Chapter 6 The periodic table CH 6 Outline

Chapter 6 - The periodic table.

CH 6 Outline 1) 2) 3) 4) 5) Model of the atom (Bohr and Current) Bohr pictures Mendeleev and the formation of the Periodic Table PT Families and their characteristics Trends 1) 2) 3) 4) 5) 6) Atomic Radius Electronegativity Ionization Energy Valence EActivity Metallic character
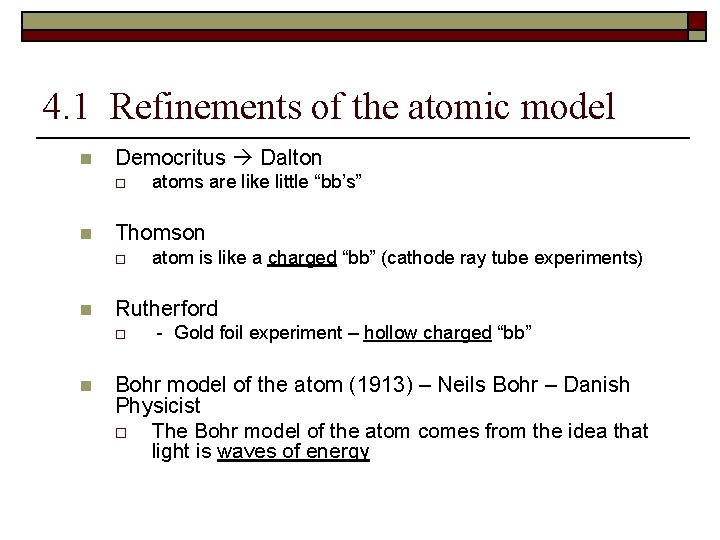
4. 1 Refinements of the atomic model n Democritus Dalton o n Thomson o n atom is like a charged “bb” (cathode ray tube experiments) Rutherford o n atoms are like little “bb’s” - Gold foil experiment – hollow charged “bb” Bohr model of the atom (1913) – Neils Bohr – Danish Physicist o The Bohr model of the atom comes from the idea that light is waves of energy

Bohr Model 1 -20 atoms and ions

Heisenberg Uncertainty Principle o There are limits to our ability to measure both a particle’s velocity and its position at the same instant
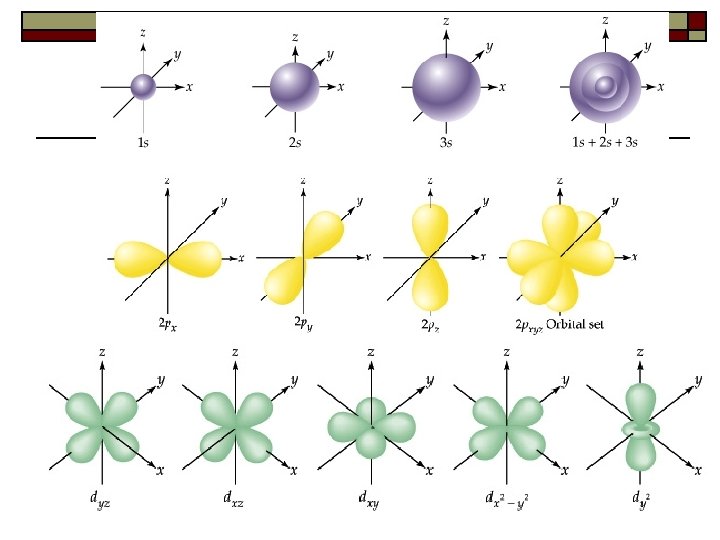

F-Orbital

Crash Course Periodic Table Video
Origin of the Periodic Table o Dimitri Mendeleev - publish first real periodic table – 1869 n n Based on chemical and physical properties Listed elements in increasing atomic mass order Left spaces for undiscovered elements His basic rule: the elements in any group, of the table are similar to their column-mates.
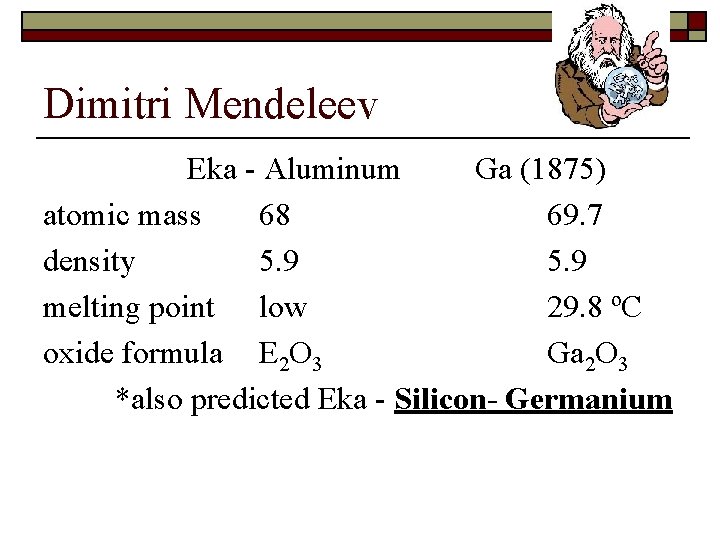
Dimitri Mendeleev Eka - Aluminum Ga (1875) atomic mass 68 69. 7 density 5. 9 melting point low 29. 8 ºC oxide formula E 2 O 3 Ga 2 O 3 *also predicted Eka - Silicon- Germanium
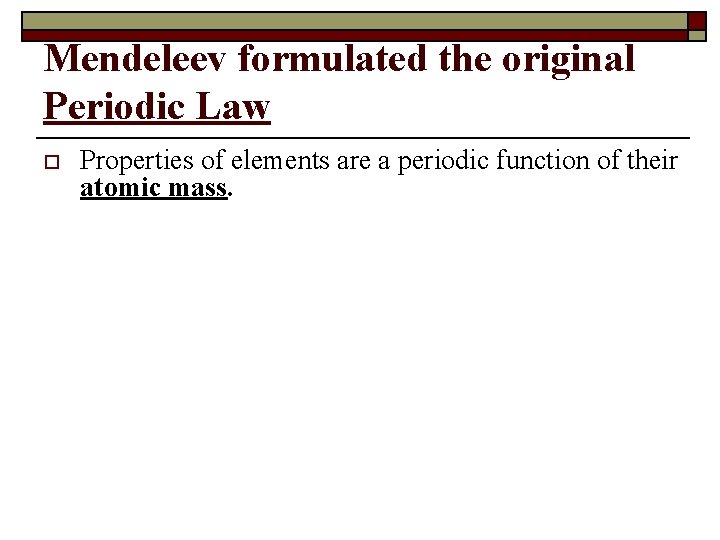
Mendeleev formulated the original Periodic Law o Properties of elements are a periodic function of their atomic mass.
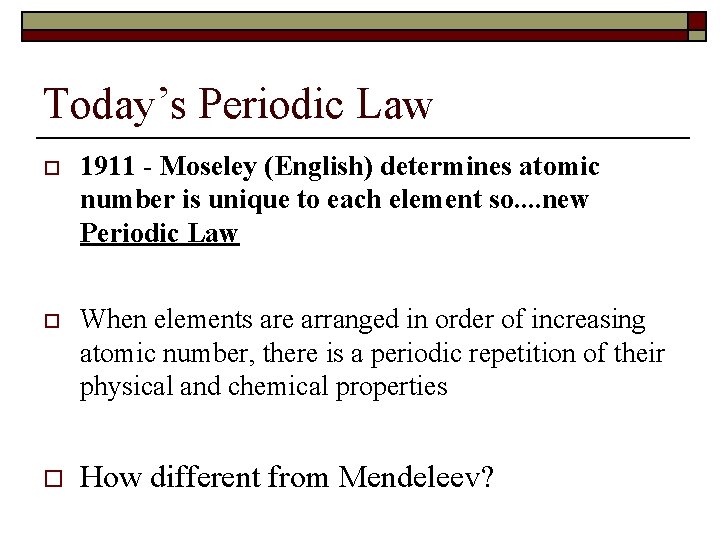
Today’s Periodic Law o 1911 - Moseley (English) determines atomic number is unique to each element so. . new Periodic Law o When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties o How different from Mendeleev?

o *** History lesson - After his brilliant discovery, Moseley was drafted into the infantry to fight for the crown in WW I. He was killed. Only after the war was it realized that scientists should probably not be drafted into combat roles. That policy exists to this day.

Review o o o Metals, Non Metals and Metalloid location Period vs Family Group Names
Periodic Table Puzzle
Valence e- of periodic table
Metals o o o Good conductors of heat and electricity Shiny (high luster) Ductile Malleable Solid at room temperature

Non Metals o o Mostly gases at room temperature (solids and liquids do exist) In general: n n Poor conductors of heat and electricity (insulators) Brittle

Metalloid o o B, Si, As, Te, At, Ge, Sb Sometimes behaves like metal, other times like a non metal

Names of Families (brainiac video) o o o Group 1 - Alkali Metals - These compounds are not found alone in nature - why? explosive with water they are stored under kerosene - very reactive. They react with nonmetals to form salts. They are silvery, shiny (luster), have a low melting point, and are soft (so soft, you can cut them with a knife). They are malleable (able to flattened into a sheet) and ductile (able to be drawn into a wire). Sodium and Potassium are particularly important in body chemistry.

Names of Families o o o Group 2 - Alkaline Earth Metals - 2 nd most reactive elements. Also not “lone state” elements. Harder, denser than group 1. Common in sea salts.
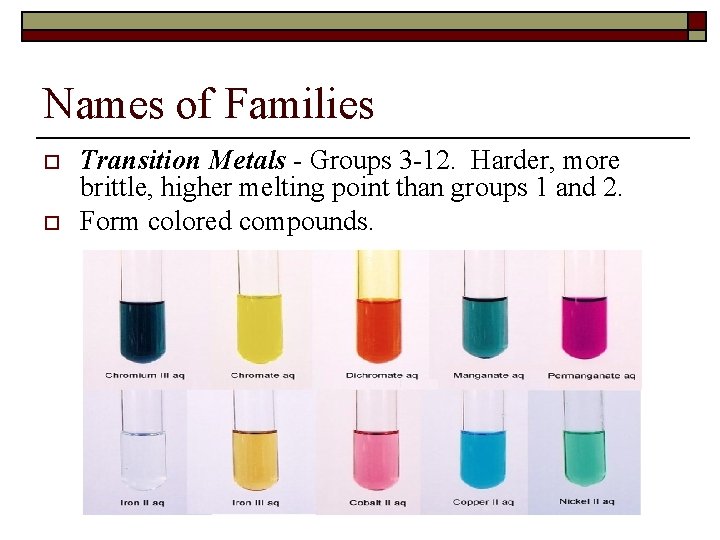
Names of Families o o Transition Metals - Groups 3 -12. Harder, more brittle, higher melting point than groups 1 and 2. Form colored compounds.

Transition Metals continued o o They can't be divided neatly into groups; all of them have very similar properties. Also, they don't always use the same number of valence electrons in chemical reactions. Iron (Fe), for example, sometimes likes to give away two electrons, and sometimes three.
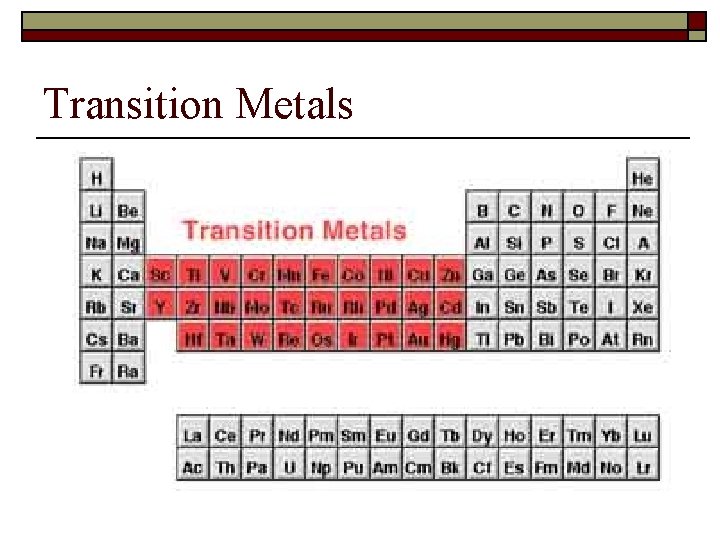
Transition Metals

Names of Families o Halogens - Group 17 - most reactive of the nonmetals. Not found free in nature. Solids, liquids, and gases in this group. Widespread sea salts, minerals, living tissue. Many applications - bleach, photography, plastics, insecticides, non-stick kitchen items.

Names of Families o Noble Gases - Group 18 - Least reactive elements - used in air conditioners, double pane windows, lights, balloons.
Names of Families o Lanthanides - f block - rare earth elements (not really rare) - shiny, silver, reactive, make TV’s glow. n Some have very interesting properties. For example, gadolinium (Gd) is the only rare earth that's ferromagnetic--that is, it sticks to magnets, the way iron does. Lanthanum is the only superconductor among them; at very low temperatures, it loses all resistance to the flow of electricity.
Names of Families o Actinides - f block - unstable, radioactive - all but 4 are artificially created. n f block elements are called inner transition elements - they were put into their current position by Glenn Seaborg - the only living person ever to have an element named after him.
With a Partner o - Point to an element on the periodic table, partner has to identify it as one of the following Alkali Metals Alkaline Earth Metals Halogens Nobel Gases - Metalloids Transition Metals Lanthanides Actinides

Study Skills Discussion 1) 2) What is working? What is not working?

Study Skills Review Big Picture 1) Chunking 2) # of times > minutes studied 3) more connections the better 4) Recall > recognition Small Picture - location/surroundings - resources used - active studying - study in blocks of time

7 Day Study Schedule A) Test next Monday B) Schedule Must include - What content you are going to study - How you will study? - Resources (notes, flashcards, book, etc) - How will you use the resource?

CH 6 Outline 1) 2) 3) 4) 5) Model of the atom (Bohr and Current) Bohr pictures Mendeleev and the formation of the Periodic Table PT Families and their characteristics Trends 1) 2) 3) 4) 5) 6) Atomic Radius Electronegativity Ionization Energy Valence EActivity Metallic character
Electron Configuration and Periodic Properties o Periodic Trends: n n n For all of the following periodic trends you should: know the definition be able to draw the trend on periodic table drawings with arrows explain why the trend happens relate the trend to other trends apply the trends on an “AB” sheet

1. Atomic Radius o o o basic idea is “how big an atom is” - atoms are not spheres with outer boundaries 2 trends: size- size increases going ↓ natural, logical - add more shells - Size decreases going → not logical! why? from left to right – n n more protons are added, but not more shells. Higher charged nucleus pulls electrons closer.

Atomic Radius - Trend looks like…

Act It Out

Warm Up- Which is a larger atom? 1) K or Na 2) K or Zn 3) Fe or Mn 4) Mn or Cs
Warm Up: Act out Atomic Radius as a Class
2. Electronegativityo o Defn - the ability of an atom to attract electrons while in a molecule (Linus Pauling) Electronegativity is related to atomic size Which atom attracts electrons better? The smaller the atom, the more pull it has on electrons while in a bond and therefore the more electronegative
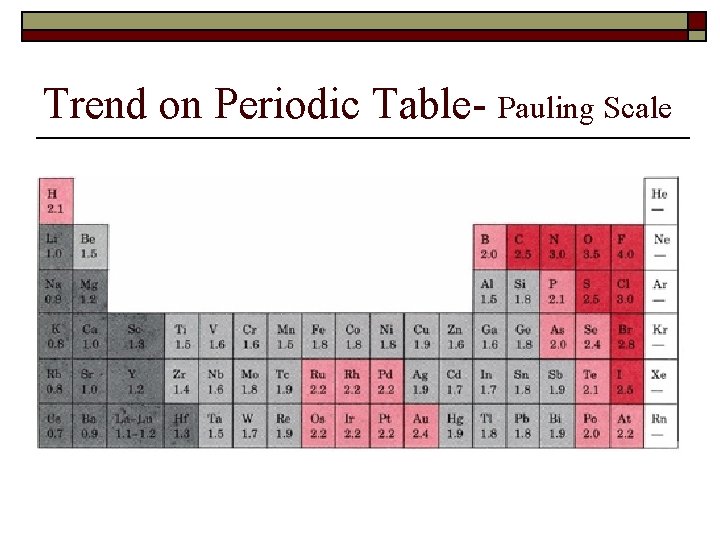
Trend on Periodic Table- Pauling Scale

Which is more electronegative? 1) 2) 3) Na or K Na or Al P or As

How does electronegativity relate to atomic radius?

Wks Pkt

Ionization Energy Intro o Act out with e- in various shells n Small vs large atom o Which is the easiest to take (lowest E needed)? o How will E change for 2 nd or third electron?
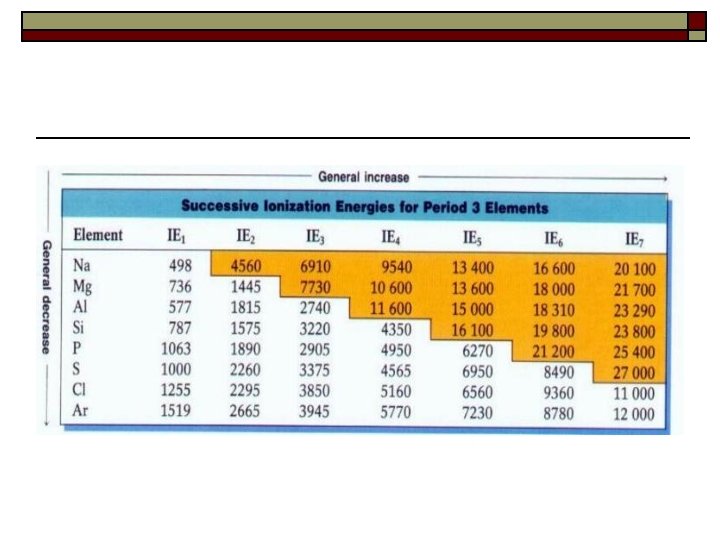
3. Ionization energy o energy required to remove the most loosely held electron from the outer energy level of an atom. The closer the electron to the nucleus the higher the Ionization energy. o IE is related to atomic radius - 2 reasons why smaller going down the table n n o o 1. greater distance from the nucleus - less attraction 2. kernel electrons “shield” outer electrons from the nucleus There is also a 2 nd and 3 rd IE - always higher than the first. IE of elements greatly increases when the outer shell has been emptied. Which has a higher 2 nd IE, Na or Mg? Which has a higher 3 rd IE - Al or Mg?
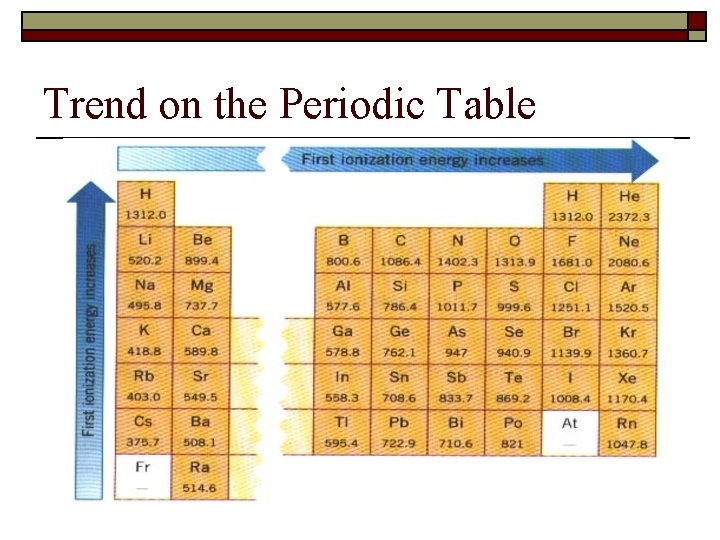
Trend on the Periodic Table

Act It Out
Which has a higher Ionization energy? 1) K or Na 2) K or Ga 3) N or P 4) S or Cl

o How is atomic radius related to Ionization Energy?
Define and Draw the Periodic Table Trend (without notes) 1) Atomic Radius 2) Electronegativity 3) Ionization Energy

Act it Out 1) 2) 3) Atomic Radius Electronegativity Ionization Energy
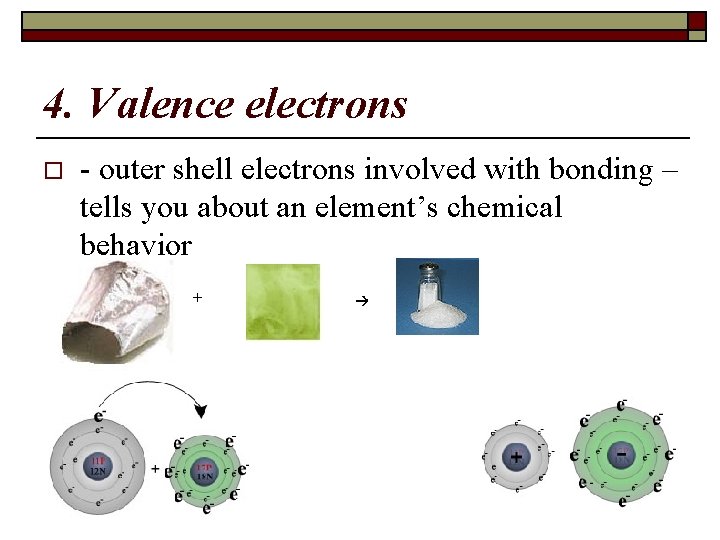
4. Valence electrons o - outer shell electrons involved with bonding – tells you about an element’s chemical behavior +

Fill in the Lewis Dot Structures in the notes
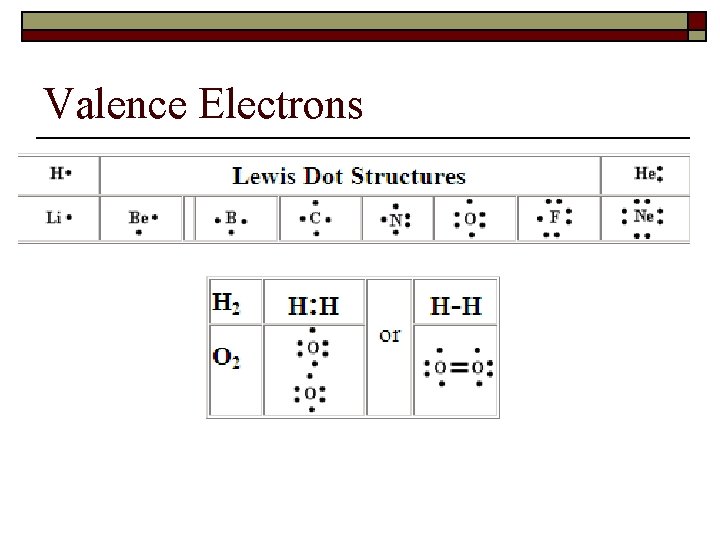
Valence Electrons

5. Activity (Reactivity) o o for metals - larger atoms are more active why? - they lose electrons more easily for nonmetals - smaller more active - why? – they gain electrons more easily n metal activity trend nonmetal activity trend

Activity/Reactivity o o o Most active metals + most active nonmetals = most stable compounds ex: Rb. F - very stable Li. Br - less stable
Which is more stable? o Ca. S or KCl? o Ba. Cl 2 or Ra. Cl 2?

6. Metallic character o some metals are said to be more metallic than others - really it is just a statement about their activity. If they are more active, they are said to be more metallic
Define and Draw Trend 1) 2) 3) 4) 5) Atomic Radius Electronegativity Ionization Energy Activity Metallic character

Which is more metallic? 1) K or Rb? 2) Ca or Sr?

Group the Trends 1) 2) 3) 4) Increase going right? Increasing going left? Increase going down? Increase going up? o o o Atomic Radius Electronegativity Ionization Energy Valence EActivity Metallic character
Define and Draw Trend 1) 2) 3) 4) 5) Atomic Radius Electronegativity Ionization Energy Activity Metallic character

Act It Out 1) 2) 3) 4) 5) Atomic Radius Electronegativity Ionization Energy Activity Metallic character
- Slides: 66