Chapter 6 Moles Molar Mass Percent Composition and









- Slides: 29

Chapter 6: Moles, Molar Mass, Percent Composition and Formulas From moles to mass and to the moon!

AMU (Atomic Mass Units) l The mass of Carbon-12 is 12 AMU. l But wait, when I look on the periodic table, the atomic mass is listed as 12. 01078 AMU? ? ? WHY? Why, cruel world? l Ww. WWwwhh. HHHhhyy. YYYYyy? ? ?

6. 1 Atoms and Moles Amedeo Avogadro l Avogadro’s Number l 6. 022 x 1023 l Avogadro discovered that there are 6. 022 x 1023 atoms in 1 gram of hydrogen. Count Lorenzo Romano Amedeo Carlo Avogadro di Quaregna e Cerreto

Be able to explain and use the concept of the “mole” l This number is called a “mole. ” l The word “mole” is just like the word “dozen”. Dozen means “ 12”. You can have a dozen of anything. You can also have a mole of anything. Hmmm… I shall call 6. 022 x 1023. . . a “mole”. Yes…that has a nice ring to it.

So How Big is a “MOLE” l Ummm… NO! Don’tout be l Here it is written Aaaiiie cruel now… e 602, 200, 000, 000 That’s 602 billion groups of a trillion! l Let’s just do an example with paper clips. l If you have a mole of paper clips and made them into a chain, how many times could you go to the moon and back with your chain?

l Assume a paper clip ( still folded) is about 3 cm long. l To find the total distance of the paper clips we use the following equation: Notice the unit “clips” cancels!!! Isn’t that Great… Anyone see the greatness? ? ? Man I love Conversions!

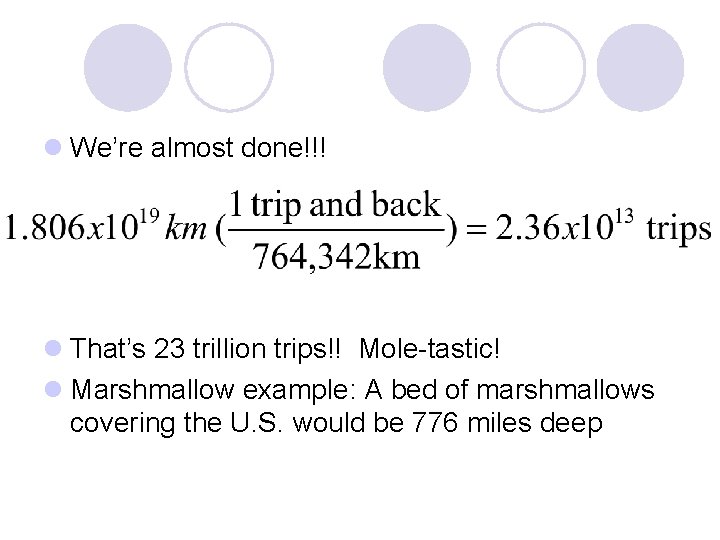
The moon is 382, 171 km from Earth, so to the moon and back would be 764, 342 km. l So we need to convert our cm into km… … oh how fun… this is a metric conversion l This of course is a “ 2 -step conversion” because both units have a prefix I love conversions!

l We’re almost done!!! l That’s 23 trillion trips!! Mole-tastic! l Marshmallow example: A bed of marshmallows covering the U. S. would be 776 miles deep

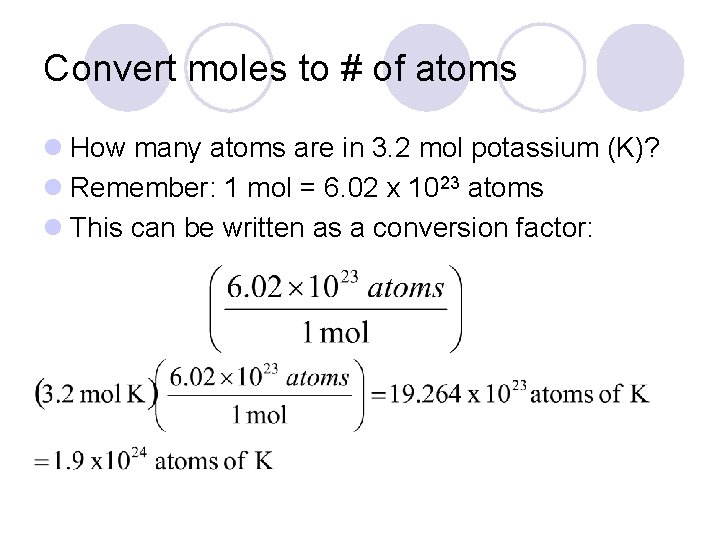
Convert moles to # of atoms l How many atoms are in 3. 2 mol potassium (K)? l Remember: 1 mol = 6. 02 x 1023 atoms l This can be written as a conversion factor:

How do we use the “Mole” in chemistry? l The atomic mass of an element is the grams of 1 mole of that atom l Why do chemists use moles? ¡It’s fun. ¡It’s impossible to count atoms with your hands. ¡You can easily measure the mass (in grams) of a chemical.

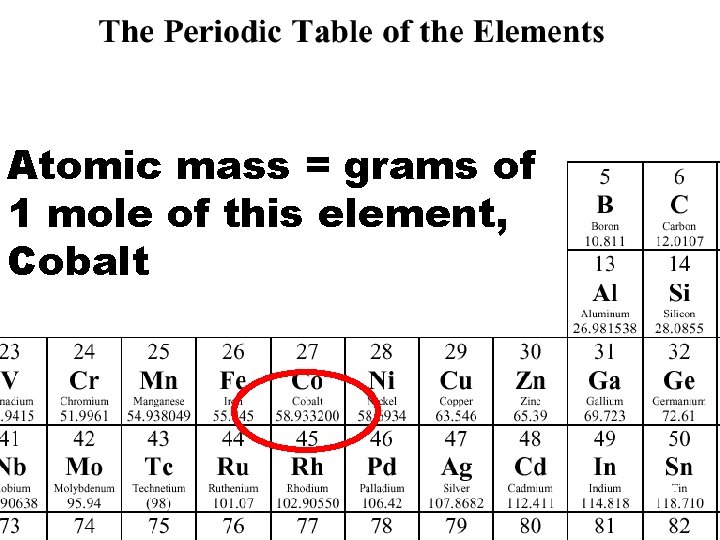
Atomic mass = grams of 1 mole of this element, Cobalt

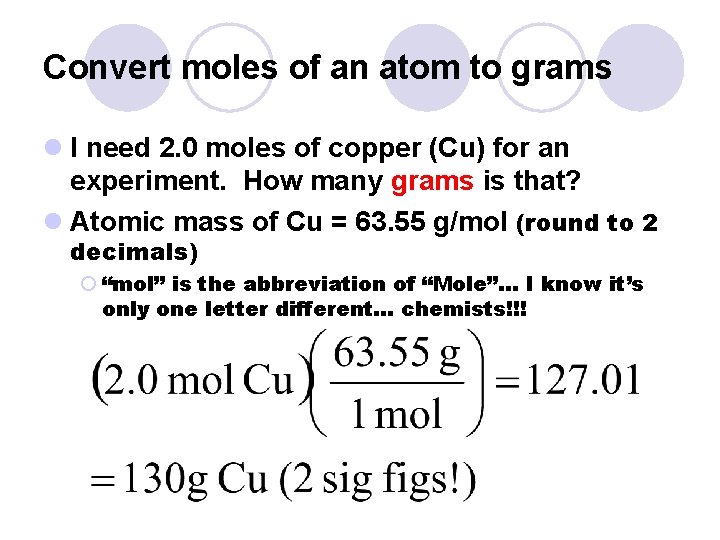
Convert moles of an atom to grams l I need 2. 0 moles of copper (Cu) for an experiment. How many grams is that? l Atomic mass of Cu = 63. 55 g/mol (round to 2 decimals) ¡ “mol” is the abbreviation of “Mole”… I know it’s only one letter different… chemists!!!

Converting grams to moles l I have 302 grams of silver (Ag). How many moles of silver do I have? ¡ Step 1: Atomic mass of Ag = 107. 87 g/mol ¡ Step 2: Calculate

6. 2 Molar Mass and Percent Composition l Atomic Mass = mass of one mole of an atom l Molar Mass= mass of one mole of a substance

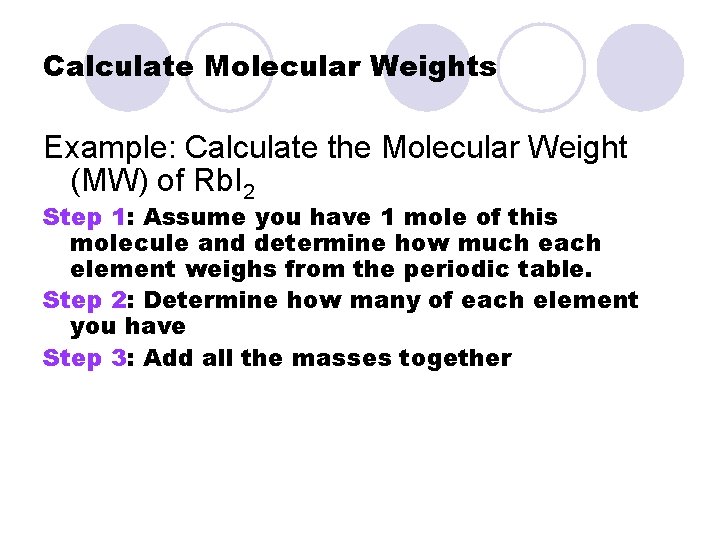
Calculate Molecular Weights Example: Calculate the Molecular Weight (MW) of Rb. I 2 Step 1: Assume you have 1 mole of this molecule and determine how much each element weighs from the periodic table. Step 2: Determine how many of each element you have Step 3: Add all the masses together

Step 1: Find how much each element weighs from the periodic table l Rb is atomic # 37. How much does each mole of Rb weigh? ¡ 85. 47 grams/mol Rb l I is atomic # 53. How much does it weigh? ¡ 126. 90 g/mol I

Step 2: Determine how many of each element you have Look at the formula: We have 1 “Rb” atom and 2 “I” atoms Rb. I 2

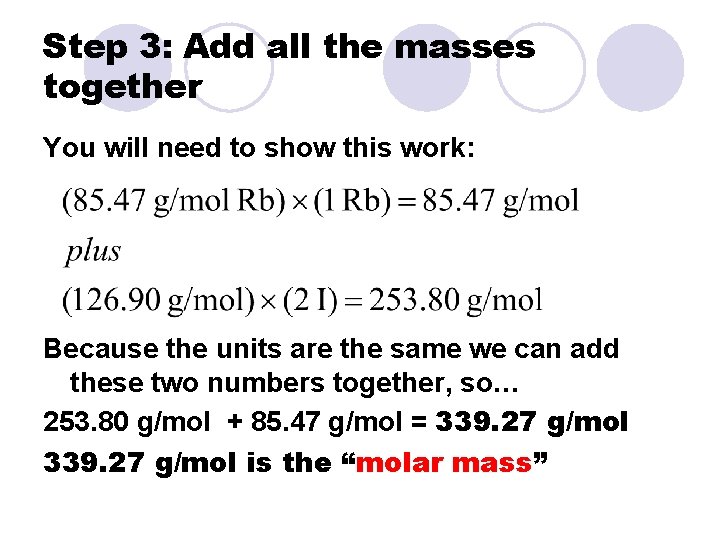
Step 3: Add all the masses together You will need to show this work: Because the units are the same we can add these two numbers together, so… 253. 80 g/mol + 85. 47 g/mol = 339. 27 g/mol is the “molar mass”

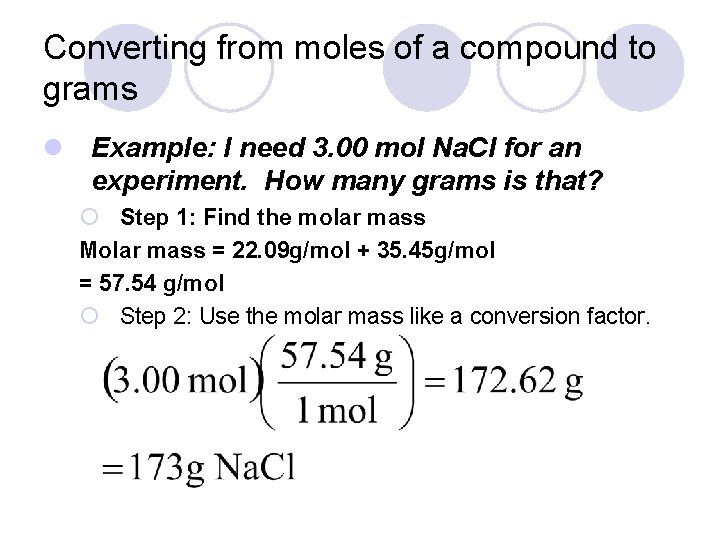
Converting from moles of a compound to grams l Example: I need 3. 00 mol Na. Cl for an experiment. How many grams is that? ¡ Step 1: Find the molar mass Molar mass = 22. 09 g/mol + 35. 45 g/mol = 57. 54 g/mol ¡ Step 2: Use the molar mass like a conversion factor.

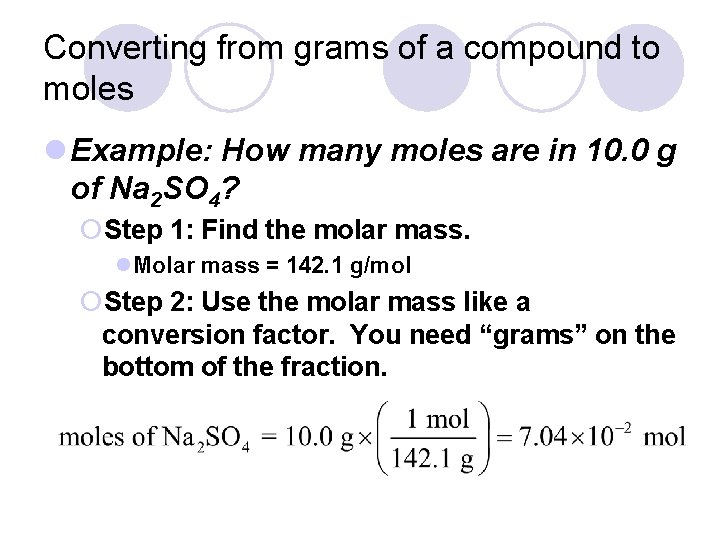
Converting from grams of a compound to moles l Example: How many moles are in 10. 0 g of Na 2 SO 4? ¡Step 1: Find the molar mass. l. Molar mass = 142. 1 g/mol ¡Step 2: Use the molar mass like a conversion factor. You need “grams” on the bottom of the fraction.

6. 3 Formulas of Compounds l Calculate “percent composition” ¡Just like any other % ¡Stuff = grams of elements

Calculate “percent composition” l. Ex: calculate % of Cu and S in Cu 2 S l Stuff = grams Cu ¡ (63. 55 g/mol Cu)(2 mol Cu) = 127. 1 g Cu l Total stuff = grams Cu + grams S = 127. 1 g + 32. 07 g = 159. 17 g = 159. 2 g

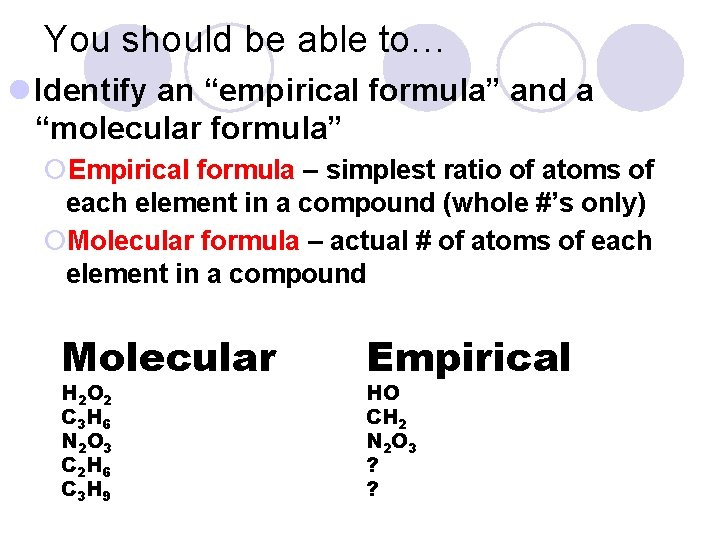
You should be able to… l Identify an “empirical formula” and a “molecular formula” ¡Empirical formula – simplest ratio of atoms of each element in a compound (whole #’s only) ¡Molecular formula – actual # of atoms of each element in a compound Molecular H 2 O 2 C 3 H 6 N 2 O 3 C 2 H 6 C 3 H 9 Empirical HO CH 2 N 2 O 3 ? ?

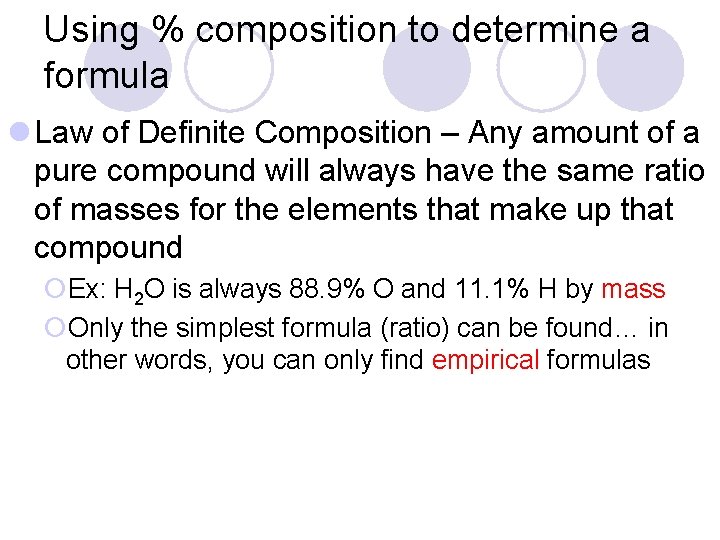
Using % composition to determine a formula l Law of Definite Composition – Any amount of a pure compound will always have the same ratio of masses for the elements that make up that compound ¡Ex: H 2 O is always 88. 9% O and 11. 1% H by mass ¡Only the simplest formula (ratio) can be found… in other words, you can only find empirical formulas

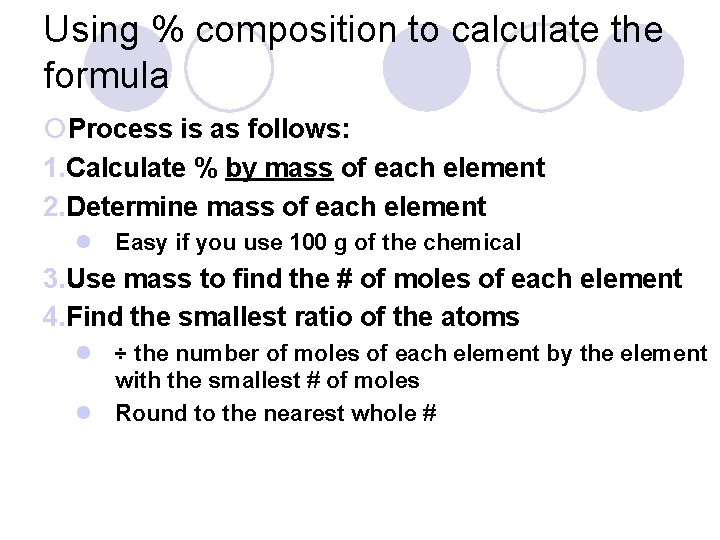
Using % composition to calculate the formula ¡Process is as follows: 1. Calculate % by mass of each element 2. Determine mass of each element l Easy if you use 100 g of the chemical 3. Use mass to find the # of moles of each element 4. Find the smallest ratio of the atoms l ÷ the number of moles of each element by the element with the smallest # of moles l Round to the nearest whole #

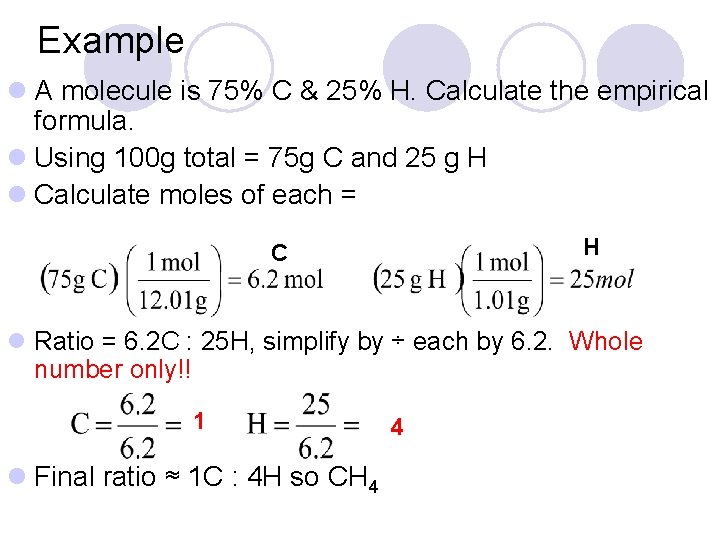
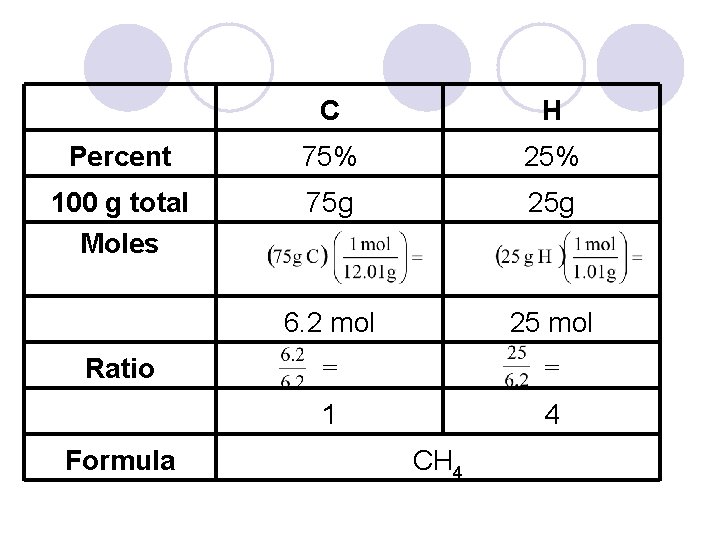
Example l A molecule is 75% C & 25% H. Calculate the empirical formula. l Using 100 g total = 75 g C and 25 g H l Calculate moles of each = H C l Ratio = 6. 2 C : 25 H, simplify by ÷ each by 6. 2. Whole number only!! 1 l Final ratio ≈ 1 C : 4 H so CH 4 4

C H Percent 75% 25% 100 g total Moles 75 g 25 g 6. 2 mol 25 mol = = 1 4 Ratio Formula CH 4

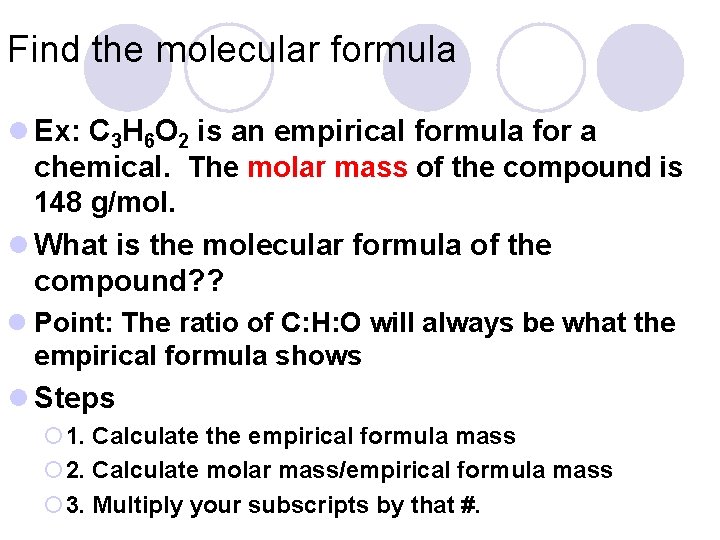
Find the molecular formula l Ex: C 3 H 6 O 2 is an empirical formula for a chemical. The molar mass of the compound is 148 g/mol. l What is the molecular formula of the compound? ? l Point: The ratio of C: H: O will always be what the empirical formula shows l Steps ¡ 1. Calculate the empirical formula mass ¡ 2. Calculate molar mass/empirical formula mass ¡ 3. Multiply your subscripts by that #.

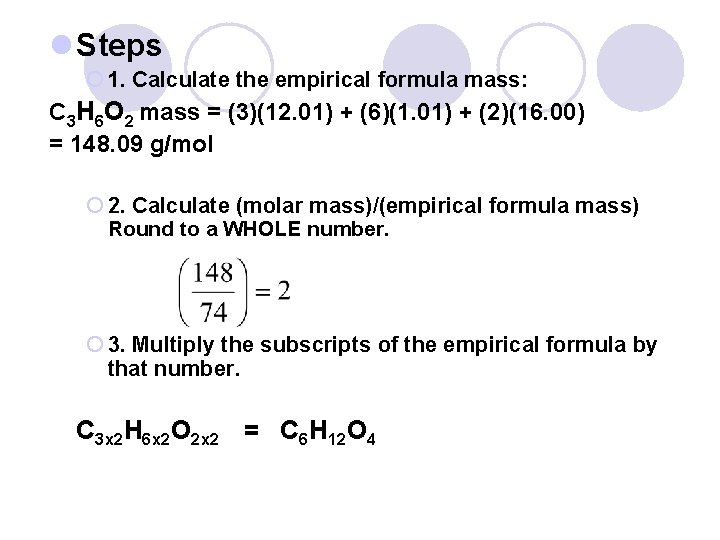
l Steps ¡ 1. Calculate the empirical formula mass: C 3 H 6 O 2 mass = (3)(12. 01) + (6)(1. 01) + (2)(16. 00) = 148. 09 g/mol ¡ 2. Calculate (molar mass)/(empirical formula mass) Round to a WHOLE number. ¡ 3. Multiply the subscripts of the empirical formula by that number. C 3 x 2 H 6 x 2 O 2 x 2 = C 6 H 12 O 4