Chapter 6 Section 4 The Building Blocks of

Chapter 6 - Section 4 The Building Blocks of Life

What do these substances have in common?
Organic Chemistry §The element carbon is a component of almost all biological molecules.
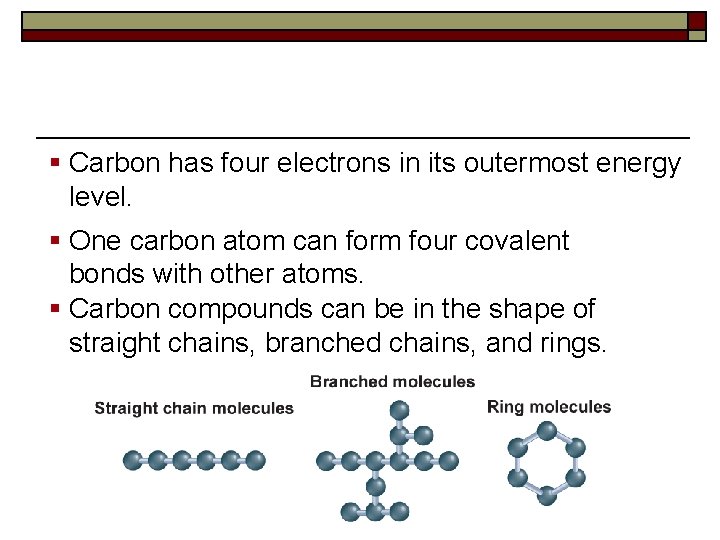
§ Carbon has four electrons in its outermost energy level. § One carbon atom can form four covalent bonds with other atoms. § Carbon compounds can be in the shape of straight chains, branched chains, and rings.
Macromolecules § Carbon atoms can be joined to form carbon molecules. § Macromolecules are large molecules formed by joining smaller organic molecules together. § Polymers are molecules made from repeating units of identical or nearly identical compounds linked together by a series of covalent bonds.
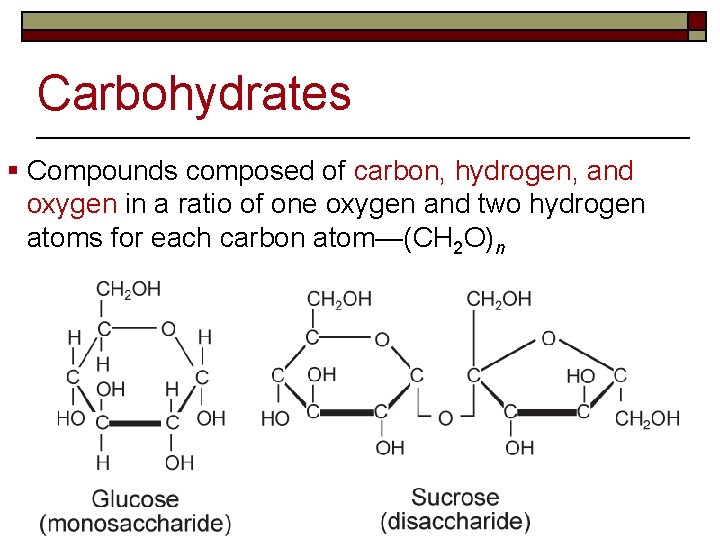
Carbohydrates § Compounds composed of carbon, hydrogen, and oxygen in a ratio of one oxygen and two hydrogen atoms for each carbon atom—(CH 2 O)n
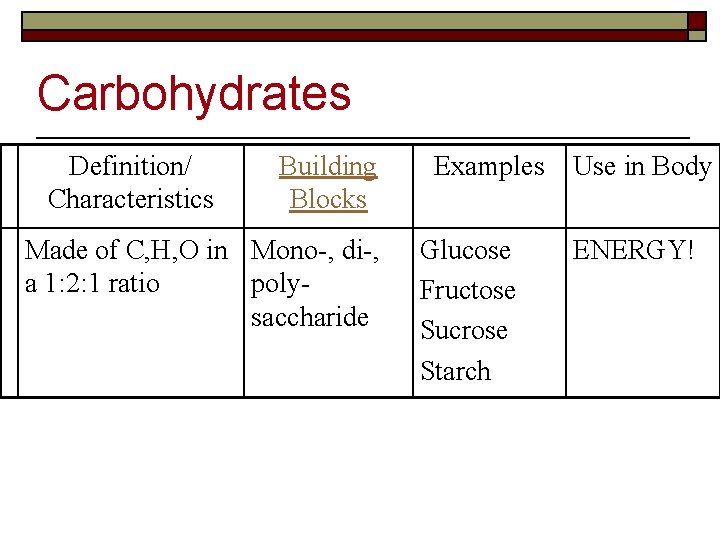
Carbohydrates Definition/ Characteristics Building Blocks Made of C, H, O in Mono-, di-, a 1: 2: 1 ratio polysaccharide Examples Glucose Fructose Sucrose Starch Use in Body ENERGY!

CARBOHYDRATE SOURCES
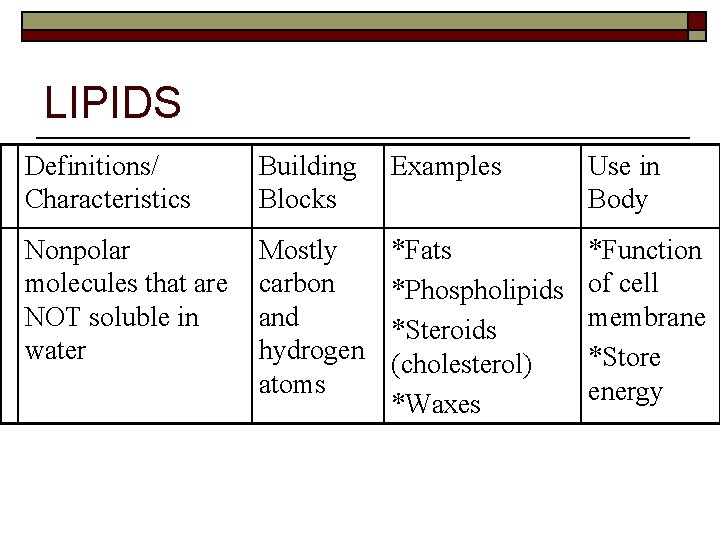
LIPIDS Definitions/ Characteristics Building Blocks Examples Use in Body Nonpolar molecules that are NOT soluble in water Mostly carbon and hydrogen atoms *Fats *Phospholipids *Steroids (cholesterol) *Waxes *Function of cell membrane *Store energy
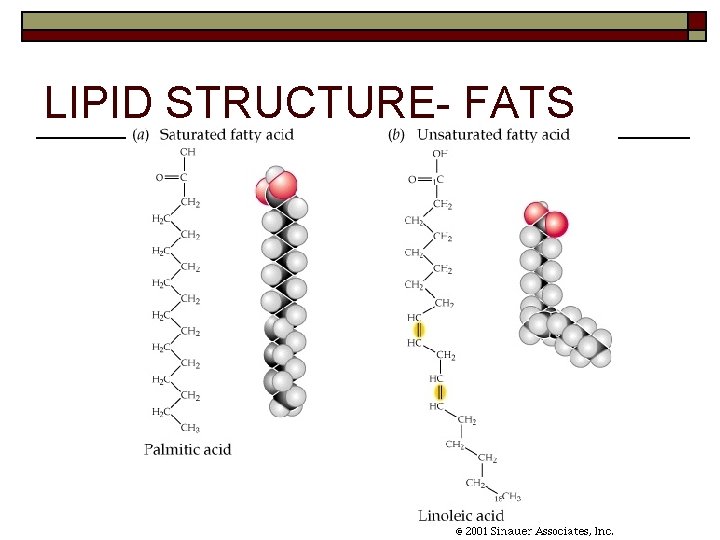
LIPID STRUCTURE- FATS
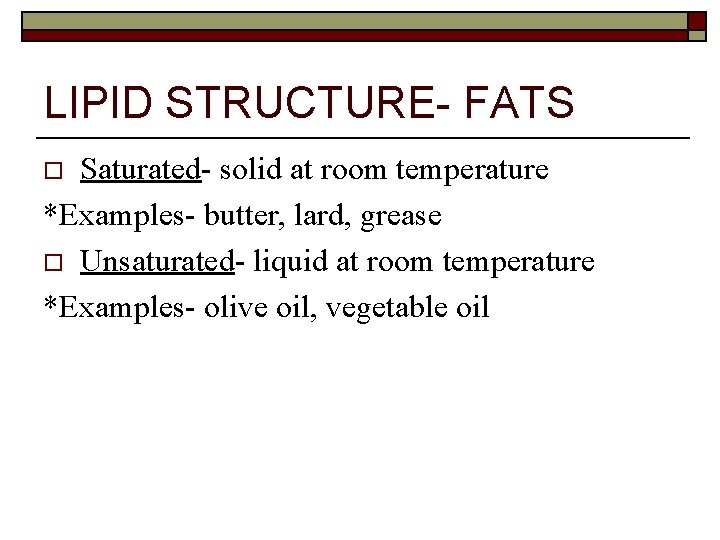
LIPID STRUCTURE- FATS Saturated- solid at room temperature *Examples- butter, lard, grease o Unsaturated- liquid at room temperature *Examples- olive oil, vegetable oil o

LIPID SOURCES
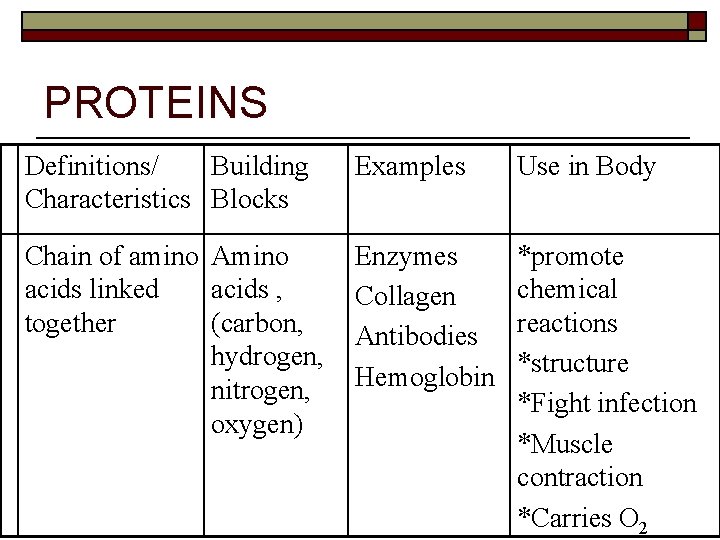
PROTEINS Definitions/ Building Characteristics Blocks Examples Use in Body Chain of amino Amino acids linked acids , together (carbon, hydrogen, nitrogen, oxygen) Enzymes Collagen Antibodies Hemoglobin *promote chemical reactions *structure *Fight infection *Muscle contraction *Carries O 2
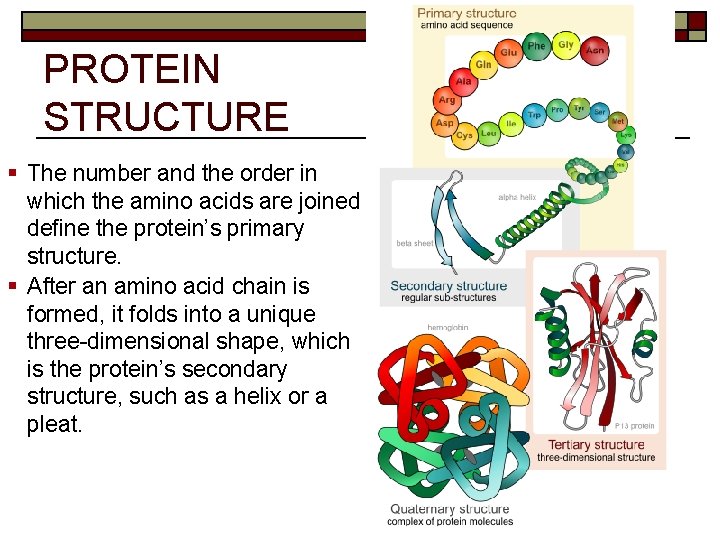
PROTEIN STRUCTURE § The number and the order in which the amino acids are joined define the protein’s primary structure. § After an amino acid chain is formed, it folds into a unique three-dimensional shape, which is the protein’s secondary structure, such as a helix or a pleat.

PROTEIN SOURCES
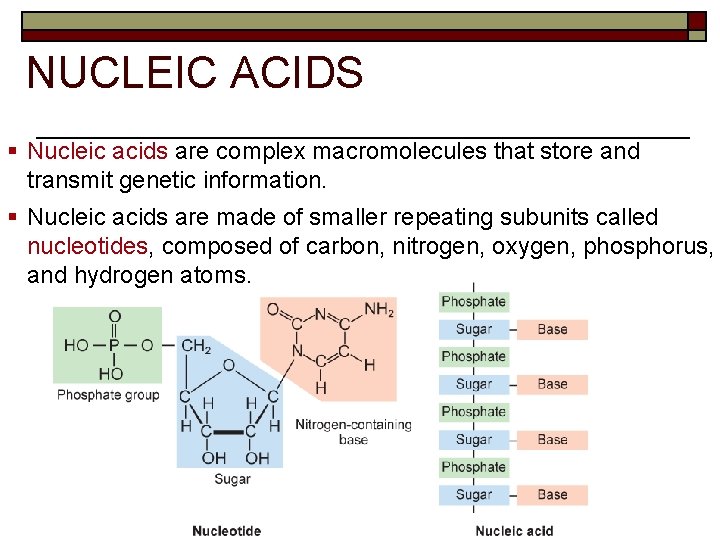
NUCLEIC ACIDS § Nucleic acids are complex macromolecules that store and transmit genetic information. § Nucleic acids are made of smaller repeating subunits called nucleotides, composed of carbon, nitrogen, oxygen, phosphorus, and hydrogen atoms.
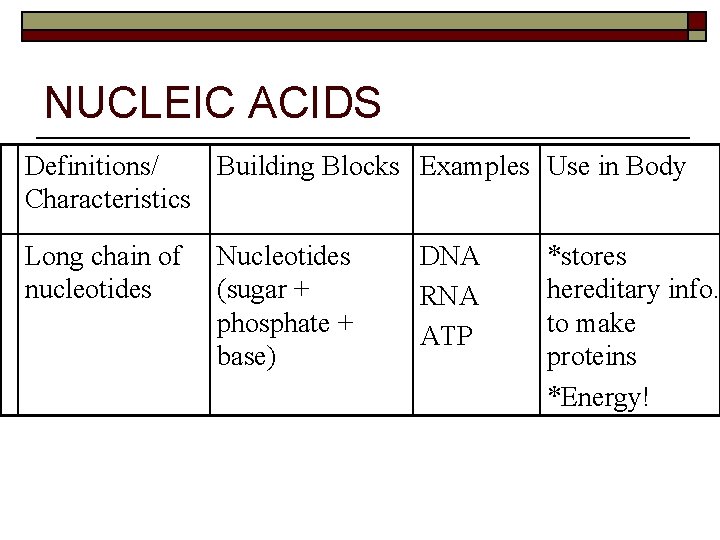
NUCLEIC ACIDS Definitions/ Building Blocks Examples Use in Body Characteristics Long chain of nucleotides Nucleotides (sugar + phosphate + base) DNA RNA ATP *stores hereditary info. to make proteins *Energy!
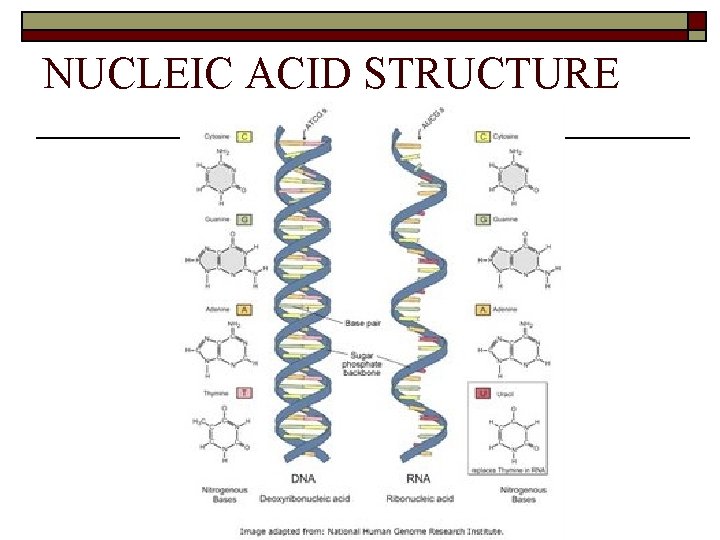
NUCLEIC ACID STRUCTURE

Journal o Your friend and Biology lab partner sits down next to you at lunch with only a bottle filled with a lemonade, cayenne pepper and honey mixture. She is in her fifth week of completing this liquid-cleanse diet, and she looks pale and very weak. You and your friends have tried to convince her to stop the diet, but because she is losing weight quickly, she refuses to stop. Use your knowledge of essential organic compounds to explain to your friend the type of damage she is doing to her body. Create a quick explanation to share with her about what is happening to her muscles and other body systems due to the lack of nutrients.

Journal o List the 4 essential organic compounds. Which one do you think is most important? Why?
Carbohydrates § Values of n ranging from three to seven are called simple sugars, or monosaccharides. § Two monosaccharides joined together form a disaccharide. § Longer carbohydrate molecules are called polysaccharides.
- Slides: 21