Chapter 6 The Periodic Table Periodic table Elements

Chapter 6 The Periodic Table
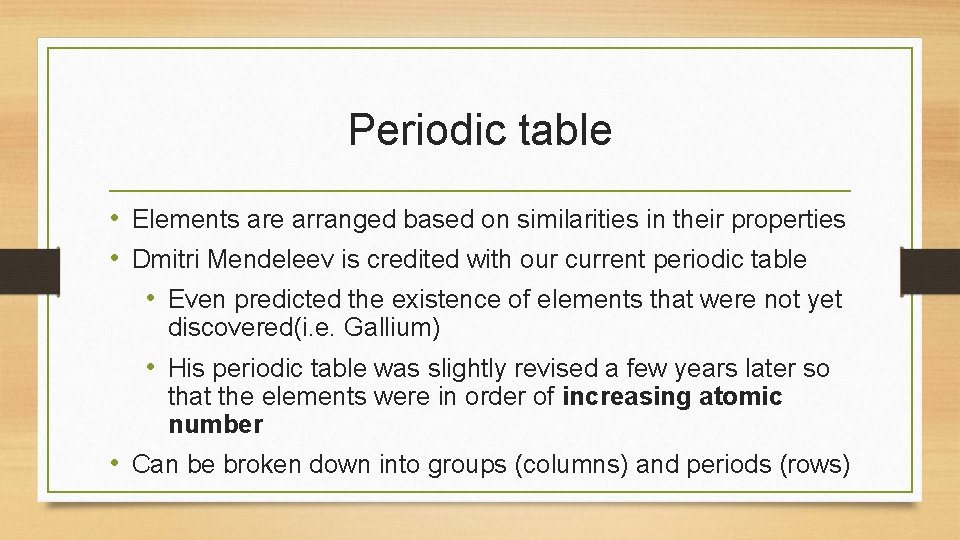
Periodic table • Elements are arranged based on similarities in their properties • Dmitri Mendeleev is credited with our current periodic table • Even predicted the existence of elements that were not yet discovered(i. e. Gallium) • His periodic table was slightly revised a few years later so that the elements were in order of increasing atomic number • Can be broken down into groups (columns) and periods (rows)

Periods • Range from 1 -7 • Indicates the highest occupied energy level in an atom
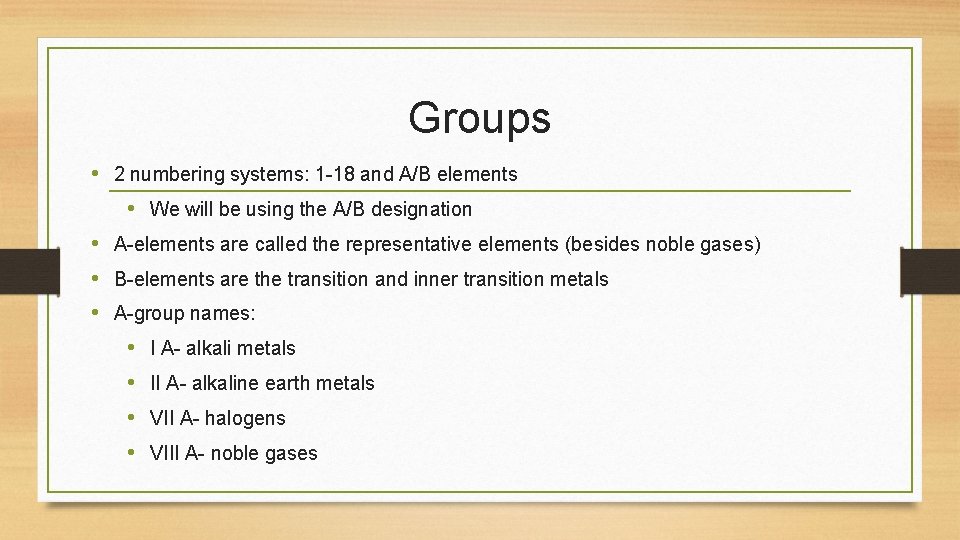
Groups • 2 numbering systems: 1 -18 and A/B elements • We will be using the A/B designation • A-elements are called the representative elements (besides noble gases) • B-elements are the transition and inner transition metals • A-group names: • I A- alkali metals • II A- alkaline earth metals • VII A- halogens • VIII A- noble gases

Zig-zag line • • Locate the zig-zag line starting at III A Metals are to the left of the line Nonmetals are to the right of the line Metalloids are those elements that border the line (except Al !!)
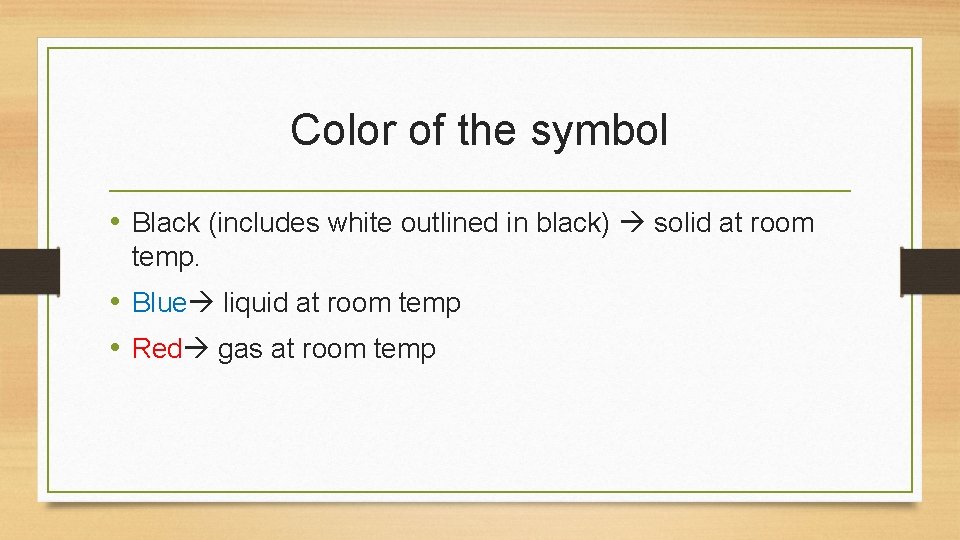
Color of the symbol • Black (includes white outlined in black) solid at room temp. • Blue liquid at room temp • Red gas at room temp

Periodic Trends Chapter 6
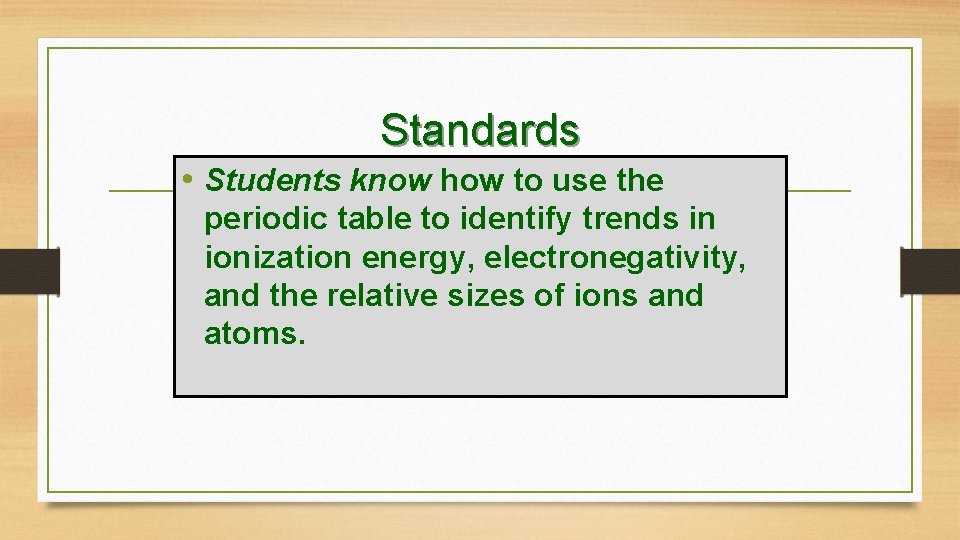
Standards • Students know how to use the periodic table to identify trends in ionization energy, electronegativity, and the relative sizes of ions and atoms.
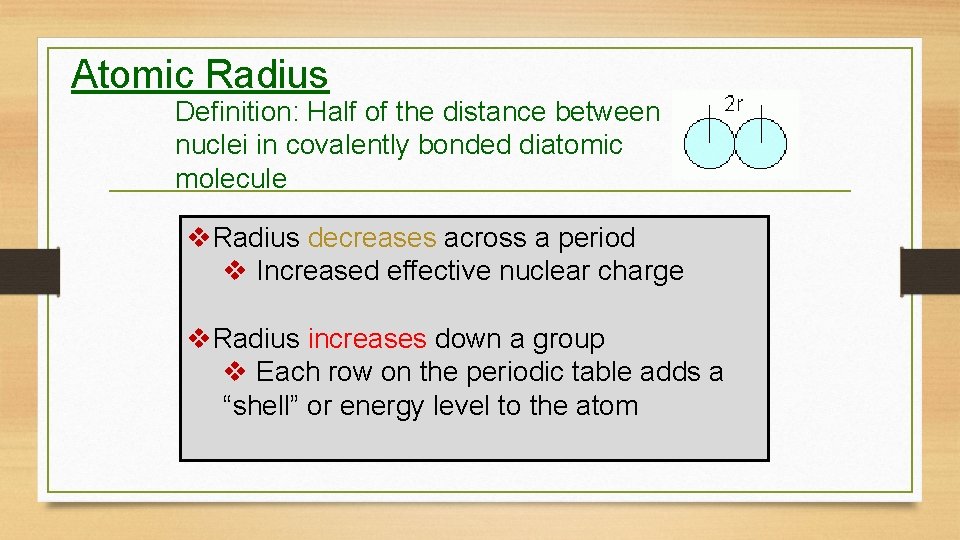
Atomic Radius Definition: Half of the distance between nuclei in covalently bonded diatomic molecule v. Radius decreases across a period v Increased effective nuclear charge v. Radius increases down a group v Each row on the periodic table adds a “shell” or energy level to the atom
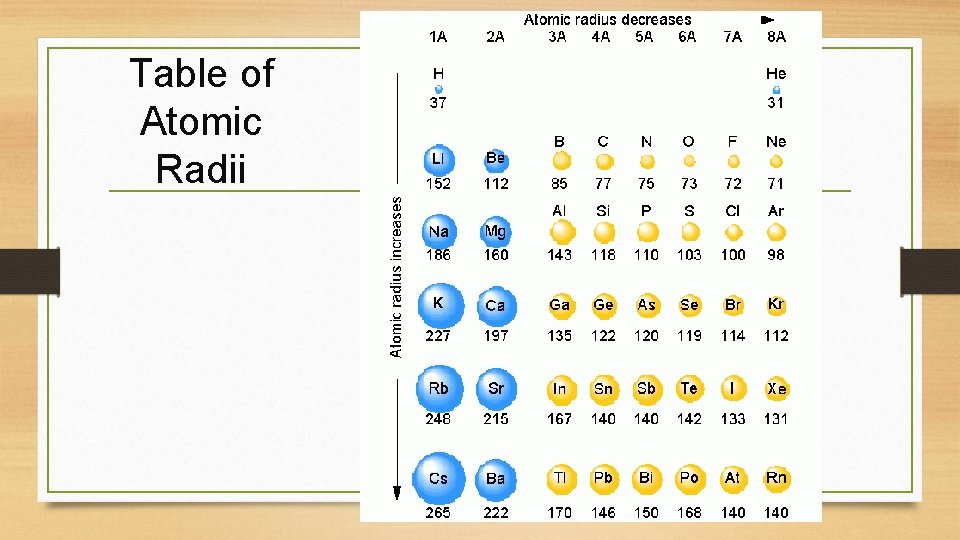
Table of Atomic Radii
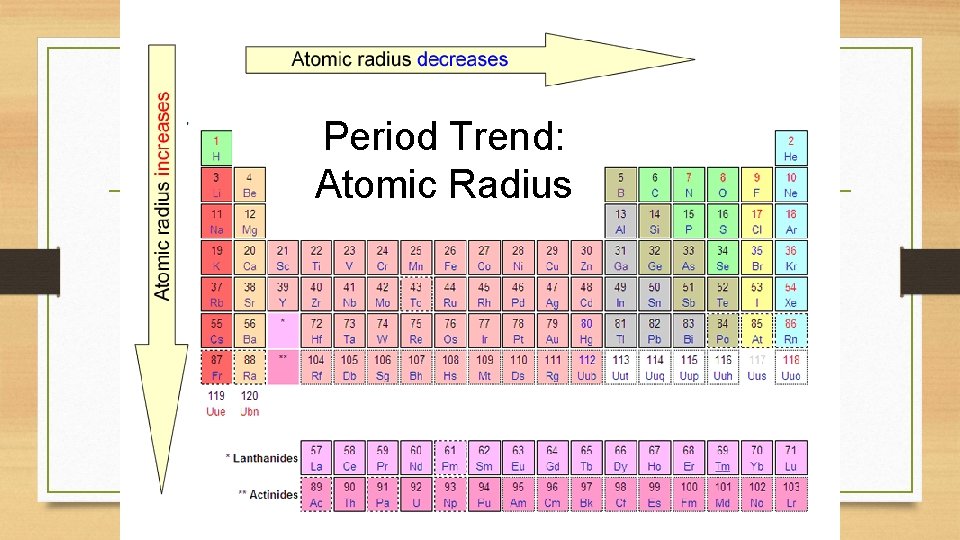
Period Trend: Atomic Radius

Ionization Energy Definition: the energy required to remove an electron from an atom q Tends to increase across a period q As radius decreases across a period, the electron you are removing is closer to the nucleus and harder to remove q Tends to decrease down a group q Outer electrons are farther from the nucleus and easier to remove
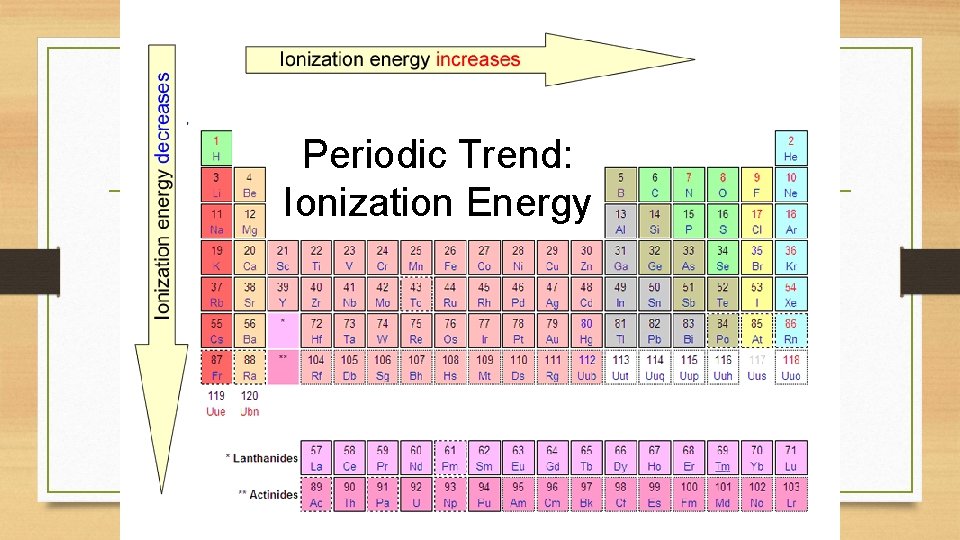
Periodic Trend: Ionization Energy
Electronegativity Definition: A measure of the ability of an atom in a chemical compound to attract electrons o Tends to increase across a period o As radius decreases, electrons get closer to the bonding atom’s nucleus o Tends to decrease down a group o. As radius increases, electrons are farther from the bonding atom’s nucleus
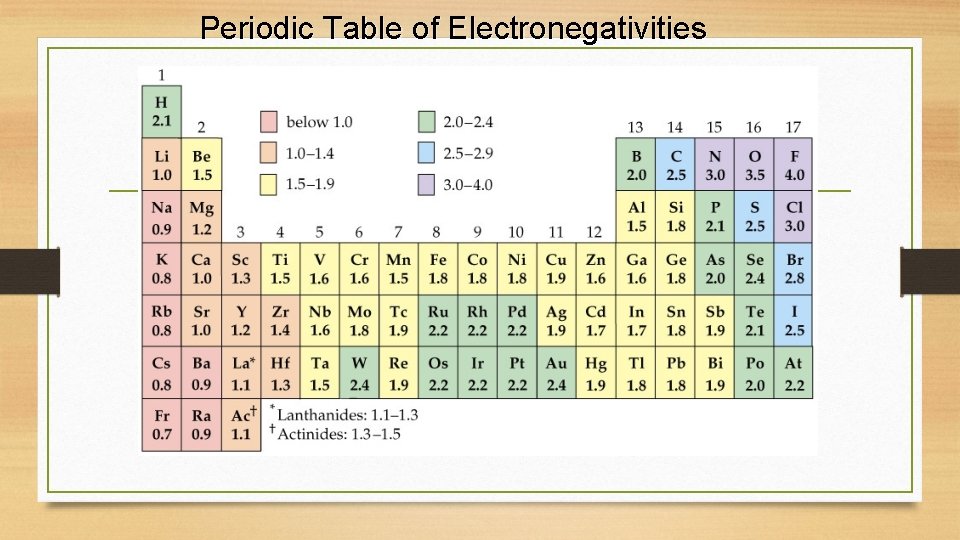
Periodic Table of Electronegativities
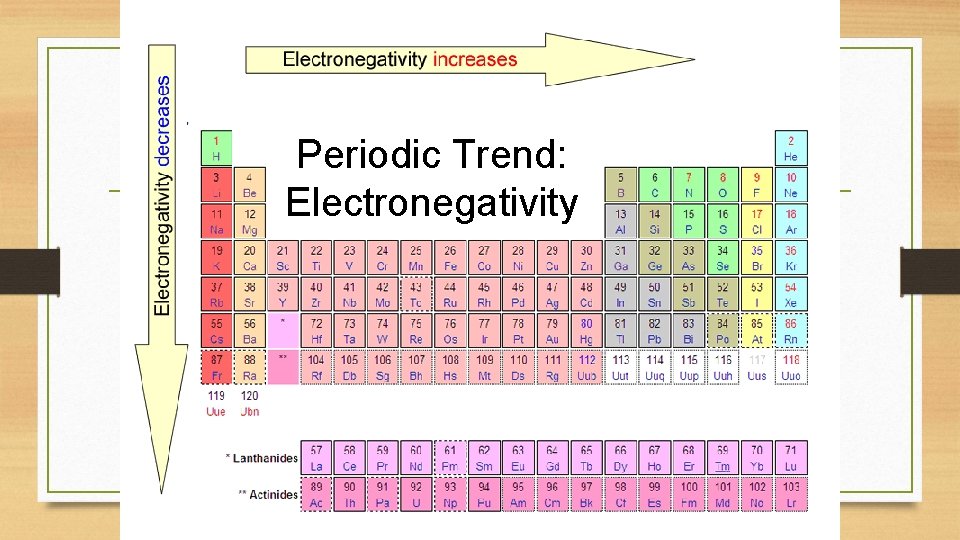
Periodic Trend: Electronegativity
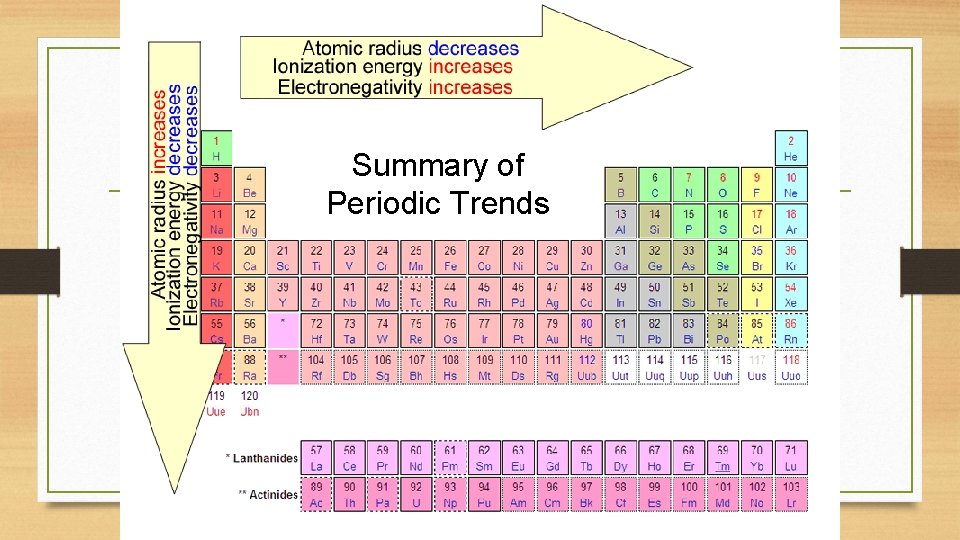
Summary of Periodic Trends
Ionic Radii q Positively charged ions formed when an atom of a metal loses one or Cations more electrons q Smaller than the corresponding atom q Negatively charged ions formed when nonmetallic atoms gain one Anions or more electrons q Larger than the corresponding atom
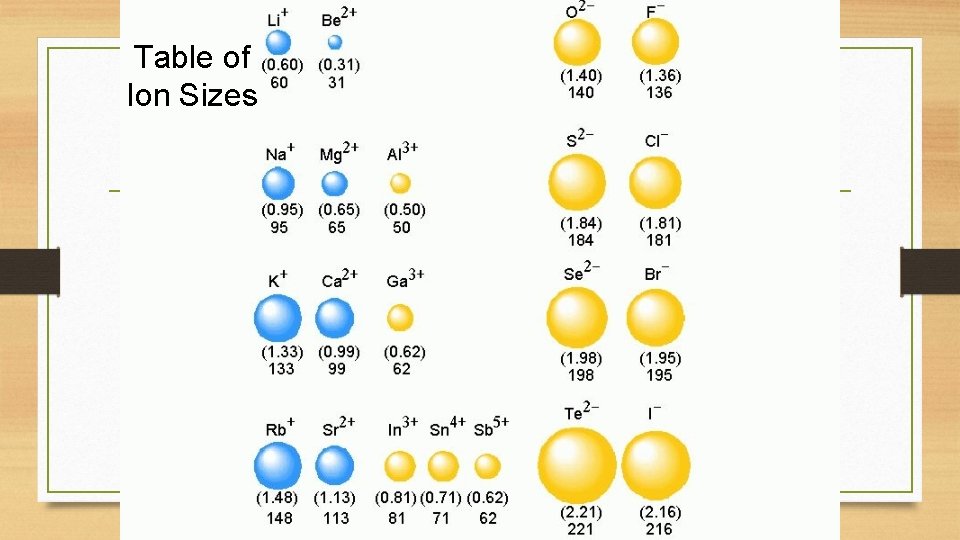
Table of Ion Sizes
Practice • Identify each of the following: • Which has a larger atomic radius? • F , O • Na , Li • Which has a higher ionization energy? • Li , B • Mg , Sr • Which has a higher electronegativity value? • Na , K • C , N • Which particle has to largest radium in each atom/ion pair? • Na , Na+ • S , S-2
- Slides: 20