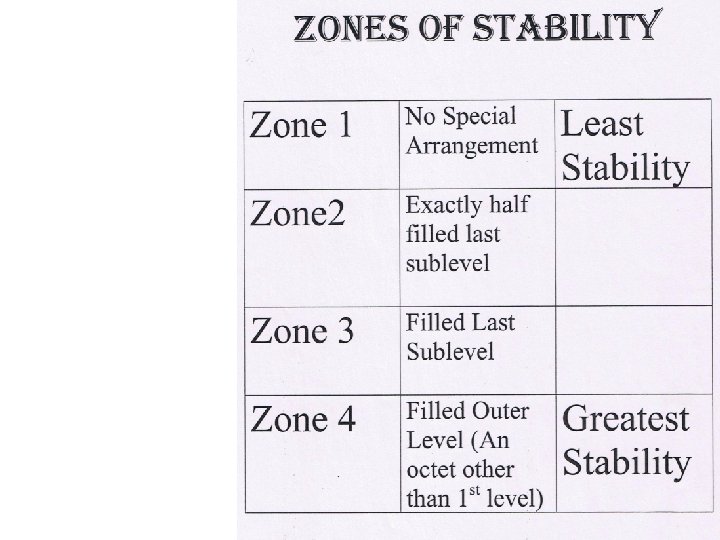
Chapter 6 Stability and Electron Configuration D Stability

Chapter 6 Stability and Electron Configuration
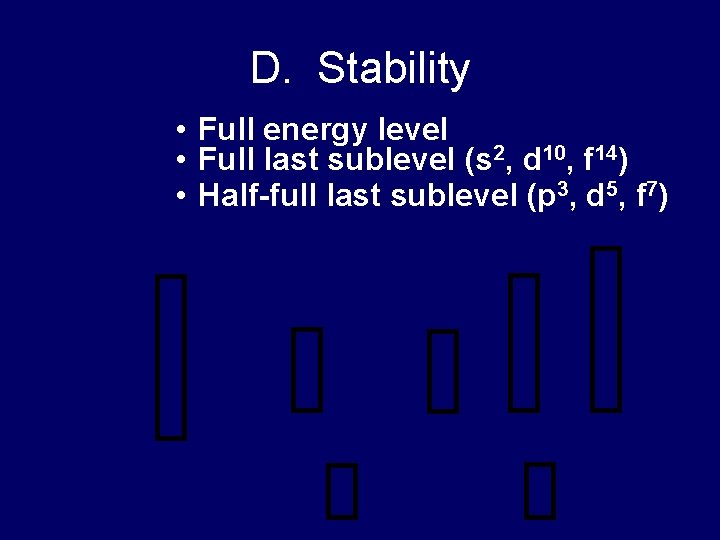
D. Stability • Full energy level • Full last sublevel (s 2, d 10, f 14) • Half-full last sublevel (p 3, d 5, f 7)

Atoms can increase their stability in one of two ways: 1. Reacting with other elements (will be covered in later chapters). 2. Electron promotion.

Electron Promotion • When the last sublevel within an electron configuration is a d or f sublevel that is one or two electrons away from being filled or half – filled an electron or two may be promoted from the s sublevel. • This makes the atom more stable
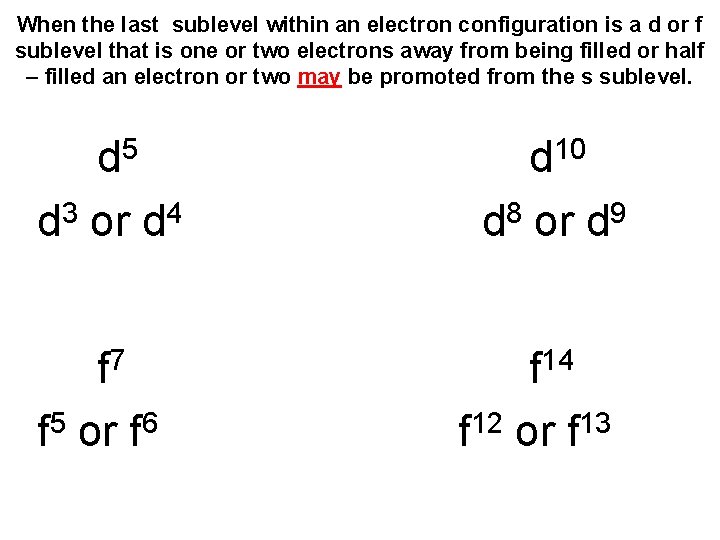
When the last sublevel within an electron configuration is a d or f sublevel that is one or two electrons away from being filled or half – filled an electron or two may be promoted from the s sublevel. 5 d 10 d d 3 or d 4 d 8 or d 9 f 7 f 5 or f 6 f 14 f 12 or f 13
![D. Stability • Electron Configuration Exceptions – Chromium (Z = 24) EXPECTED: [Ar] 4 D. Stability • Electron Configuration Exceptions – Chromium (Z = 24) EXPECTED: [Ar] 4](http://slidetodoc.com/presentation_image_h2/53dfa4ee8e6cfcb68e22c9fa3290541d/image-7.jpg)
D. Stability • Electron Configuration Exceptions – Chromium (Z = 24) EXPECTED: [Ar] 4 s 2 3 d 4 ACTUALLY: [Ar] 4 s 1 3 d 5 – Chromium gains stability with a half-full d-sublevel.
![D. Stability • Electron Configuration Exceptions – Palladium (Z = 46) EXPECTED: [Kr] 5 D. Stability • Electron Configuration Exceptions – Palladium (Z = 46) EXPECTED: [Kr] 5](http://slidetodoc.com/presentation_image_h2/53dfa4ee8e6cfcb68e22c9fa3290541d/image-8.jpg)
D. Stability • Electron Configuration Exceptions – Palladium (Z = 46) EXPECTED: [Kr] 5 s 24 d 8 ACTUALLY: [Kr] 4 d 10 – Palladium gains stability with a full d-sublevel.

Homework • Worksheet: Stability and Electron Configuration (Due Friday). • Study Guide Chapter 6 (Due Monday)
- Slides: 9