Chapter 6 Nomenclature of Inorganic Compounds This seashell





















- Slides: 50

Chapter 6 Nomenclature of Inorganic Compounds This seashell is formed from the chemical calcium carbonate, commonly called limestone. It is the same chemical used in many calcium supplements for our diets. Introduction to General, Organic, and Biochemistry 10 e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan Arena

Chapter Outline 6. 1 Common and Systematic Names 6. 2 Elements and Ions 6. 4 Naming Binary Compounds 6. 5 Naming Compounds Containing Polyatomic Ions 6. 3 Writing Formulas from 6. 6 Acids Names of Ionic Compounds Copyright 2012 John Wiley & Sons, Inc

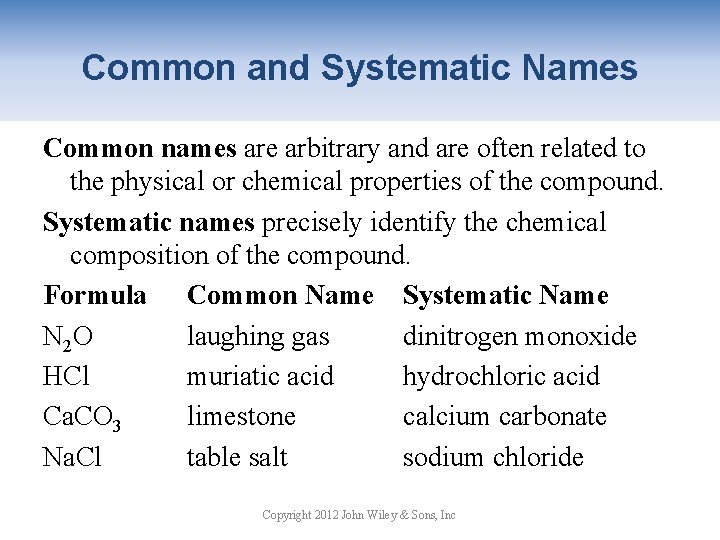
Common and Systematic Names Common names are arbitrary and are often related to the physical or chemical properties of the compound. Systematic names precisely identify the chemical composition of the compound. Formula Common Name Systematic Name N 2 O laughing gas dinitrogen monoxide HCl muriatic acid hydrochloric acid Ca. CO 3 limestone calcium carbonate Na. Cl table salt sodium chloride Copyright 2012 John Wiley & Sons, Inc

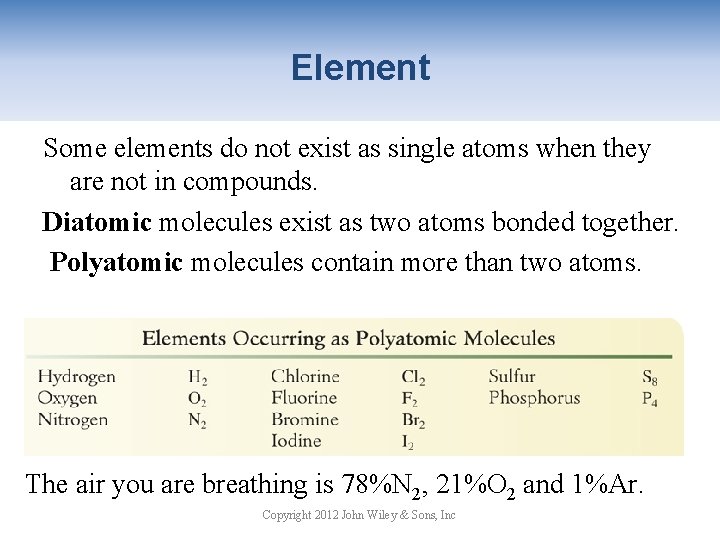
Element Some elements do not exist as single atoms when they are not in compounds. Diatomic molecules exist as two atoms bonded together. Polyatomic molecules contain more than two atoms. The air you are breathing is 78%N 2, 21%O 2 and 1%Ar. Copyright 2012 John Wiley & Sons, Inc

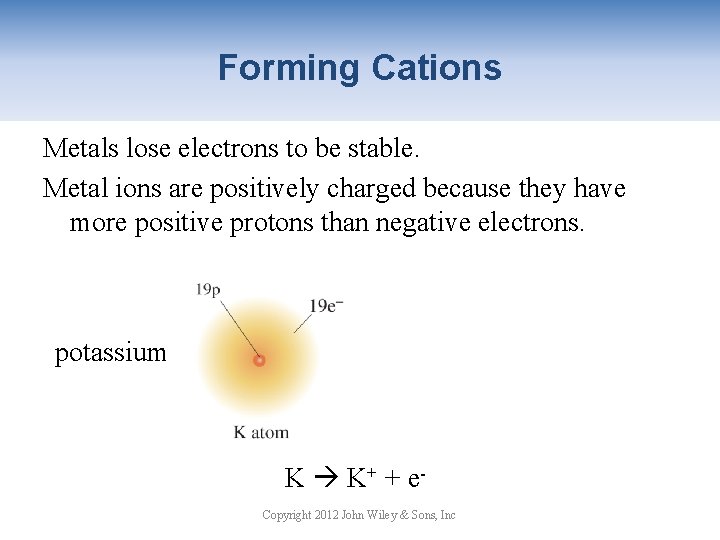
Forming Cations Metals lose electrons to be stable. Metal ions are positively charged because they have more positive protons than negative electrons. potassium ion K K+ + e Copyright 2012 John Wiley & Sons, Inc

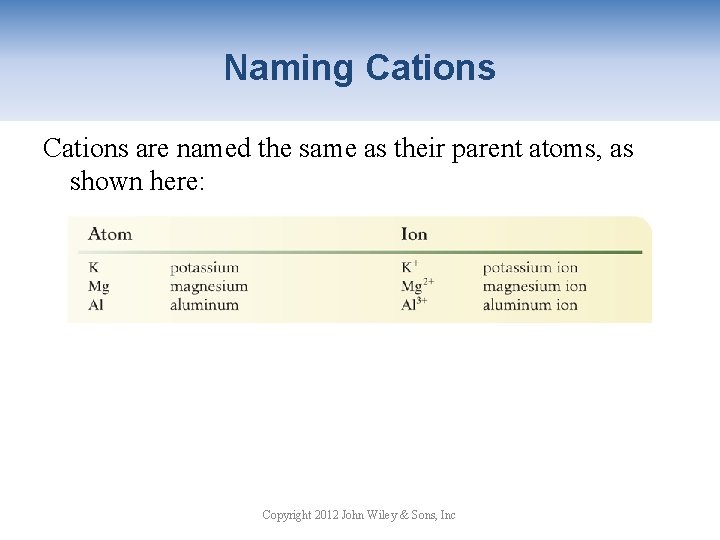
Naming Cations are named the same as their parent atoms, as shown here: Copyright 2012 John Wiley & Sons, Inc

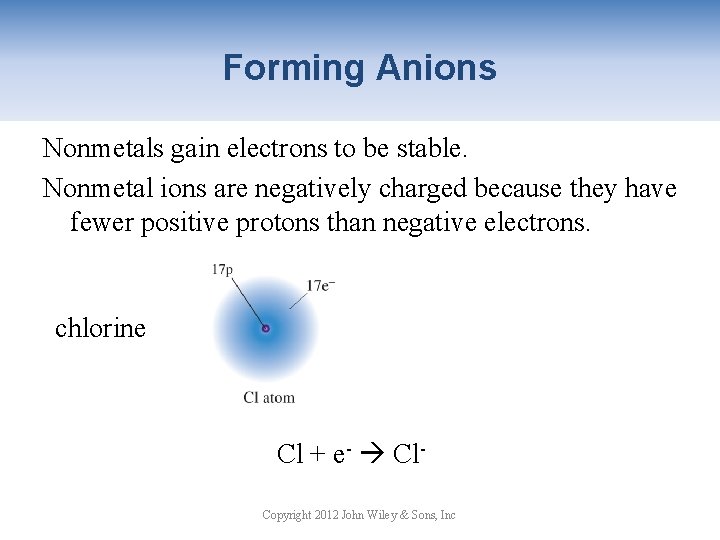
Forming Anions Nonmetals gain electrons to be stable. Nonmetal ions are negatively charged because they have fewer positive protons than negative electrons. chlorine chloride ion Cl + e- Cl. Copyright 2012 John Wiley & Sons, Inc

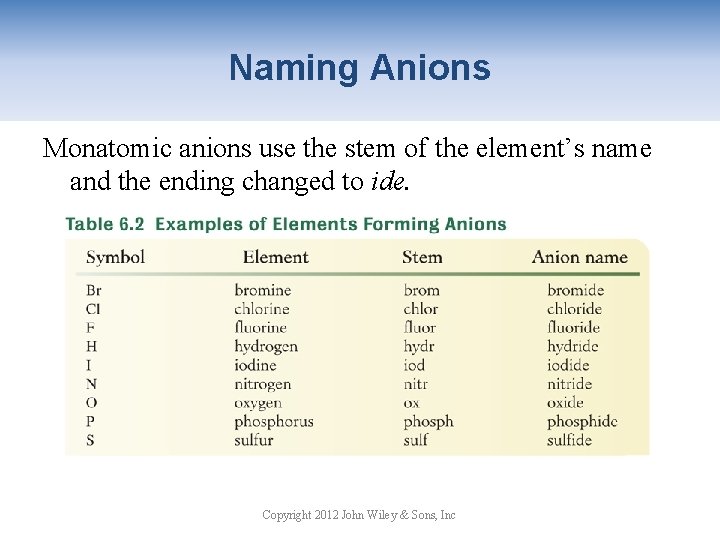
Naming Anions Monatomic anions use the stem of the element’s name and the ending changed to ide. Copyright 2012 John Wiley & Sons, Inc

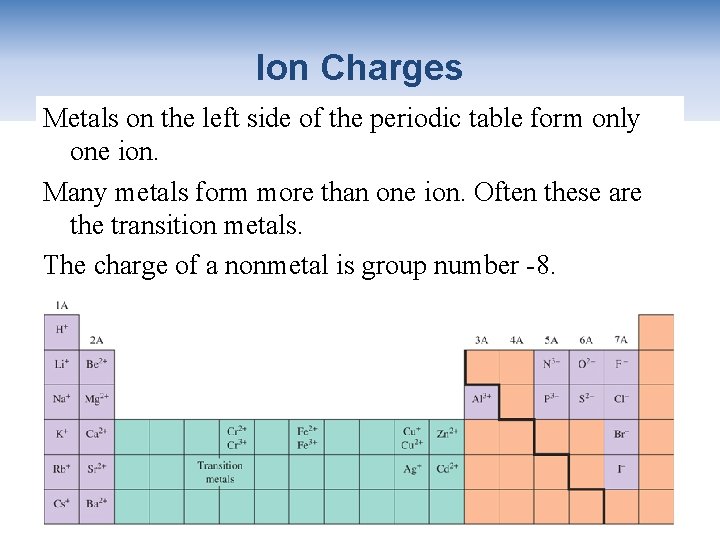
Ion Charges Metals on the left side of the periodic table form only one ion. Many metals form more than one ion. Often these are the transition metals. The charge of a nonmetal is group number -8. Copyright 2012 John Wiley & Sons, Inc

Your Turn! Calcium is an element in group 2 A. Which of the following statements is correct about calcium forming an ion? a. Ca gains two electrons, forming Ca 2+ b. Ca gains two electrons, forming Ca 2 c. Ca loses two electrons, forming Ca 2 d. Ca loses two electrons, forming Ca 2+ Copyright 2012 John Wiley & Sons, Inc

Your Turn! Phosphorus is a nonmetal in group 5 A. The charge on the phosphide ion is a. -3 because the element lost 3 electrons. b. -3 because the element gained 3 electrons. c. +3 because the element lost 3 electrons. d. +3 because the element gained 3 electrons. Copyright 2012 John Wiley & Sons, Inc

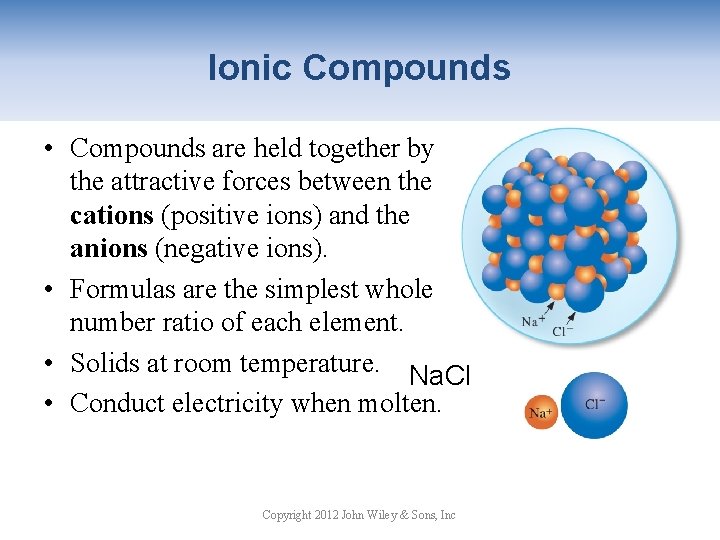
Ionic Compounds • Compounds are held together by the attractive forces between the cations (positive ions) and the anions (negative ions). • Formulas are the simplest whole number ratio of each element. • Solids at room temperature. Na. Cl • Conduct electricity when molten. Copyright 2012 John Wiley & Sons, Inc

Writing Formulas for Ionic Compounds 1. Write the formula for the metal ion followed by the formula for the nonmetal ion. 2. Combine the smallest numbers of each ion needed to give the charge sum equal to zero. 3. Write the formula for the compound as the symbol for the metal and nonmetal each followed by a subscript of the number determined in step 2. Copyright 2012 John Wiley & Sons, Inc

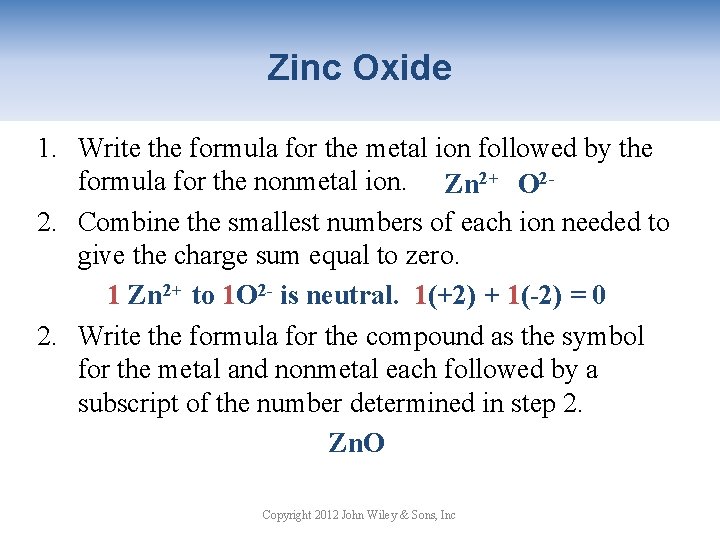
Zinc Oxide 1. Write the formula for the metal ion followed by the formula for the nonmetal ion. Zn 2+ O 22. Combine the smallest numbers of each ion needed to give the charge sum equal to zero. 1 Zn 2+ to 1 O 2 - is neutral. 1(+2) + 1(-2) = 0 2. Write the formula for the compound as the symbol for the metal and nonmetal each followed by a subscript of the number determined in step 2. Zn. O Copyright 2012 John Wiley & Sons, Inc

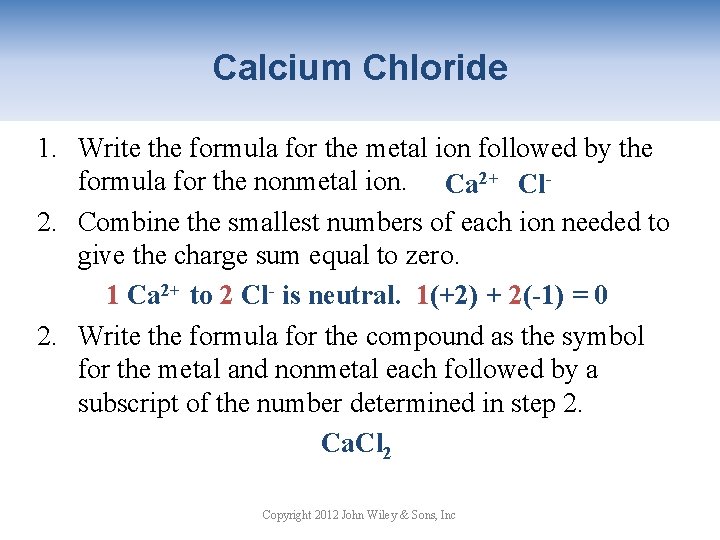
Calcium Chloride 1. Write the formula for the metal ion followed by the formula for the nonmetal ion. Ca 2+ Cl 2. Combine the smallest numbers of each ion needed to give the charge sum equal to zero. 1 Ca 2+ to 2 Cl- is neutral. 1(+2) + 2(-1) = 0 2. Write the formula for the compound as the symbol for the metal and nonmetal each followed by a subscript of the number determined in step 2. Ca. Cl 2 Copyright 2012 John Wiley & Sons, Inc

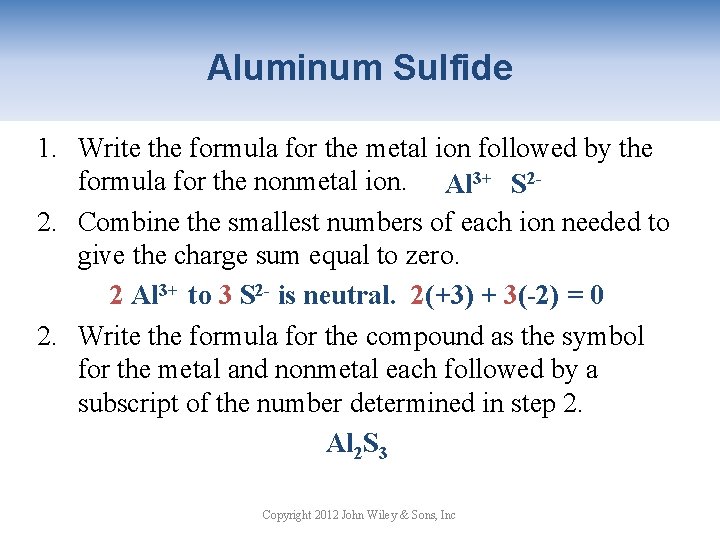
Aluminum Sulfide 1. Write the formula for the metal ion followed by the formula for the nonmetal ion. Al 3+ S 22. Combine the smallest numbers of each ion needed to give the charge sum equal to zero. 2 Al 3+ to 3 S 2 - is neutral. 2(+3) + 3(-2) = 0 2. Write the formula for the compound as the symbol for the metal and nonmetal each followed by a subscript of the number determined in step 2. Al 2 S 3 Copyright 2012 John Wiley & Sons, Inc

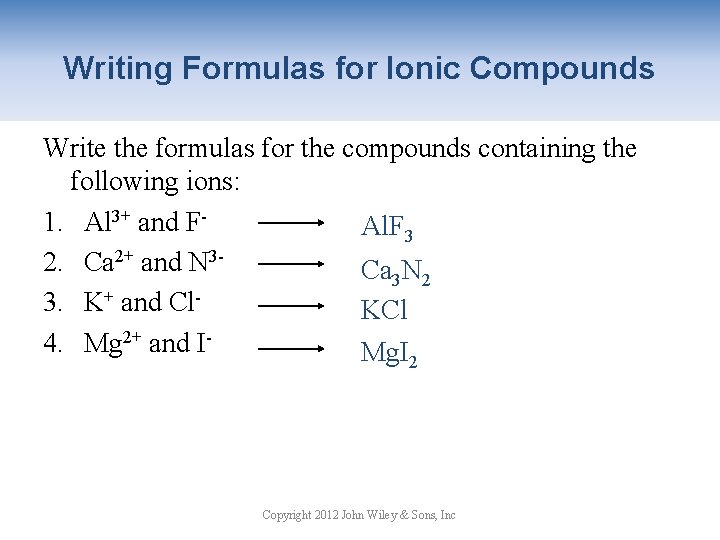
Writing Formulas for Ionic Compounds Write the formulas for the compounds containing the following ions: 1. Al 3+ and FAl. F 3 2. Ca 2+ and N 3 Ca 3 N 2 3. K+ and Cl. KCl 4. Mg 2+ and IMg. I 2 Copyright 2012 John Wiley & Sons, Inc

Your Turn! What is the correct formula for the compound beryllium fluoride? a. Be. F b. Be 2 F c. Be. F 2 d. Be 2 F 2 Copyright 2012 John Wiley & Sons, Inc

Your Turn! What is the correct formula for the compound silver sulfide? a. Ag. S b. Ag. S 2 c. Ag 2 S d. 2 Ag. S Copyright 2012 John Wiley & Sons, Inc

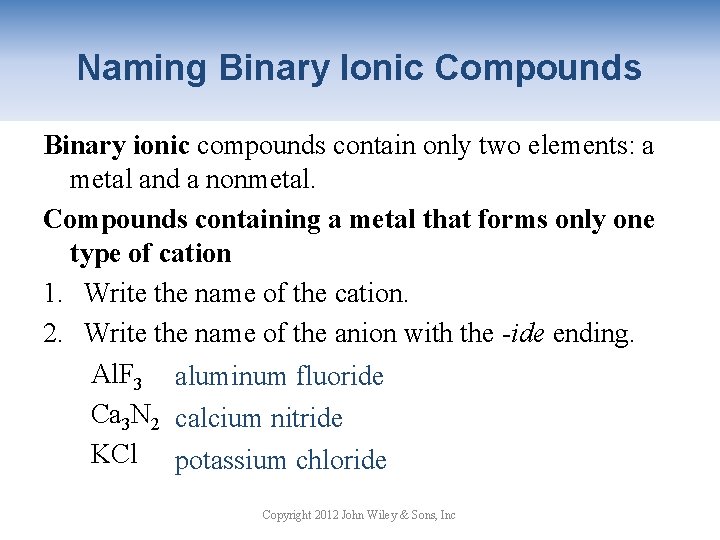
Naming Binary Ionic Compounds Binary ionic compounds contain only two elements: a metal and a nonmetal. Compounds containing a metal that forms only one type of cation 1. Write the name of the cation. 2. Write the name of the anion with the -ide ending. Al. F 3 aluminum fluoride Ca 3 N 2 calcium nitride KCl potassium chloride Copyright 2012 John Wiley & Sons, Inc

Naming Binary Ionic Compounds Common metals with only one type of cation: All metals in Group 1 A, Group 2 A, Al, Zn, Ag and Cd. Their charge is the group number. Name these compounds: 1. Ba. I 2 barium iodide 2. Li 2 O lithium oxide 3. Ca. C 2 calcium carbide 4. Ag 2 S silver sulfide 5. Rb 3 N rubidium nitride Copyright 2012 John Wiley & Sons, Inc

Your Turn! What is the correct name for Cd. F 2? a. Cadmium flourine b. Cadmium flouride c. Cadmium fluorine d. Cadmium fluoride Copyright 2012 John Wiley & Sons, Inc

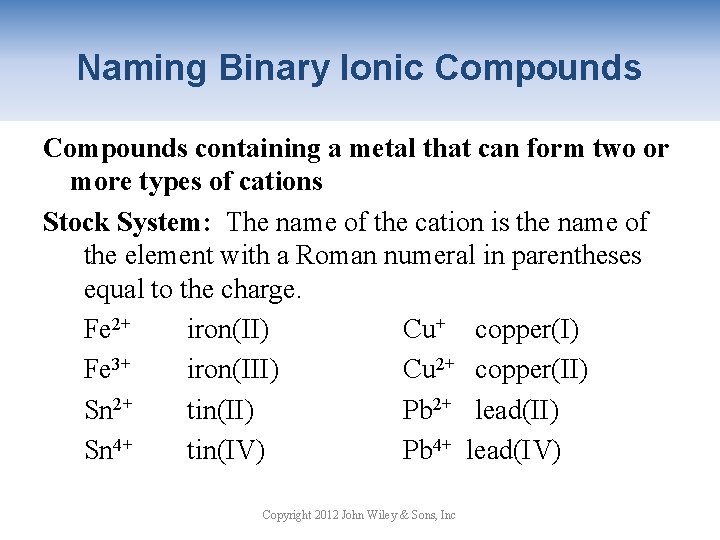
Naming Binary Ionic Compounds containing a metal that can form two or more types of cations Stock System: The name of the cation is the name of the element with a Roman numeral in parentheses equal to the charge. Fe 2+ iron(II) Cu+ copper(I) Fe 3+ iron(III) Cu 2+ copper(II) Sn 2+ tin(II) Pb 2+ lead(II) Sn 4+ tin(IV) Pb 4+ lead(IV) Copyright 2012 John Wiley & Sons, Inc

Naming Binary Ionic Compounds 1. Write the name of the cation. 2. Write the charge on the cation as a Roman numeral in parenthesis. 3. Write the name of the anion with suffix –ide. Co. Cl 3 cobalt(III) chloride Fe 3 P 2 iron(II) phosphide Cu. O copper(II) oxide Sn. Br 4 tin(IV) bromide Copyright 2012 John Wiley & Sons, Inc

Naming Binary Ionic Compounds More Practice 1. Co. Cl 3 cobalt(III) chloride 2. K 2 S potassium sulfide 3. Hg. F 2 mercury(II) fluoride 4. Ag. Br silver bromide 5. Fe 3 P 2 iron(II) phosphide 6. Pb. I 4 lead(IV) iodide Copyright 2012 John Wiley & Sons, Inc

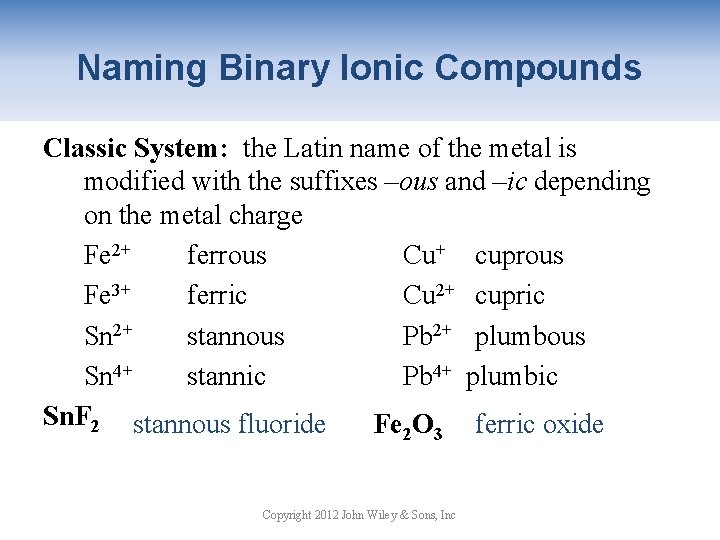
Naming Binary Ionic Compounds Classic System: the Latin name of the metal is modified with the suffixes –ous and –ic depending on the metal charge Fe 2+ ferrous Cu+ cuprous Fe 3+ ferric Cu 2+ cupric Sn 2+ stannous Pb 2+ plumbous Sn 4+ stannic Pb 4+ plumbic Sn. F 2 stannous fluoride Fe O ferric oxide 2 3 Copyright 2012 John Wiley & Sons, Inc

Your Turn! Cu. Cl 2 is a. Copper chloride b. Copper (I) chloride c. Copper (II) chloride d. Cuprous chloride e. Copper chloride (II) Copyright 2012 John Wiley & Sons, Inc

Your Turn! Hg. S is a. mercury(II) sulfide b. mercury(I) sulfide c. mercury sulfide d. mercurous sulfide Copyright 2012 John Wiley & Sons, Inc

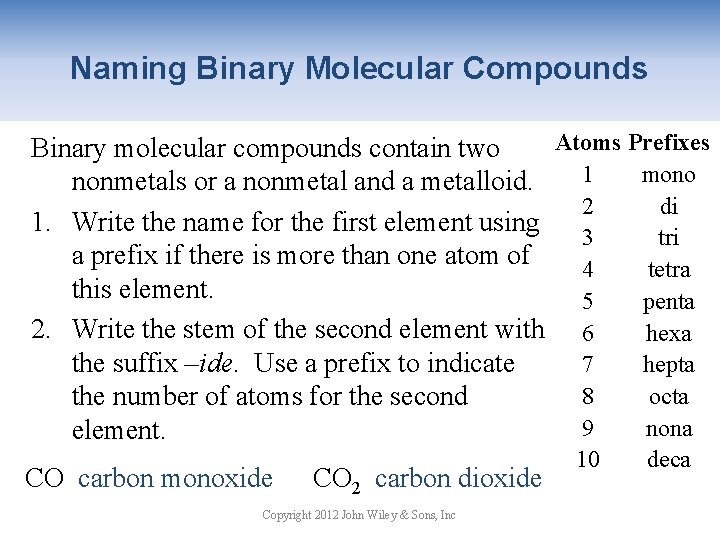
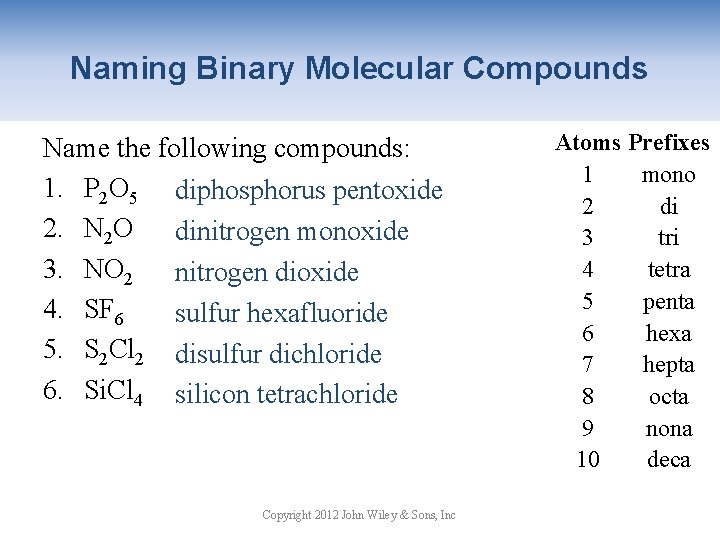
Naming Binary Molecular Compounds Atoms Prefixes Binary molecular compounds contain two 1 mono nonmetals or a nonmetal and a metalloid. 2 di 1. Write the name for the first element using 3 tri a prefix if there is more than one atom of 4 tetra this element. 5 penta 2. Write the stem of the second element with 6 hexa the suffix –ide. Use a prefix to indicate 7 hepta 8 octa the number of atoms for the second 9 nona element. CO carbon monoxide CO 2 carbon dioxide Copyright 2012 John Wiley & Sons, Inc 10 deca

Naming Binary Molecular Compounds Name the following compounds: 1. P 2 O 5 diphosphorus pentoxide 2. N 2 O dinitrogen monoxide 3. NO 2 nitrogen dioxide 4. SF 6 sulfur hexafluoride 5. S 2 Cl 2 disulfur dichloride 6. Si. Cl 4 silicon tetrachloride Copyright 2012 John Wiley & Sons, Inc Atoms Prefixes 1 mono 2 di 3 tri 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona 10 deca

Your Turn! Arsenic pentachloride is a. As. Cl 5 b. As 5 Cl c. As 2 Cl 5 d. As. Cl Copyright 2012 John Wiley & Sons, Inc

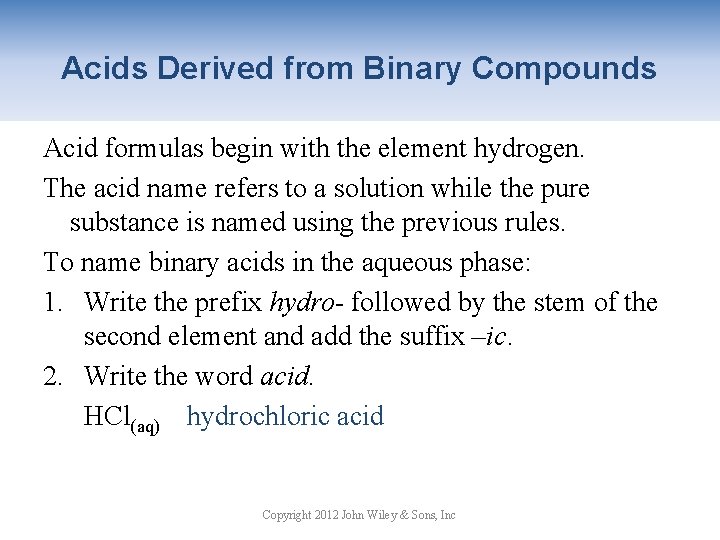
Acids Derived from Binary Compounds Acid formulas begin with the element hydrogen. The acid name refers to a solution while the pure substance is named using the previous rules. To name binary acids in the aqueous phase: 1. Write the prefix hydro- followed by the stem of the second element and add the suffix –ic. 2. Write the word acid. HCl(aq) hydrochloric acid Copyright 2012 John Wiley & Sons, Inc

Acids Derived from Binary Compounds Name the following compounds: 1. HBr(g) hydrogen bromide gas 2. HBr(aq) hydrobromic acid 3. H 2 S(aq) hydrosulfuric acid 4. HF(aq) hydrofluoric acid 5. HI(aq) hydroiodic acid Copyright 2012 John Wiley & Sons, Inc

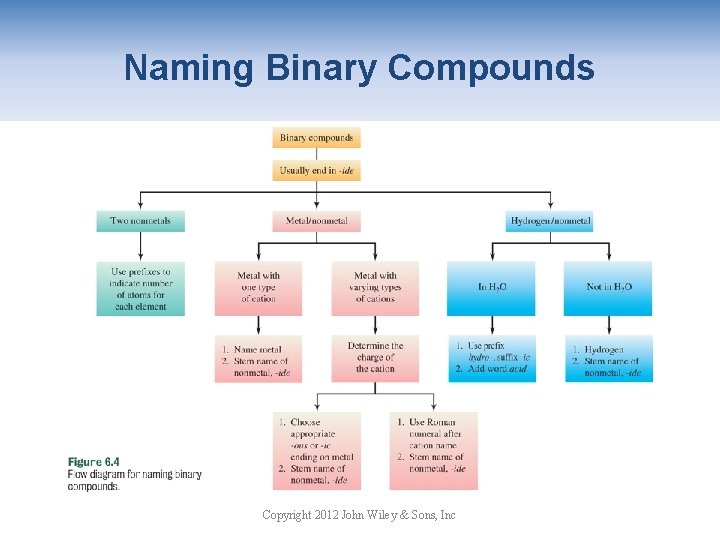
Naming Binary Compounds Copyright 2012 John Wiley & Sons, Inc

Your Turn! V 2 O 5 is a. divanadium pentoxide b. vanadium pentoxide c. vanadium(II) oxide d. vanadium(V) oxide Copyright 2012 John Wiley & Sons, Inc

Your Turn! Sulfur dioxide is a. SO b. S 2 O c. SO 2 Copyright 2012 John Wiley & Sons, Inc

Your Turn! A solution containing HF should be named a. hydrogen fluoride b. hydrofluoric acid c. hydrofluoride acid Copyright 2012 John Wiley & Sons, Inc

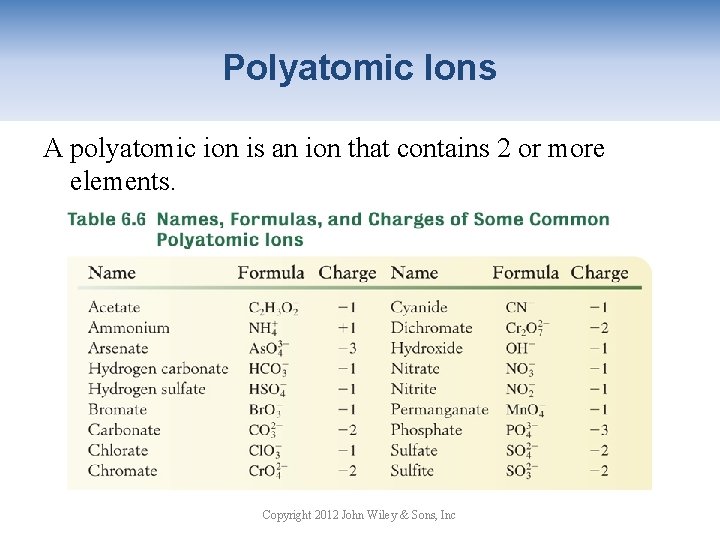
Polyatomic Ions A polyatomic ion is an ion that contains 2 or more elements. Copyright 2012 John Wiley & Sons, Inc

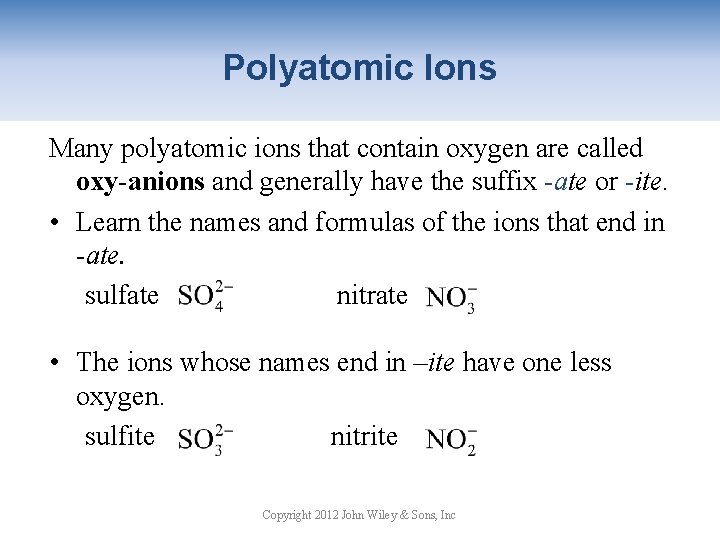
Polyatomic Ions Many polyatomic ions that contain oxygen are called oxy-anions and generally have the suffix -ate or -ite. • Learn the names and formulas of the ions that end in -ate. sulfate nitrate • The ions whose names end in –ite have one less oxygen. nitrite sulfite Copyright 2012 John Wiley & Sons, Inc

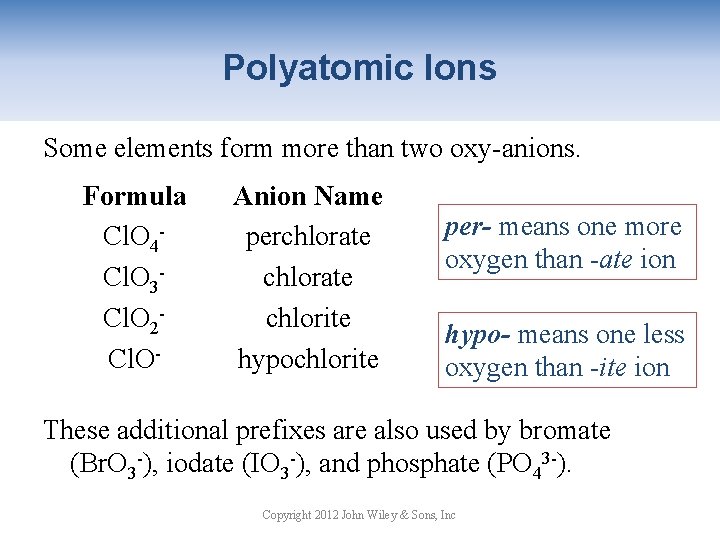
Polyatomic Ions Some elements form more than two oxy-anions. Formula Cl. O 4 Cl. O 3 Cl. O 2 Cl. O- Anion Name perchlorate chlorite hypochlorite per- means one more oxygen than -ate ion hypo- means one less oxygen than -ite ion These additional prefixes are also used by bromate (Br. O 3 -), iodate (IO 3 -), and phosphate (PO 43 -). Copyright 2012 John Wiley & Sons, Inc

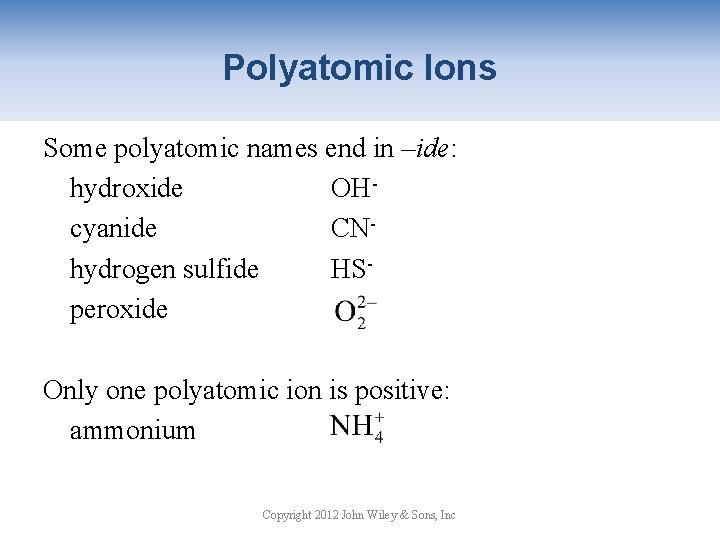
Polyatomic Ions Some polyatomic names end in –ide: hydroxide OHcyanide CNhydrogen sulfide HSperoxide Only one polyatomic ion is positive: ammonium Copyright 2012 John Wiley & Sons, Inc

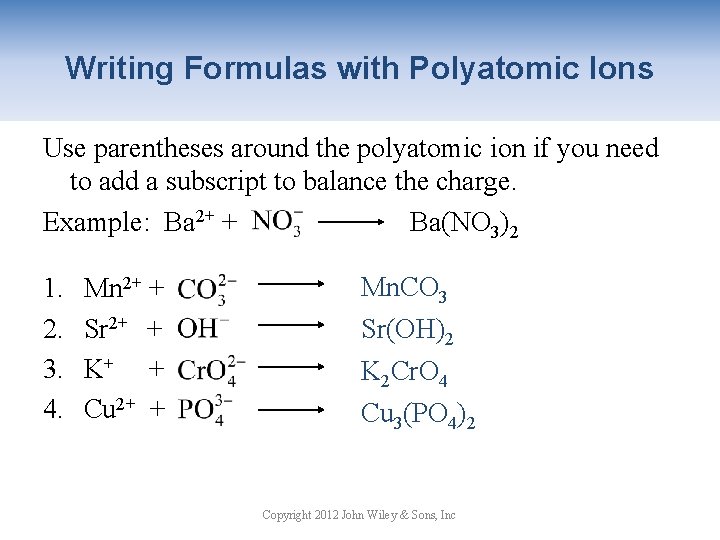
Writing Formulas with Polyatomic Ions Use parentheses around the polyatomic ion if you need to add a subscript to balance the charge. Example: Ba 2+ + Ba(NO 3)2 1. 2. 3. 4. Mn 2+ + Sr 2+ + K+ + Cu 2+ + Mn. CO 3 Sr(OH)2 K 2 Cr. O 4 Cu 3(PO 4)2 Copyright 2012 John Wiley & Sons, Inc

Naming Compounds Containing Polyatomic Ions 1. Write the name of the cation. 2. Write the name of the anion. Name these compounds: Hg(Cl. O 2)2 mercury(II) chlorite Zn 3(PO 4)2 zinc phosphate NH 4 NO 3 ammonium nitrate Pb(C 2 H 3 O 2)2 lead(II) acetate Copyright 2012 John Wiley & Sons, Inc

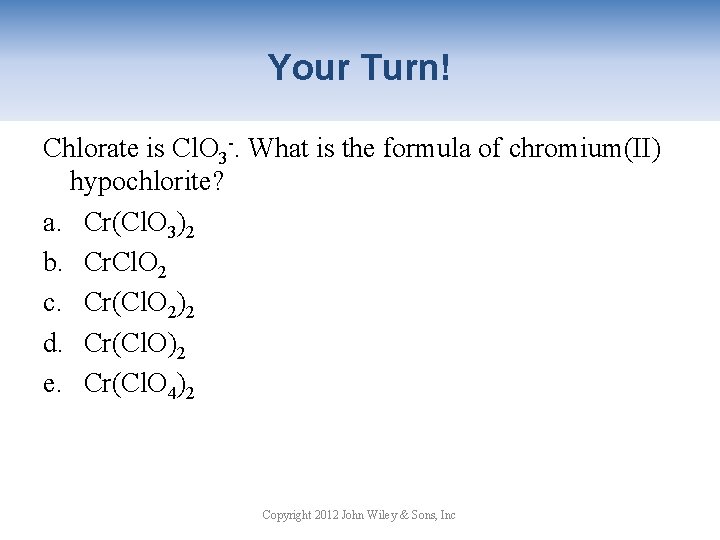
Your Turn! Chlorate is Cl. O 3 -. What is the formula of chromium(II) hypochlorite? a. Cr(Cl. O 3)2 b. Cr. Cl. O 2 c. Cr(Cl. O 2)2 d. Cr(Cl. O)2 e. Cr(Cl. O 4)2 Copyright 2012 John Wiley & Sons, Inc

Your Turn! Sulfate is SO 42 -. Name the compound Fe. SO 4. a. iron sulfate b. iron(I) sulfate c. iron(II) sulfate d. iron(IV) sulfate Copyright 2012 John Wiley & Sons, Inc

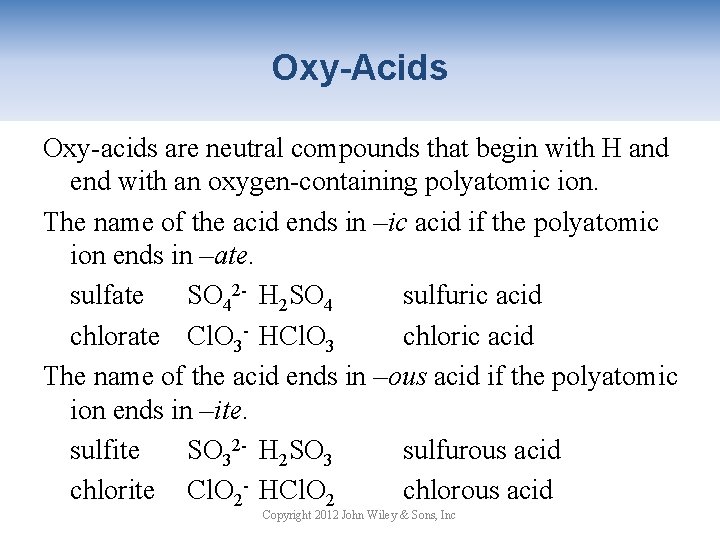
Oxy-Acids Oxy-acids are neutral compounds that begin with H and end with an oxygen-containing polyatomic ion. The name of the acid ends in –ic acid if the polyatomic ion ends in –ate. sulfate SO 42 - H 2 SO 4 sulfuric acid chlorate Cl. O 3 - HCl. O 3 chloric acid The name of the acid ends in –ous acid if the polyatomic ion ends in –ite. sulfite SO 32 - H 2 SO 3 sulfurous acid chlorite Cl. O 2 - HCl. O 2 chlorous acid Copyright 2012 John Wiley & Sons, Inc

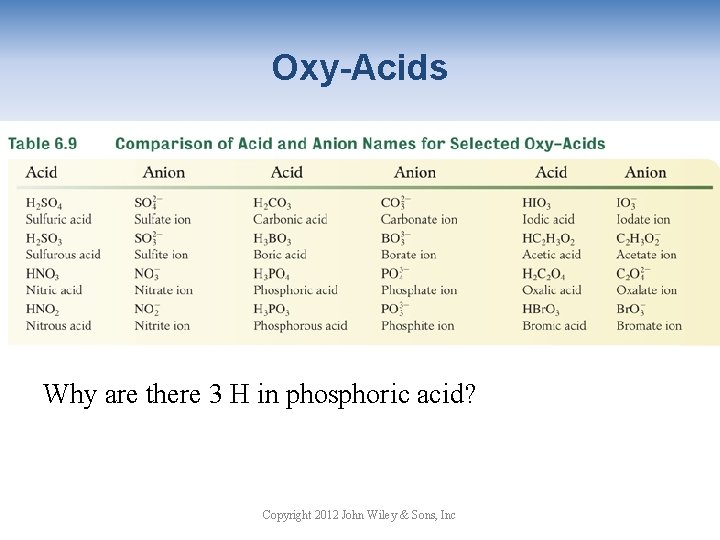
Oxy-Acids Why are there 3 H in phosphoric acid? Copyright 2012 John Wiley & Sons, Inc

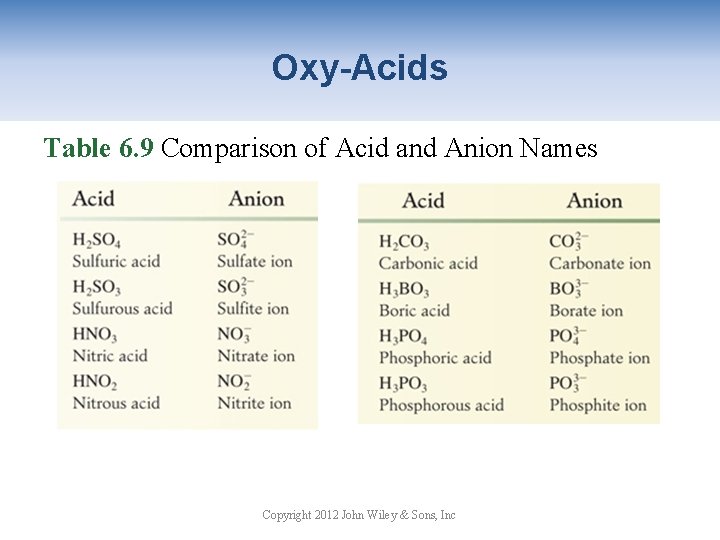
Oxy-Acids Table 6. 9 Comparison of Acid and Anion Names Copyright 2012 John Wiley & Sons, Inc

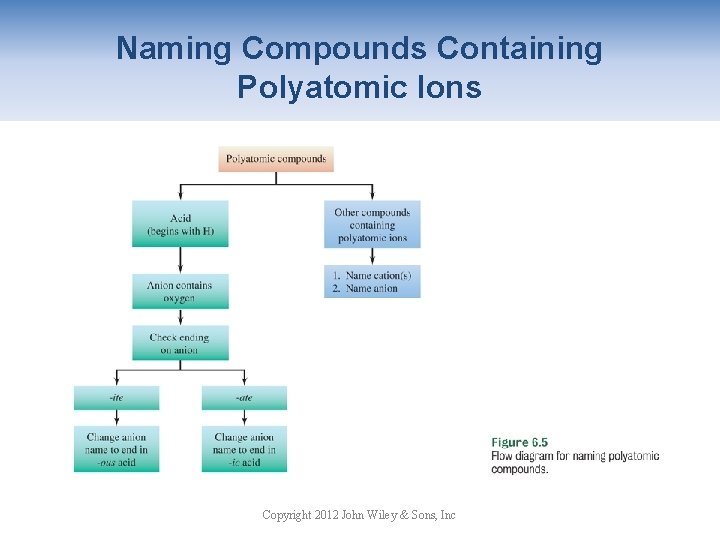
Naming Compounds Containing Polyatomic Ions Copyright 2012 John Wiley & Sons, Inc

Your Turn! Nitrate is NO 3 -. HNO 2 is a. Hydrogen nitrite b. Hydrogen nitrogen dioxide c. Nitric acid d. Nitrous acid Copyright 2012 John Wiley & Sons, Inc