Chapter 6 Section 2 Describing Chemical Reactions Chemical

Chapter 6, Section 2 Describing Chemical Reactions
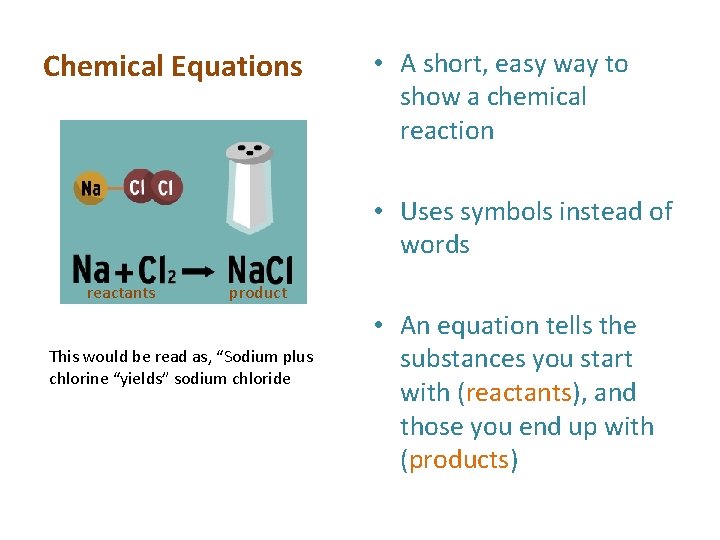
Chemical Equations • A short, easy way to show a chemical reaction • Uses symbols instead of words reactants product This would be read as, “Sodium plus chlorine “yields” sodium chloride • An equation tells the substances you start with (reactants), and those you end up with (products)
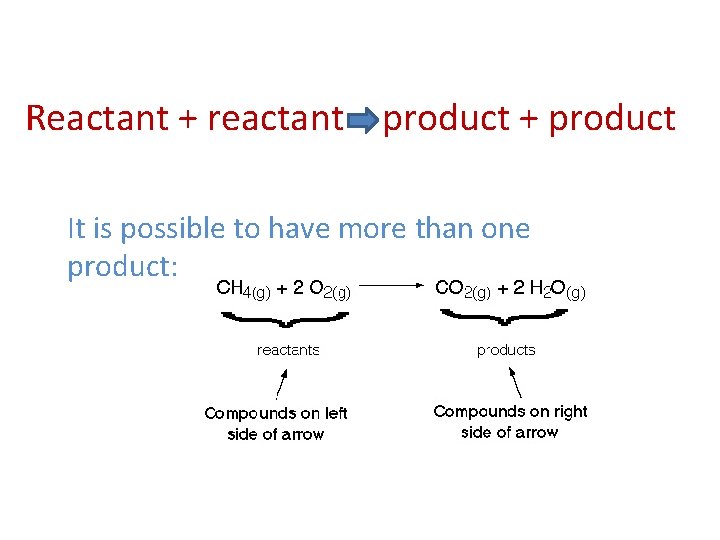
Reactant + reactant = product + product It is possible to have more than one product:

Conservation of Matter • During a chemical reaction, matter is neither created or destroyed • The total mass of the reactants must equal the total mass of the products Table Talk: If you want to measure all the matter before and after a reaction, what must you do?

Balancing Chemical Equations To describe a reaction accurately, a chemical equation must show the same number of each type of atom on both sides of the equation It’s not THIS bad ; )
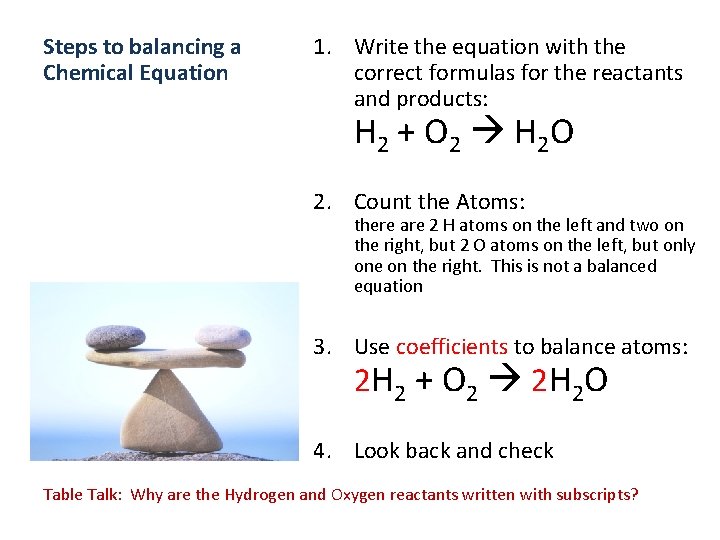
Steps to balancing a Chemical Equation 1. Write the equation with the correct formulas for the reactants and products: H 2 + O 2 H 2 O 2. Count the Atoms: there are 2 H atoms on the left and two on the right, but 2 O atoms on the left, but only one on the right. This is not a balanced equation 3. Use coefficients to balance atoms: 2 H 2 + O 2 2 H 2 O 4. Look back and check Table Talk: Why are the Hydrogen and Oxygen reactants written with subscripts?

Now you try it! 1. 2. 3. 4. Write the equation Mg + O 2 Mg. O Count the atoms Use coefficients Look back and check 2 Mg + O 2 2 Mg. O
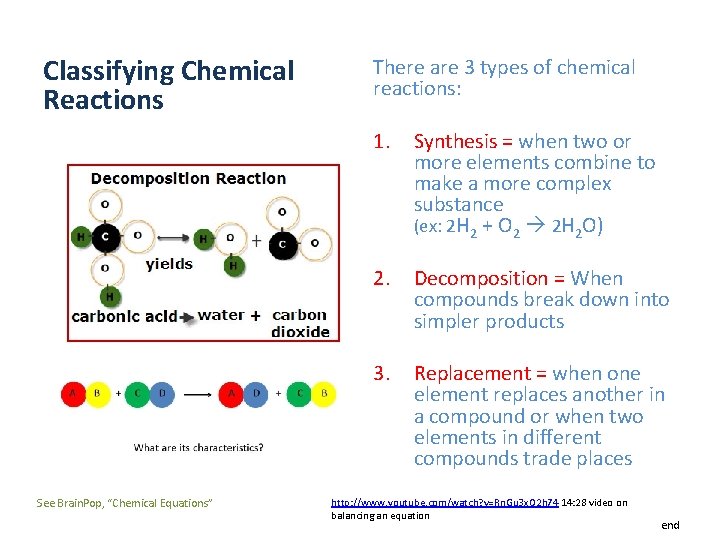
Classifying Chemical Reactions See Brain. Pop, “Chemical Equations” There are 3 types of chemical reactions: 1. Synthesis = when two or more elements combine to make a more complex substance (ex: 2 H 2 + O 2 2 H 2 O) 2. Decomposition = When compounds break down into simpler products 3. Replacement = when one element replaces another in a compound or when two elements in different compounds trade places http: //www. youtube. com/watch? v=Rn. Gu 3 x. O 2 h 74 14: 28 video on balancing an equation end
- Slides: 9