CHAPTER 6 Staining and Examination of Blood films

CHAPTER 6 Staining and Examination of Blood films

Acknowledgements n n n n Addisa Ababa University Jimma University Hawassa University Haramaya University of Gondar American Society for Clinical Pathology Center for Disease Control and Prevention-Ethiopia

Learning Objectives At the end of this chapter, the students will be able to: n Explain the general principle of staining blood films in hematology n List commonly used Romanowsky dyes n Perform the technique of staining thin blood films with Romanowsky dyes n Prepare Wright and Leishman stain in the right concentration n Describe the appearance of cells and cell components in Romanowsky-stained blood films

Objectives cont’d n n n n Prepare Giemsa and Field stains in the right concentration Stain thick blood films with Giemsa and Field stain Explain the principle of staining with Giemsa and Field stains Define panoptic staining Stain thin blood films with the panoptic stains Identify the advantage of panoptic staining over the simple Romanowsky dyes List the problems that arise in staining and the possible remedies Perform quality control for staining

Outline n n Introduction Principle of staining Romanowsky Stains Panoptic stains

6. 1. Introduction n There is little consistency between laboratories in the precise stain used to prepare a blood film for microscopic examination, but the multiple stains in use are based on the Romanowsky stain n Ehrlich was the first to use aniline dyes at first in sequence and latter as a premixed acidic – basic stains (neutral dyes). n Jenner (1880) found that the precipitate formed when eosin and methylene blue are mixed could be dissolved in methyl alcohol to form a useful stain combining certain properties of both parent dye stuffs.

Introduction cont’d n n Romanowsky stain was developed by the Russian protozoologist in the late nineteenth century (1890) He used a mixture of old methylene blue and eosin to stain the nucleus of a malarial parasite purple and the cytoplasm blue. Subsequently, Giemsa modified the stain, combining methylene azure and eosin. The stain most commonly used in the UK is a combination of Giemsa’s stain with May-Grünwald stain; it is therefore designated the May-Grünwald–Giemsa (MGG) stain.

Introduction cont’d n The stain most commonly used in North America is Wright’s stain, which contains methylene blue n Romanowsky found that when old (ripened and therefore "polychromed") methylene blue solution is mixed with eosin and the precipitate dissolved in methyl alcohol, a stain results that has a wider range than Jenner’s staining cell nuclei and platelet granules (which Jenner’s mixture failed to stain).

6. 2. Principle of Staining n n n Acidic dyes such as eosin unite with the basic components of the cell (cytoplasm) and hence the cytopolasm is eosinophilic (acidic). Conversely, basic stains like methylene blue are attracted to and combine with the acidic parts of the cell (nucleic acid and nucleoproteins of the nucleus) and hence these structures are called basophilic. Other structures stained by combination of the two are neutrophilic

6. 3. Romanowsky Stains n n n Contain: ¨ eosin Y an acidic anionic dye and ¨ azure B and other thiazine dyes derived from the oxidation or polychroming of methylene blue are basic cationic dyes When Romanowsky dye is diluted with buffered water, ionization occurs Eosin ions are negatively charged and stain the basic components of blood cells ¨ e. g. hemoglobin stains pink –red, and the granules of eosinophils stain orange red

Romanowsky Stains cont’d n n Azure B and other methylene blue derived dyes are positively charged and stain the acidic component of cells. ¨ Nucleic acids and nucleoprotein, stain various shades of mauve-purple and violet ¨ Granules of basophils stain dark blue –violet ¨ Cytoplasm of monocytes and lymphocytes stain blue or blue gray. The staining reaction of Romanowsky stains is p. Hdependent, that is why the stains are diluted in buffered water of specific p. H.

6. 3. 1 Romanowsky Stains in Common Use n Modern Romanowsky stains in common use are: ¨ Wright stain ¨ Leishman stain ¨ Giemsa stain n Wright and Leishman are basically similar to Romanowsky’s original method, the difference being the method of polychroming the methylene blue.

6. 3. 1. 1. Wright Stain n n Methylene blue is polychromed by heating with sodium carbonate It is purchased as a solution ready to use or as a powder Staining properties of Wright’s stain deteriorate rapidly ¨ when the stain absorbs moisture or ¨ if stored at high temperatures or in bright sunlight. Wright’s stain should also be renewed every 3 months and should be left for 3– 5 days before being used p. H of buffered water should be 6. 8

Wright staining procedure 1. 2. Place the air-dried smear film side up on a staining rack (two parallel glass rods kept 5 cm apart). Cover the smear with undiluted stain and leave for 1 minute ¨ Note: The undiluted stain not only acts as a fixative but also partially stains the smear. This stage is required to obtain the best possible staining results. ¨ The methyl alcohol fixes the smear. ¨ When an aqueous or diluted stain is used, the air dried smear must first be fixed by flooding with absolute methanol for 3 -5 minutes ¨ if films are left unfixed for a day or more, it will be found that the background of dried plasma stains pale blue

Wright staining procedure cont’d 3. Add equal the volume of p. H 6. 8 -buffered water (i. e. , the same number of drops as the stain) 4. Mix by blowing until a metallic sheen appears. 5. Allow the diluted stain to act for 3 -5 minutes 6. Wash off the stain with running tap water/wash bottle ¨ Don’t tip off the stain, because this will leave a fine deposit covering the film. 7. Wipe the back of the slide clean and stand it in a draining rack for the smear to dry (head part down). 8. The blood film should appear neither too pink nor too blue (check the results microscopically)

Wright staining procedure cont’d n Note: ¨ Diluting the stain in buffered water brings about full staining of the blood cells ¨ The exact staining time to use should be decided when a new batch of stain is prepared ¨ It should be checked also at the beginning of each week. Each Lab should determine the optimal staining time!!!

6. 3. 1. 2. Leishman Stain ¨ In its preparation, the methylene blue is polychromed by heating a 1% solution with 0. 5% sodium carbonate at 65 o. C for 12 hours ¨ a further ripening is allowed to proceed for 10 days before it is mixed with an equal volume of 0. 1% eosin B

Leishman Staining n similar to that used in Wright stain except for step 3 ¨ i. e. with Leishman stain, dilution is effected with approximately two volume of distilled water to one volume of stain n the best guide is the appearance of a metallic scum

6. 3. 2. Appearance of cells and cell components in Romanowsky-stained blood films n n Films stained with either Wright or Leishman stain are pinkish in color when viewed with the naked eye. Microscopically, ¨ Red cells – pink with a central pale area ¨ Nuclei of leucocytes – blue to purple (light purple in monocytes) ¨ Eosinophilic granules – red orange each distinctly visible ¨ Basophilic granules – dark blue ¨ Platelets – violet granules

Appearance of cells and cell components in Romanowsky-stained blood films: n n n Cytoplasm ¨ Monocytes – gray blue with fine reddish granules ¨ Neutrophils: light pink with lilac (pale purple) granules ¨ Lymphocytes: varying shades of blue Malaria parasites – sky blue cytoplasm and red purple chromatin Note: if the stain fails, it is the size of the granules that will mark the cell

6. 3. 1. 3. Giemsa stain n n Giemsa Stain ¨ employs various azure compounds (thionine and its methyl derivative) with eosin and methylene blue ¨ Is an alcohol-based Romanowsky stain that requires dilution in p. H 7. 1 -7. 2 buffered water ¨ It is excellent in staining malaria parasites in thick films. Commonly used in combination with Jenner or May – Grünwald stains constituting “panoptic staining”
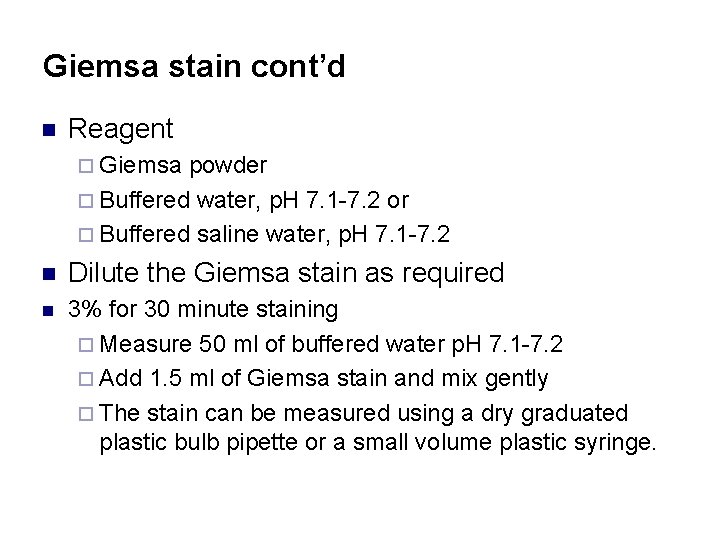
Giemsa stain cont’d n Reagent ¨ Giemsa powder ¨ Buffered water, p. H 7. 1 -7. 2 or ¨ Buffered saline water, p. H 7. 1 -7. 2 n Dilute the Giemsa stain as required n 3% for 30 minute staining ¨ Measure 50 ml of buffered water p. H 7. 1 -7. 2 ¨ Add 1. 5 ml of Giemsa stain and mix gently ¨ The stain can be measured using a dry graduated plastic bulb pipette or a small volume plastic syringe.

Giemsa stain cont’d n n 10% solution for 10 minute staining ¨ Measure 45 ml of buffered water in 50 ml cylinder ¨ Add 5 ml of Giemsa stain to 50 ml mark ¨ Mix gently Place the slides in a staining rod/rack Do not fix the films before staining. Cover the air-dried smear with a 1: 10 diluted Giemsa using buffered distilled water at p. H 7. 2 (recommended for malaria parasites in order to stain schuffner’s granules) as a diluent ¨ 1: 10 Giemsa =1 part of stock Giemsa + 9 parts buffered water

Giemsa stain cont’d n n n stain the slides as follows: ¨ 30 min if using 3% stain solution ¨ 10 min if using 10% stain solution Wash the stain from the slide gently using clean water (not necessarily distilled water or buffered water) Wipe the back of each slide clean and place it in draining rack for the Do NOT fix thick preparation to air dry. films before Giemsa staining

6. 4. Panoptic Stain n Consists of a combination of a Romanowsky stain with another stain, e. g. , Giemsa with Jenner It improves the staining of cytoplasmic granules and other bodies like nucleoli of blast cells Popular methods are: ¨ Jenner-Giemsa and ¨ May–Grunwald-Giemsa.

6. 4. 1. May-Grünwald-Giemsa staining n n n Air dry the films and fix by immersing in a jar containing methanol for 10 -20 seconds For bone marrow films leave for 20 -25 minutes Transfer the films to a staining jar containing May- Grünwald’s stain freshly diluted with an equal volume of buffered water and leave for 10 -15 minutes Transfer the slides without washing to a jar containing Giemsa’s stain freshly diluted with 9 volumes of buffered water p. H 6. 8. Allow to stain for 10 -15 minutes

May-Grünwald-Giemsa cont’d n n Transfer the slides to a jar containing buffered water, p. H 6. 8; rapidly wash in 3 or 4 changes of water allow to stand undisturbed in water for 2 -5 minutes for differentiation to take place Place the slides on vertical end to dry

6. 4. 2. Jenner-Giemsa staining n n n Air dry the films fix by immersing in a jar containing methanol for 10 -20 seconds Transfer the films to a staining jar containing Jenner’s stain freshly diluted with 4 volumes of buffered water Leave for 4 minutes. Transfer the slides (without washing) to a jar containing Giemsa stain freshly diluted with 9 volumes of buffered water p. H 6. 8. Allow to stain for 7 -10 minutes.

Jenner-Giemsa cont’d n n Transfer the slides to a jar containing buffered water, p. H 6. 8; Rapidly wash in 3 or 4 changes of water Allow to stand undisturbed in water for 2 -5 minutes for differentiation to take place. Place the slides on vertical end to dry.

6. 5. Field’s Stain n n n Is a water based Romanowsky stain composed of two solutions ¨ Field stain A ¨ Field stain B Was introduced to provide a quick method for staining thick films for malaria parasites. should be buffered to the correct p. H Neither solution requires dilution when staining thick films Field stain B requires dilution in staining thin solution More stable compared to Giemsa working stain Stain well fresh blood films particularly thick films

Thin film Field’s staining n Required ¨ Field’s stain A ¨ Field’s stain B, diluted 1 in 5 ¨ Buffered water (p. H 7. 1 -7. 2)

Thin film Field’s staining cont’d n n n n n Place slide on staining rack Fix with methanol Cover with 0. 5 ml of diluted Field’s stain B Add immediately an equal volume of Field stain A Mix using 1 ml graduated plastic bulb pipets Leave for 1 min Wash off the stain with clean water Wipe the back of the slide clean Allow to air dry

Thick film Field’s staining n Required ¨ Container of Field’s stain A ¨ Container of Field’s stain B ¨ Two containers of clean water (not buffered)

Thick film Field’s staining cont’d n n n n Holding the thick side facing downward, dip slide into Field stain A for 5 sec Drain off excess stain by touching a corner of the slide against the side of the coplin jar Wash gently for 5 sec in clean water and drain off excess water Dip the slide into Field stain B for 3 sec Drain off excess stain Wash gently in clean water Wipe the back of the slide Allow to air dry

Indications of the different stains for use n n n Wright stain ¨ Peripheral smears Leishamn ¨ Peripheral smears Geimsa ¨ For malaria thick films

6. 6. Staining Problems Excessively Blue Stain n n Causes: ¨ too thick films ¨ prolonged staining ¨ inadequate washing ¨ too high alkalinity of stain or diluent Appearance of cellular elements on excessively blue stained film: ¨ Erythrocytes – blue green ¨ Nuclear chromatin – deep blue to black ¨ Granules of neutrophils – deeply stained, appear large and prominent

Staining Problems cont’d n Correction: ¨ preparing films with ideal thickness ¨ reducing staining time (optimize the staining time) ¨ using less stain and more diluent ¨ prolonging washing ¨ adjust p. H of buffer or prepare a new batch of stain

Staining Problems cont’d Excessively Pink Stain n Causes: ¨ Insufficient staining time ¨ Prolonged washing ¨ Too high acidity of the stain or buffer (exposure of stain or buffer to acid fumes) n Appearance of cells: ¨ Erythrocytes – bright red or orange ¨ Nuclear chromatin – pale blue ¨ Granules of eosinophils – sparkling brilliant red

Staining Problems cont’d Excessively Pink Stain n Correction: ¨ Prolonging staining time (optimize staining time) ¨ Reducing washing ¨ Adjust p. H of buffer or prepare a new batch of stain
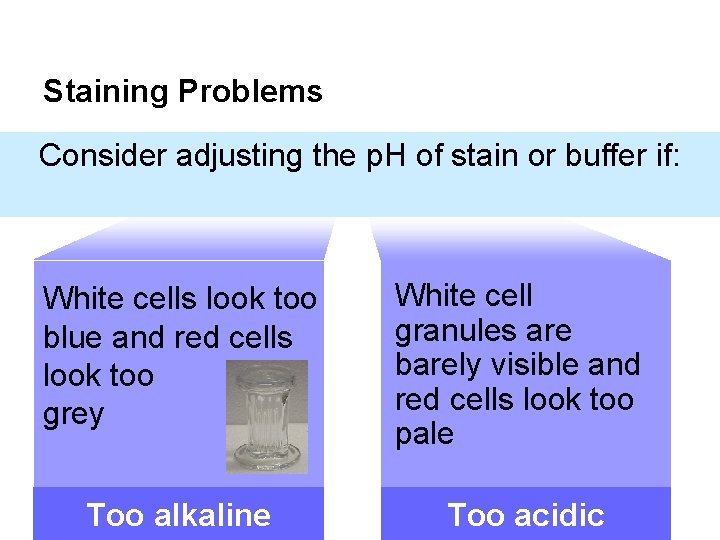
Staining Problems Consider adjusting the p. H of stain or buffer if: White cells look too blue and red cells look too grey White cell granules are barely visible and red cells look too pale Too alkaline Too acidic

Staining Problems cont’d Precipitate on the Film n Causes: ¨ Unclean slides ¨ Drying during the period of staining ¨ Inadequate washing of slide at the end of the staining period (excessive rinsing of the stained smear will cause fading of stain) ¨ Use of unfiltered or inadequately filtered stain

Staining Problems cont’d n Correction: ¨ Use n clean slides ¨ Cover the smear with generous amount of the stain and avoid drying ¨ Wash the slide until thinner parts of the film are pinkish. ¨ Filter stain N. B It is possible to re-stain the slide after washing with methanol ¨ but this should be only done when it is not possible to make a new smear ¨ If re-staining is to be done: flood smear with methanol, flood with tap water as many times as possible, restain

Quality control of staining n n When a new batch of stain is prepared, decide the best staining time to use ¨ e. g. stain films made from the same blood at different times: e. g. 5, 7, 10, 12, 15, minutes. ¨ Compare the results with a stained control blood film By checking the p. H of newly prepared buffer water and rechecking it at weekly intervals ¨ The p. H of the buffered water used to dilute the stain must be correct (6. 8) n p. H is mainly responsible for the staining reactions.

Technical tips n During preparation of staining solutions, mix the preparation 3 -4 times everyday to ensure complete dissolution of the powder ¨ Use magnetic stirrer, if it is available n Ripening of the prepared stain gives good results n Always filter staining solutions before use Proper rinsing is critical Water artifact can interfere with smear evaluation Keep stain jars tightly covered when not in use n n n

Case Study 1: Analyzing a Smear A technician prepares to do a set of differential counts n She notices that the red cells look pale and washed out and that the white cell granules are barely visible on every slide n At this point, identification of 1 minute! white cells is difficult Question: n What is the proper course of action? n
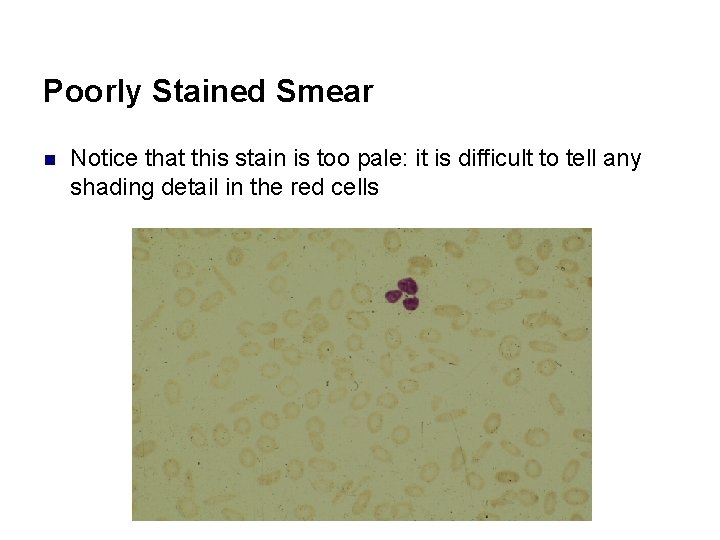
Poorly Stained Smear n Notice that this stain is too pale: it is difficult to tell any shading detail in the red cells

Case Study 1 Answer: Analyzing a Smear Question: What is the proper course of action? Answer: n A stain that is too pale indicates stain may be too acidic due to a buffer problem ¨ Stain a second slide from the patient n Increase the p. H, stain, and then assess for color of granules and red cell color
6. 7. Microscopic Examination of Blood Films n n Every film should first be inspected at low power (10 x) before general examination is undertaken with the 100 x lens ¨ Check for even distribution of cells, staining quality, platelet clumping It is essential to mount (cover) the film with a cover glass as this permits the film to be examined with the 10 x and 40 x objectives. Thus, when the film is completely dry cover it by a rectangular cover glass permanently with a neutral mountant (DPX)

Microscopic Examination of Blood Films cont’d n n n Survey the film at 10 x magnification to get a general impression of its quality Find an area where the red cells are evenly distributed, just touching but not overlapping, and study their gross morphology at 40 x At the same time, scan the film to get an impression of the quantitative distribution of while blood cells

Microscopic Examination of Blood Films cont’d n n n identify any unusual or abnormal cells, estimate the relative proportion of platelets and note the presence of abnormally large platelets. Use the 100 x objective for studying the fine details of the cell morphology.

Review Questions/Summary 1. 2. 3. 4. 5. 6. 7. 8. What is the general principle of staining blood films with Romanowsky dyes? What are the Romanowsky dyes that are commonly used in staining blood films? Describe the appearance of cells and cell components in Romanowsky- stained thin blood films What are the staining problems that give rise to unsatisfactory results? How do you correct these problems? What is panoptic staining? What is the advantage of panoptic stains over simple Romanowsky dyes? List two dyes that are commonly used in thick blood film staining? Discuss staining quality control methods
- Slides: 51