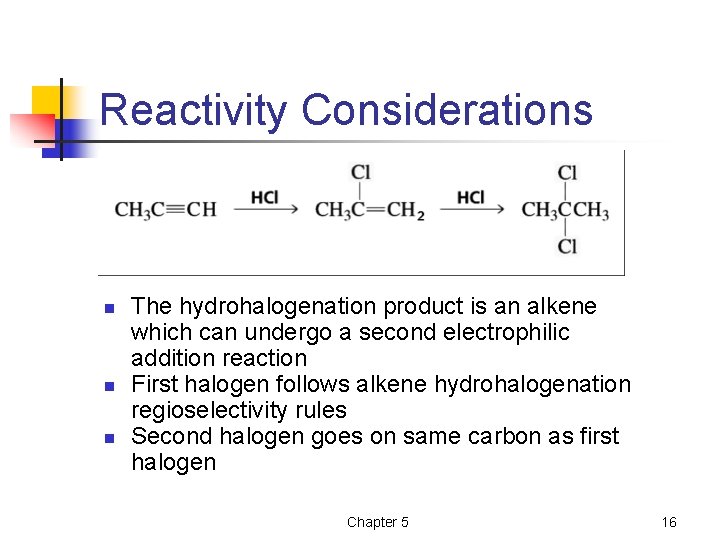
Reactivity Considerations n n n The hydrohalogenation product is an alkene which can undergo a second electrophilic addition reaction First halogen follows alkene hydrohalogenation regioselectivity rules Second halogen goes on same carbon as first halogen Chapter 5 16

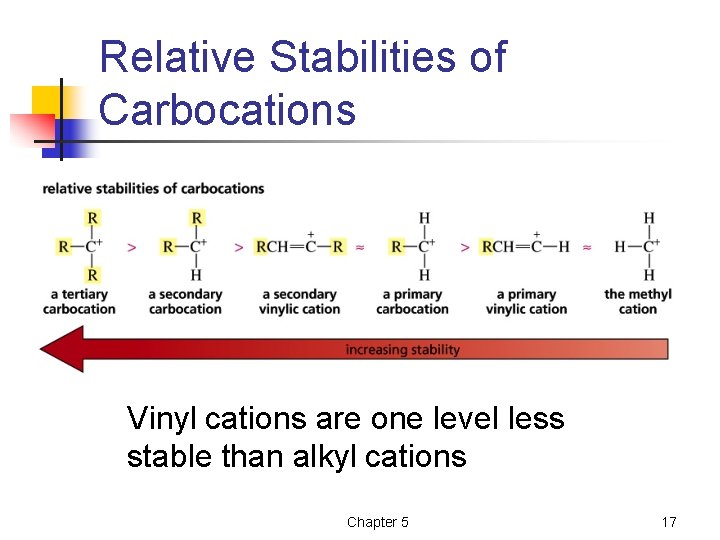
Relative Stabilities of Carbocations Vinyl cations are one level less stable than alkyl cations Chapter 5 17

Halogen Addition to Alkynes n n Halogens add to alkynes as well as to alkenes Excess halogen leads to the addition of a second equivalent Chapter 5 18

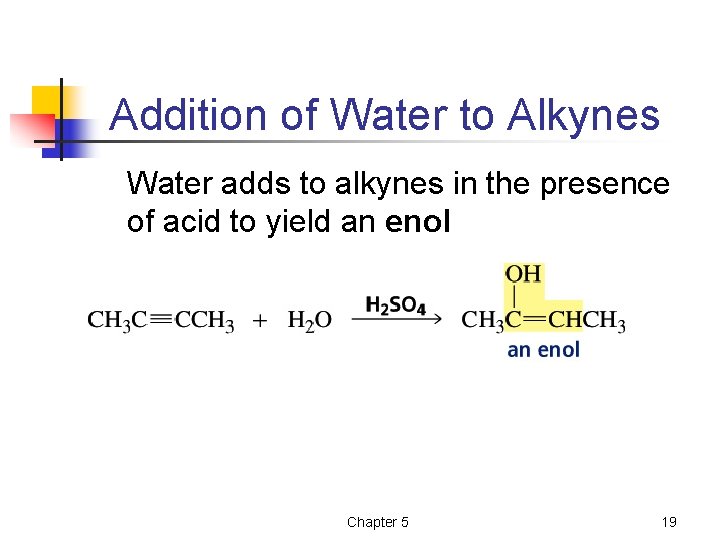
Addition of Water to Alkynes Water adds to alkynes in the presence of acid to yield an enol Chapter 5 19

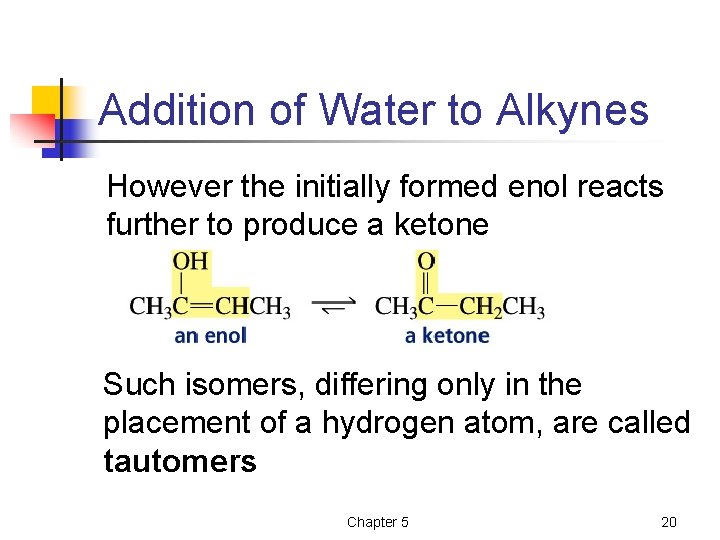
Addition of Water to Alkynes However the initially formed enol reacts further to produce a ketone Such isomers, differing only in the placement of a hydrogen atom, are called tautomers Chapter 5 20

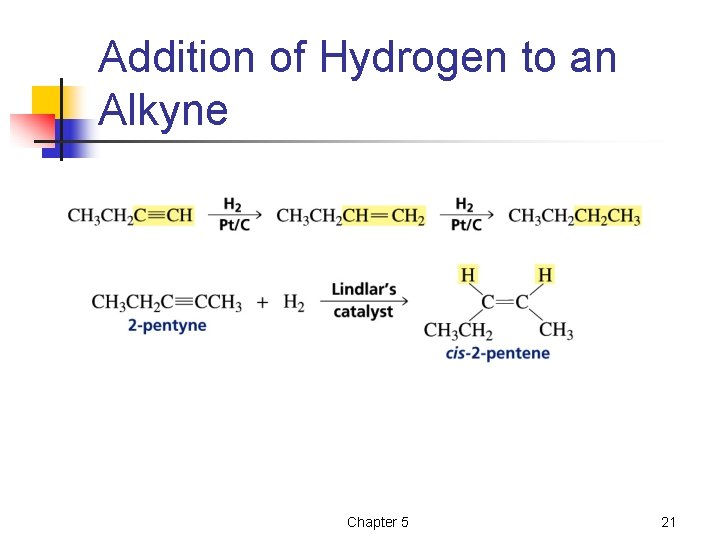
Addition of Hydrogen to an Alkyne Chapter 5 21

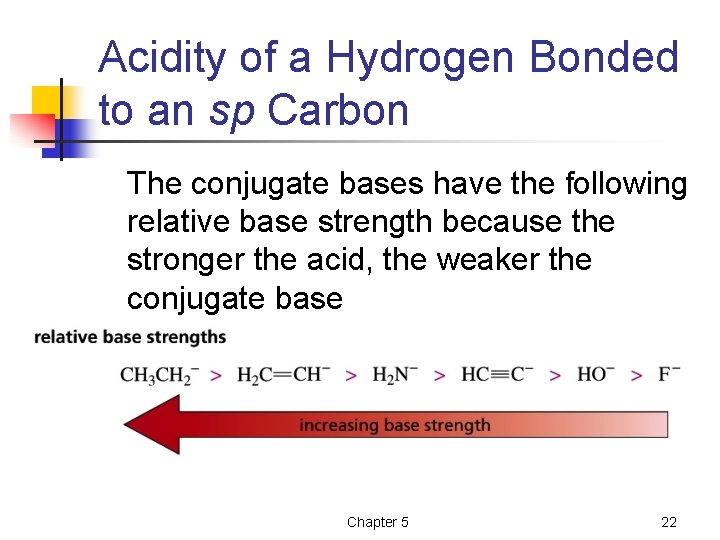
Acidity of a Hydrogen Bonded to an sp Carbon The conjugate bases have the following relative base strength because the stronger the acid, the weaker the conjugate base Chapter 5 22

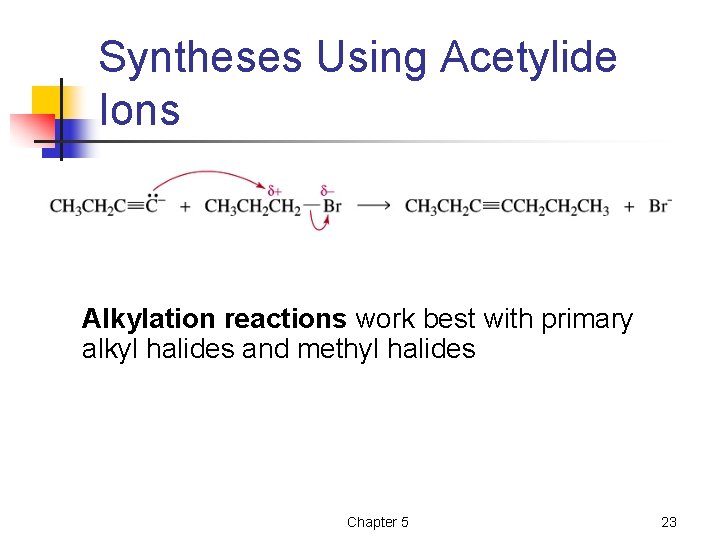
Syntheses Using Acetylide Ions Alkylation reactions work best with primary alkyl halides and methyl halides Chapter 5 23

Introduction to Multistep Synthesis The thought process is known as retrosynthetic analysis and is indicated by using open arrows Chapter 5 24

Introduction to Multistep Synthesis - Considerations n n Alkene products made from alkynes Carbonyl products made from alkynes Alkane products made from alkenes Understand issues involved in proper choice of reagents thoroughly Chapter 5 25

Introduction to Multistep Synthesis – Reagent Issues n n n Regioselectivity of HX + alkene/alkyne rxn Regioselectivity of 2 HX + alkyne rxn Stereospecificity of H 2/Lindlar reduction H 2 with Pd/C, Pt/C, or Ni does complete reduction RX + Na. NH 2 + H-C=C-R rxn works best with primary halide Chapter 5 26

Introduction to Multistep Synthesis – Practice Use retrosynthetic analysis to make these: Chapter 5 27