Chapter 6 Periodic Trends Development of the Periodic









- Slides: 19

Chapter 6: Periodic Trends

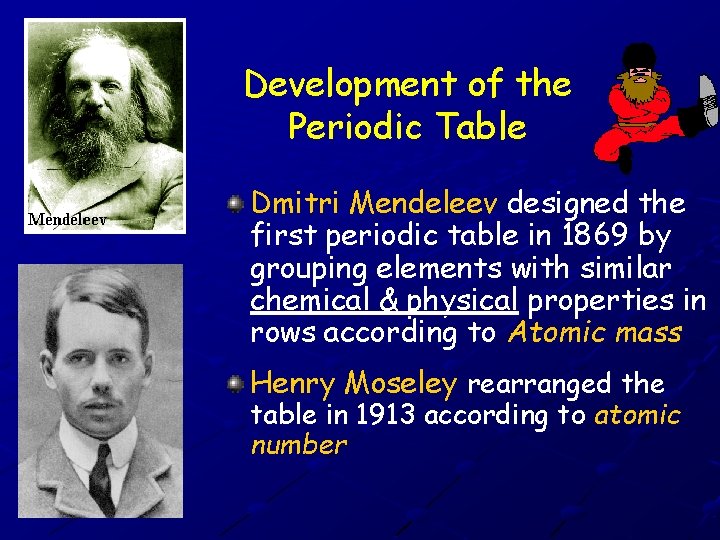
Development of the Periodic Table Dmitri Mendeleev designed the first periodic table in 1869 by grouping elements with similar chemical & physical properties in rows according to Atomic mass Henry Moseley rearranged the table in 1913 according to atomic number

The Modern Periodic Table Organized by groups (columns) and periods (rows)

Classifying Elements by Electron Configurations Representative Elements- Outermost s and p are filling n Alkali metals – Outermost s has 1 e n Alkaline earth metals – Outermost s is full n Halogens – Outermost s is full, outermost p has 5 e n Noble Gases- Outermost s and p are totally full Transition Metals -Outermost s is full and d is filling or full Inner Transition Metals- Outermost s is full and f is filling or full

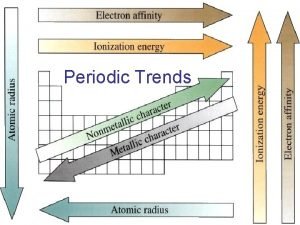
Periodic Trends The location of an element on the Periodic Table gives a lot of information about key properties of that element compared to the other elements As you move across the periodic table, you keep periodically running across the same properties. . . Hence the name periodic table.

Trend #1: Atomic Size An atoms size is determined by its atomic radius r Atomic radius is defined as half the distance between the nuclei of two like atoms

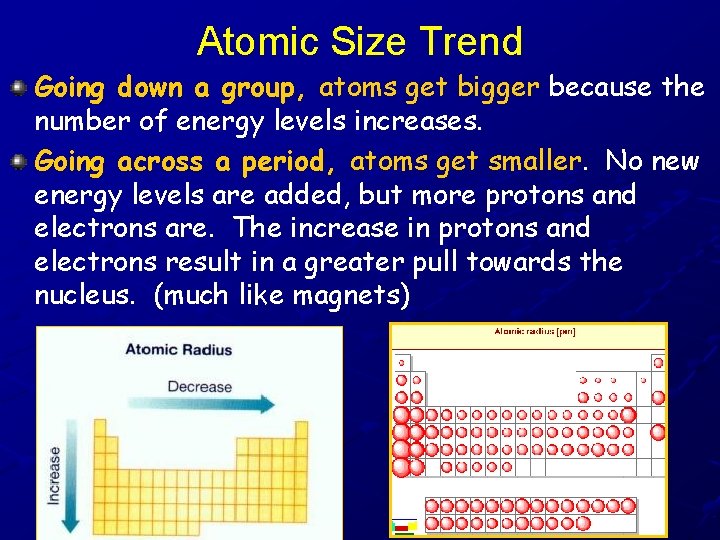
Atomic Size Trend Going down a group, atoms get bigger because the number of energy levels increases. Going across a period, atoms get smaller. No new energy levels are added, but more protons and electrons are. The increase in protons and electrons result in a greater pull towards the nucleus. (much like magnets)

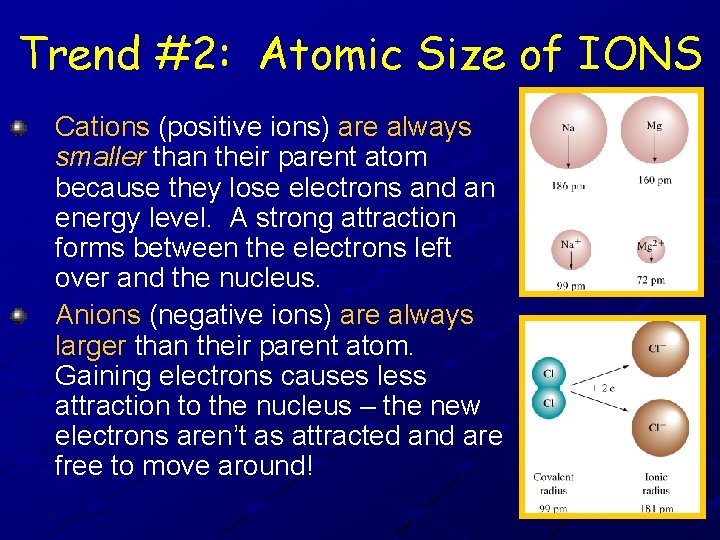
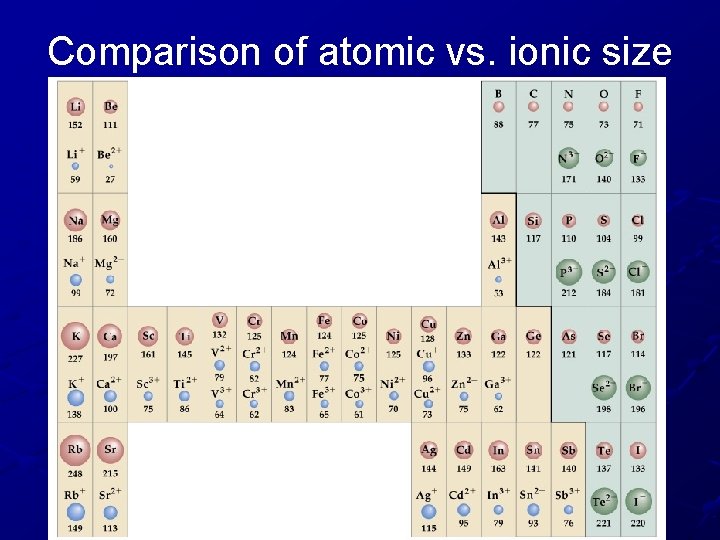
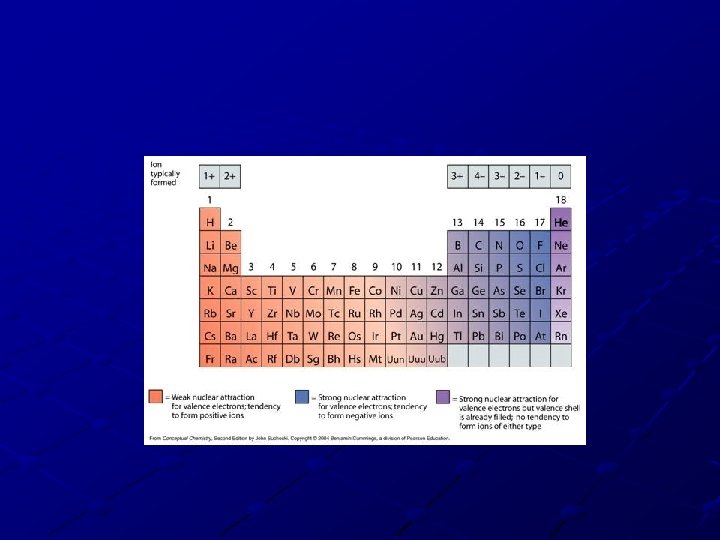
Trend #2: Atomic Size of IONS Cations (positive ions) are always smaller than their parent atom because they lose electrons and an energy level. A strong attraction forms between the electrons left over and the nucleus. Anions (negative ions) are always larger than their parent atom. Gaining electrons causes less attraction to the nucleus – the new electrons aren’t as attracted and are free to move around!

Comparison of atomic vs. ionic size

Answer this. . . Which is larger: S or S-2. Why? § S-2 is larger – Gaining electrons causes less of an attraction to the nucleus – the new electrons are free to move around. Which is smaller: Fe or Fe+4. Why? § Fe+4 is smaller – Losing electrons causes more of an attraction to the nucleus – the remaining electrons are pulled in tighter. Which is smaller: Na+ or Al 3+? Why § They both lose an energy level BUT Al 3+ loses MORE e-, nucleus pulls in the remaining electrons due to the more drastic change. . Which is smaller: Be 2+ or Na+. Why? § They both lose an energy level BUT Be 2+ loses MORE e- & goes down to a smaller energy level. An atom walks into a ‘restaurant’ and proclaims, "Hey! Somebody just stole one of my electrons!" The bartender says, "Are you sure? “ The atom replies, "Yes I'm positive!" A neutron walks into a ‘restaurant’ and says, "Hey bartender give me a drink. " The bartender gives him one and says, “No charge for you"

Trend #3: Electronegativity ELECTRONEGATIVITY - the ability for an element to attract an electron Going down a group, electronegativity decreases because the added energy levels ‘shield’ the power of the nucleus to attract electrons Going across a period, electronegativity increases because nuclear charge increases - the closer an atom is to having a full outer shell, the greater the desire to completely fill that shell by gaining electrons.

Which element would be the most electronegative? Why? Fluorine - It is located at a low energy level with a high number of electrons and protons. There is a strong attraction between the electrons and the nucleus. It wants an electron BADLY!

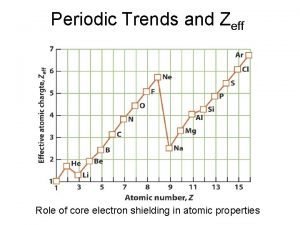
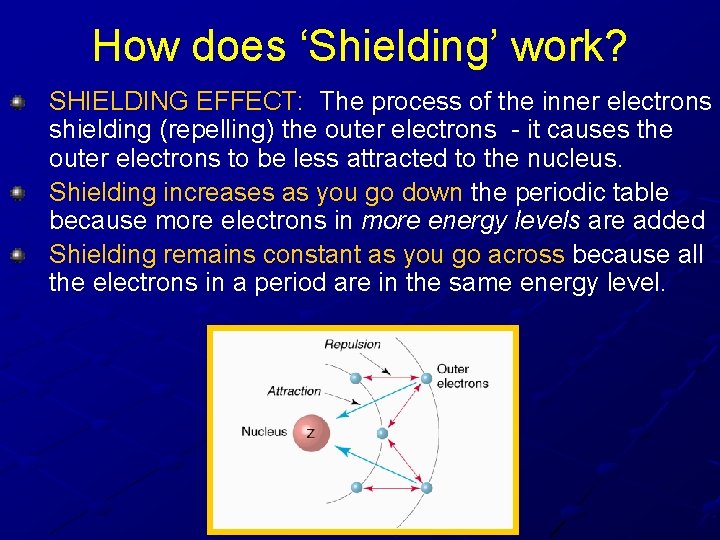
How does ‘Shielding’ work? SHIELDING EFFECT: The process of the inner electrons shielding (repelling) the outer electrons - it causes the outer electrons to be less attracted to the nucleus. Shielding increases as you go down the periodic table because more electrons in more energy levels are added Shielding remains constant as you go across because all the electrons in a period are in the same energy level.

Why aren’t noble gases assigned electronegativity numbers? They are happy the way they are – they don’t need any more electrons

Trend #4: Ionization Energy IONIZATION ENERGY- The energy needed to REMOVE an electron from the outside shell. Going down a group, ionization energy decreases because more energy levels are added. This ‘shielding’ causes the electrons to be less attracted to the nucleus little energy is needed to remove them. Going across a period, ionization energy increases because the electrons are closer to the nucleus. More energy is needed to remove them the closer they are to the nucleus.

First vs. Second First ionization energy is the energy required to remove the first electron Second ionization energy is the energy required to remove the second electron (and so forth) First ionization energy is always smaller (compared to the second or third ionization energy) because each successive electron removed is closer to the nucleus and strongly attracted to it.

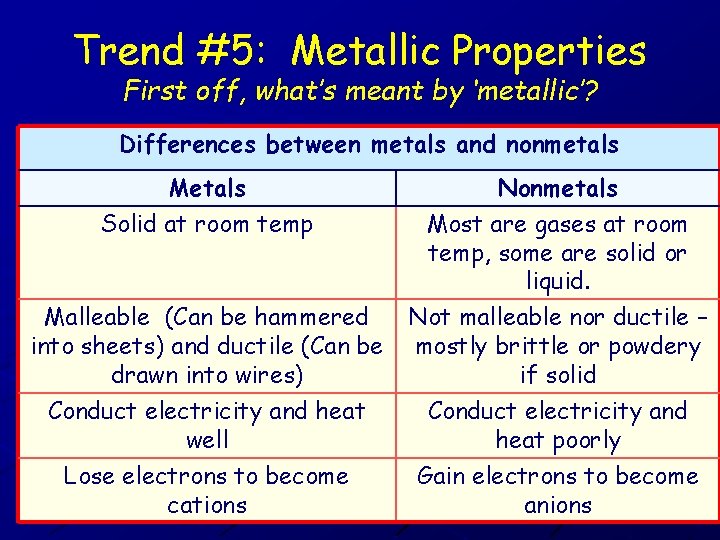
Trend #5: Metallic Properties First off, what’s meant by ‘metallic’? Differences between metals and nonmetals Metals Solid at room temp Nonmetals Most are gases at room temp, some are solid or liquid. Malleable (Can be hammered into sheets) and ductile (Can be drawn into wires) Not malleable nor ductile – mostly brittle or powdery if solid Conduct electricity and heat well Lose electrons to become cations Conduct electricity and heat poorly Gain electrons to become anions

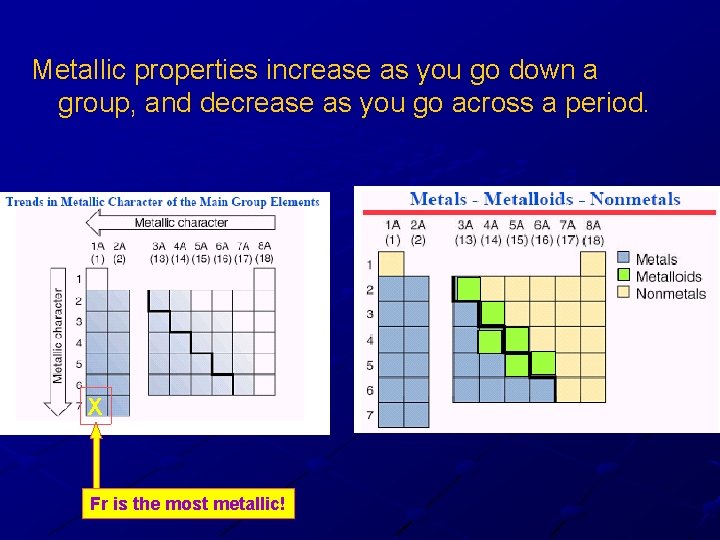
Metallic properties increase as you go down a group, and decrease as you go across a period. X Fr is the most metallic!

 Periodic table trend
Periodic table trend Boron group number
Boron group number The periodic table and periodic law chapter 6
The periodic table and periodic law chapter 6 Chapter 6 periodic table
Chapter 6 periodic table The periodic table and periodic law chapter 6
The periodic table and periodic law chapter 6 Atomic size vs atomic radius
Atomic size vs atomic radius Periodic trends
Periodic trends Periodic table trends cheat sheet
Periodic table trends cheat sheet Ap chemistry chapter 7 atomic structure and periodicity
Ap chemistry chapter 7 atomic structure and periodicity Graphing periodic trends
Graphing periodic trends Oxidation trends periodic table
Oxidation trends periodic table Periodic trends electronegativity
Periodic trends electronegativity Trend of zeff in the periodic table
Trend of zeff in the periodic table Periodic trends activity worksheet
Periodic trends activity worksheet Periodic trends practice quiz
Periodic trends practice quiz Reactivity trend periodic table
Reactivity trend periodic table Periodic trends summary
Periodic trends summary Ionization energy practice problems
Ionization energy practice problems Electron affinity worksheet
Electron affinity worksheet Periodic trends electron affinity
Periodic trends electron affinity