Chapter 6 Inorganic and Organic Compounds Names and


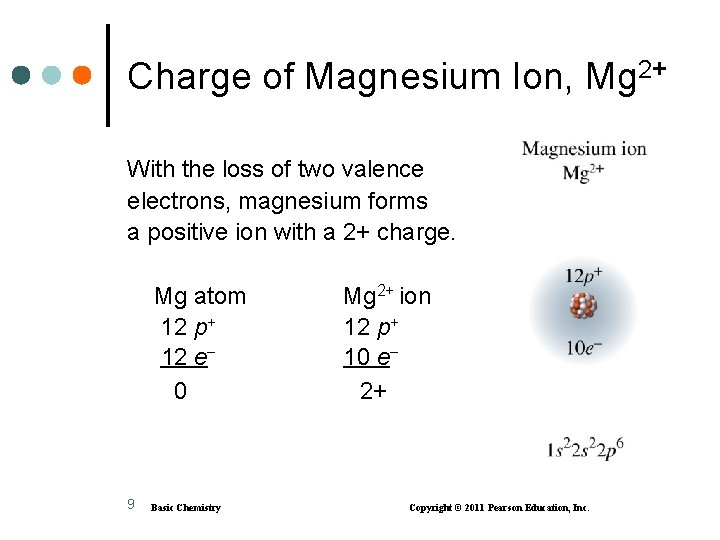























- Slides: 88

Chapter 6 Inorganic and Organic Compounds: Names and Formulas 6. 1 1 Basic Chemistry Octet Rule and Ions Copyright © 2011 Pearson Education, Inc.

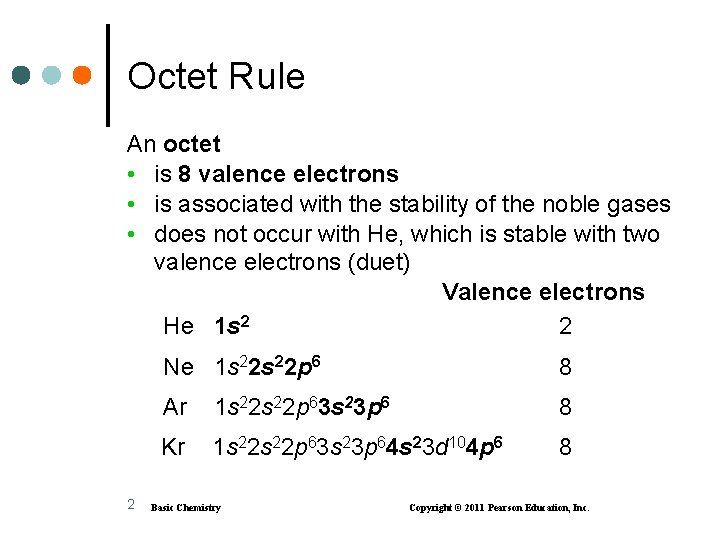
Octet Rule An octet • is 8 valence electrons • is associated with the stability of the noble gases • does not occur with He, which is stable with two valence electrons (duet) Valence electrons He 1 s 2 2 2 Ne 1 s 22 p 6 8 Ar 1 s 22 p 63 s 23 p 6 8 Kr 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 8 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Forming Octets Atoms acquire octets • to become more stable • by losing, gaining, or sharing valence electrons • by forming ionic or covalent bonds 3 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

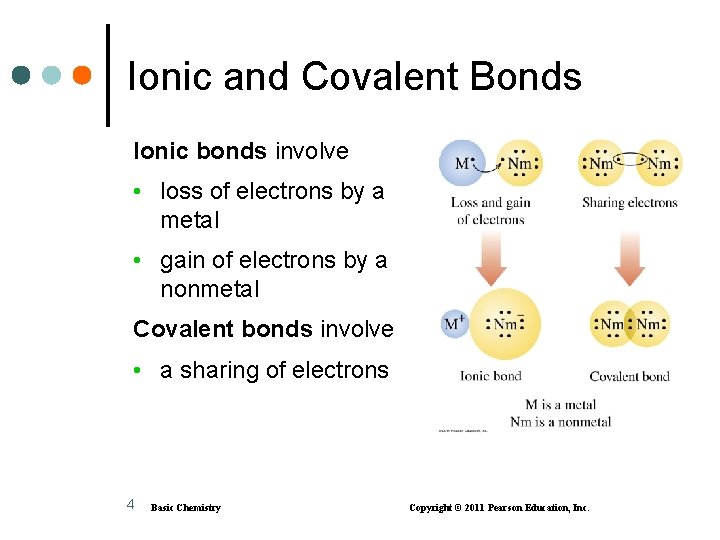
Ionic and Covalent Bonds Ionic bonds involve • loss of electrons by a metal • gain of electrons by a nonmetal Covalent bonds involve • a sharing of electrons 4 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

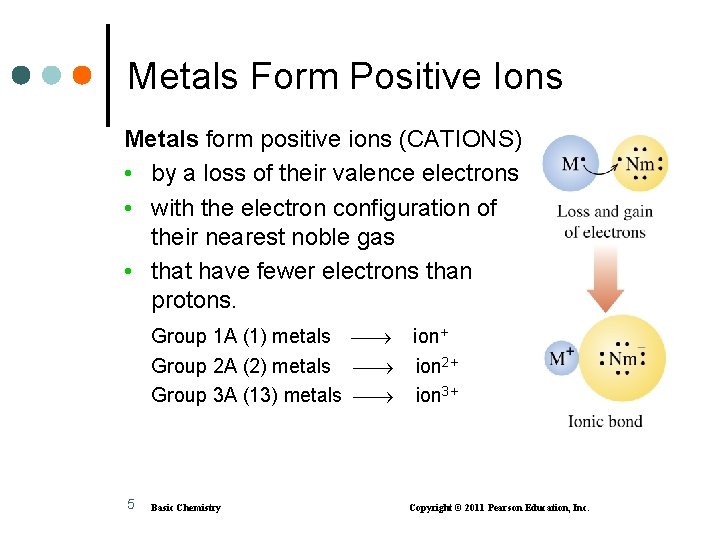
Metals Form Positive Ions Metals form positive ions (CATIONS) • by a loss of their valence electrons • with the electron configuration of their nearest noble gas • that have fewer electrons than protons. Group 1 A (1) metals Group 2 A (2) metals Group 3 A (13) metals 5 Basic Chemistry ion+ ion 2+ ion 3+ Copyright © 2011 Pearson Education, Inc.

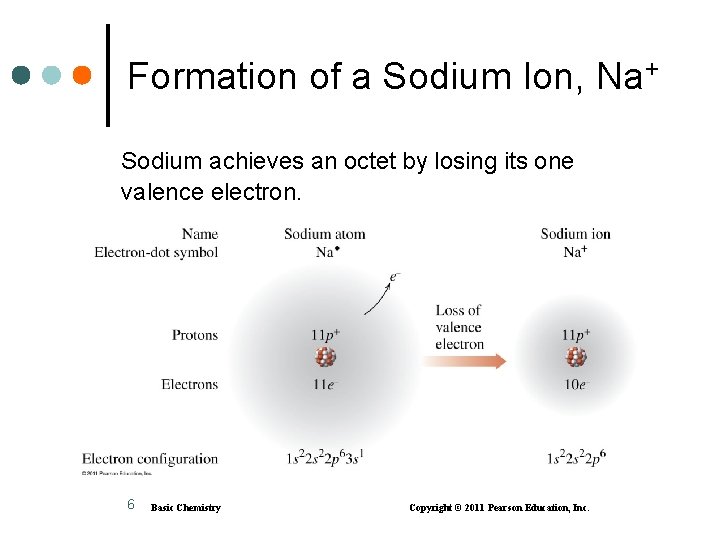
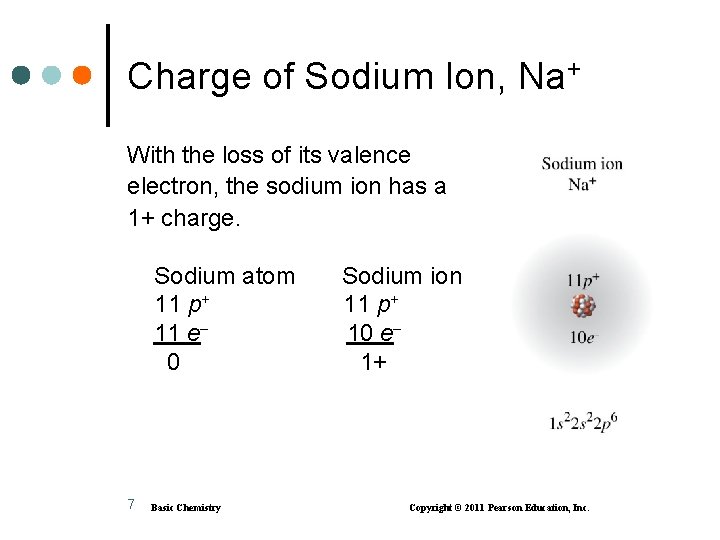
Formation of a Sodium Ion, Na+ Sodium achieves an octet by losing its one valence electron. 6 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Charge of Sodium Ion, Na+ With the loss of its valence electron, the sodium ion has a 1+ charge. Sodium atom 11 p+ 11 e 0 7 Basic Chemistry Sodium ion 11 p+ 10 e 1+ Copyright © 2011 Pearson Education, Inc.

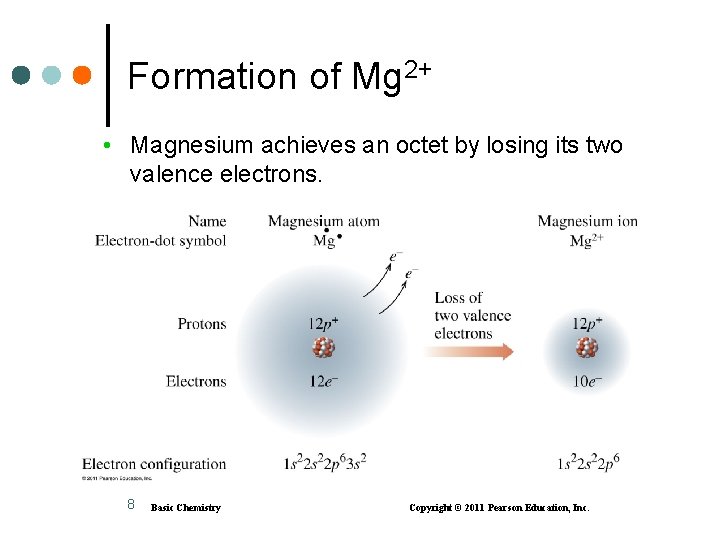
Formation of Mg 2+ • Magnesium achieves an octet by losing its two valence electrons. 8 Basic Chemistry Copyright © 2011 Pearson Education, Inc.
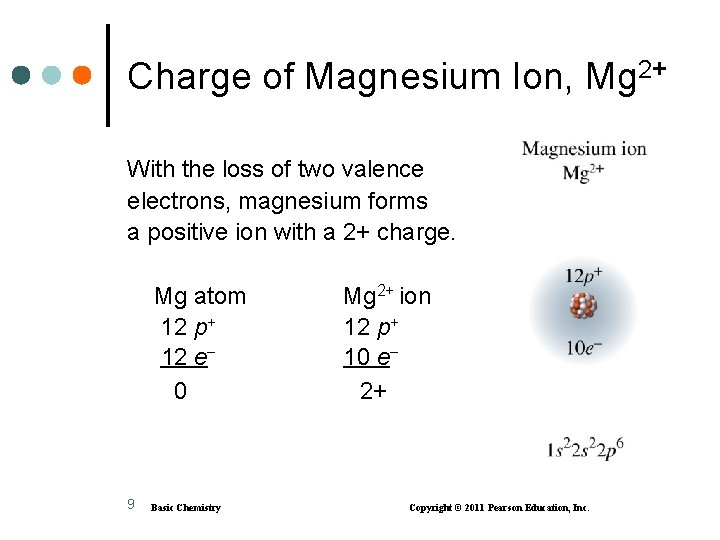
Charge of Magnesium Ion, Mg 2+ With the loss of two valence electrons, magnesium forms a positive ion with a 2+ charge. Mg atom 12 p+ 12 e 0 9 Basic Chemistry Mg 2+ ion 12 p+ 10 e 2+ Copyright © 2011 Pearson Education, Inc.

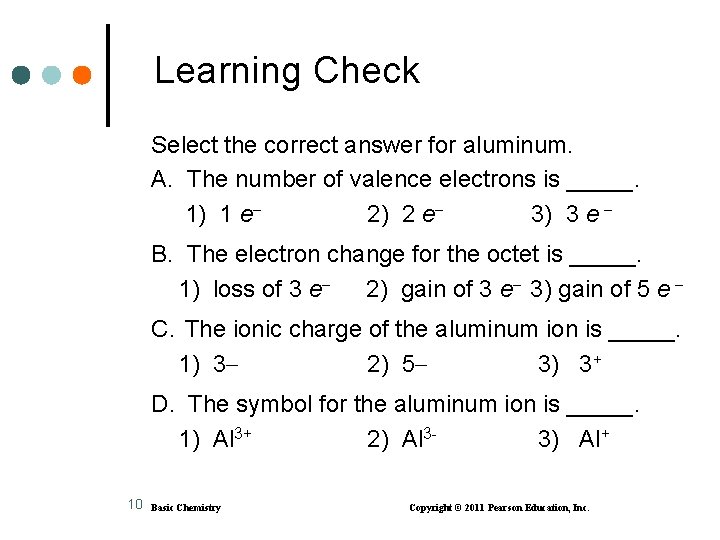
Learning Check Select the correct answer for aluminum. A. The number of valence electrons is _____. 1) 1 e 2) 2 e 3) 3 e B. The electron change for the octet is _____. 1) loss of 3 e 2) gain of 3 e 3) gain of 5 e C. The ionic charge of the aluminum ion is _____. 1) 3 2) 5 3) 3+ D. The symbol for the aluminum ion is _____. 1) Al 3+ 2) Al 33) Al+ 10 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

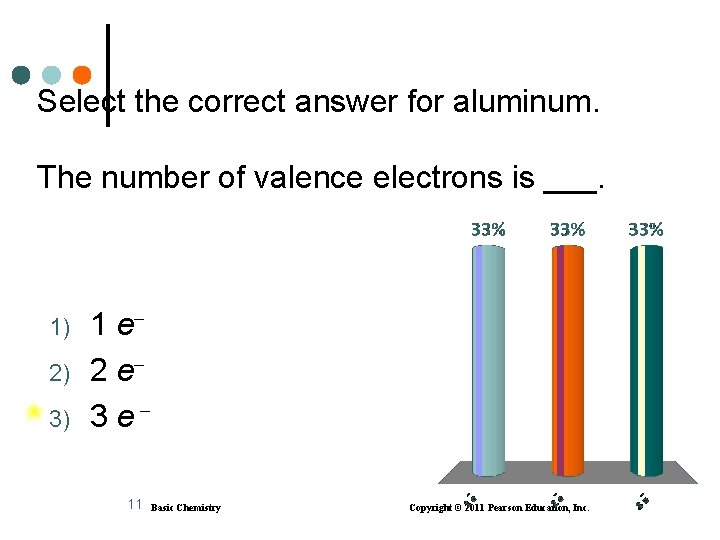
Select the correct answer for aluminum. The number of valence electrons is ___. 1) 2) 3) 1 e 2 e 3 e 11 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

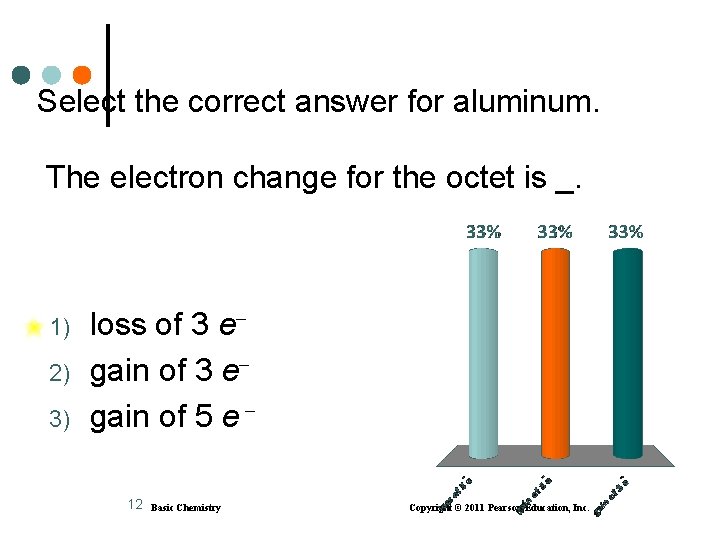
Select the correct answer for aluminum. The electron change for the octet is _. 1) 2) 3) loss of 3 e gain of 5 e 12 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

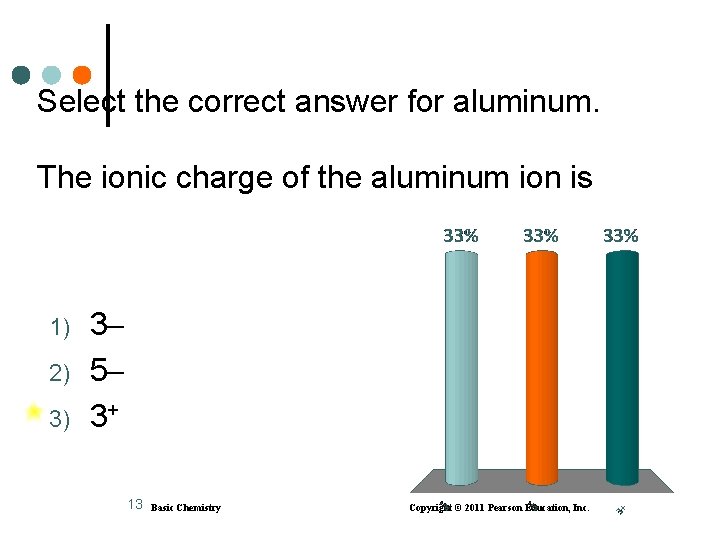
Select the correct answer for aluminum. The ionic charge of the aluminum ion is 1) 2) 3) 3 5 3+ 13 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

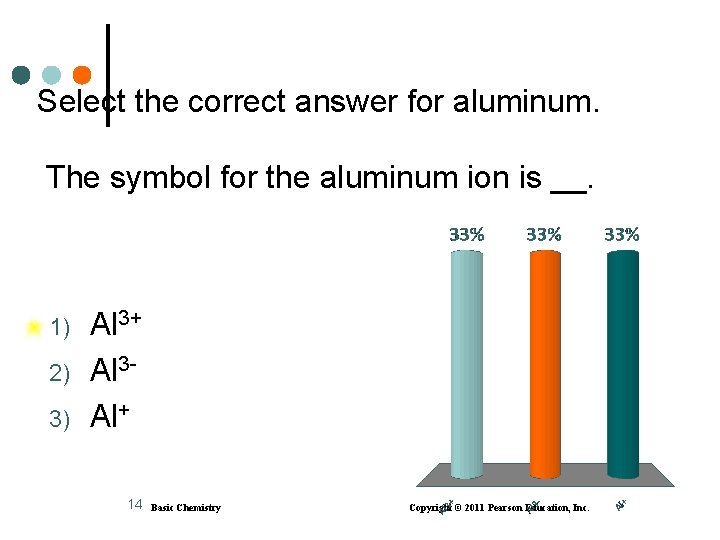
Select the correct answer for aluminum. The symbol for the aluminum ion is __. 1) 2) 3) Al 3+ Al 3 Al+ 14 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Select the correct answer for aluminum: A. The number of valence electrons is 3) 3 e B. The electron change for the octet is 1) loss of 3 e C. The ionic charge of the aluminum ion is 3) 3+ D. The symbol for the aluminum ion is 1) Al 3+ 15 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Formation of Negative Ions In ionic compounds, nonmetals (FORM ANIONS) • achieve an octet arrangement • gain electrons • form negatively charged ions with 3 , 2 , or 1 charges 16 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

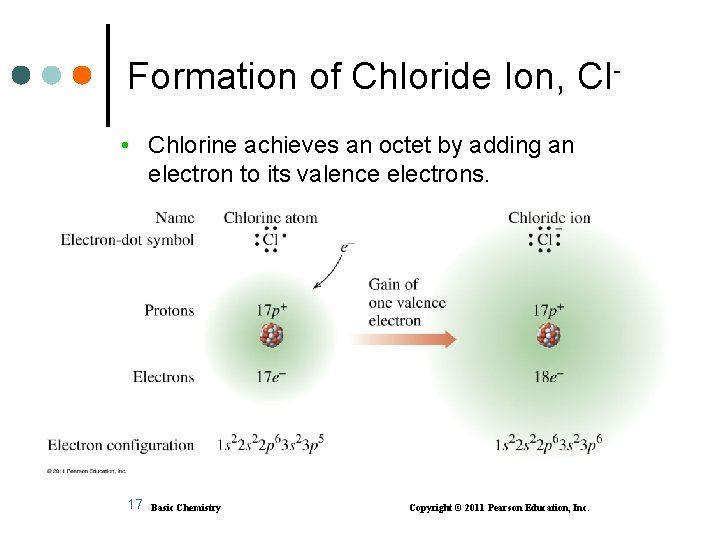
Formation of Chloride Ion, Cl • Chlorine achieves an octet by adding an electron to its valence electrons. 17 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

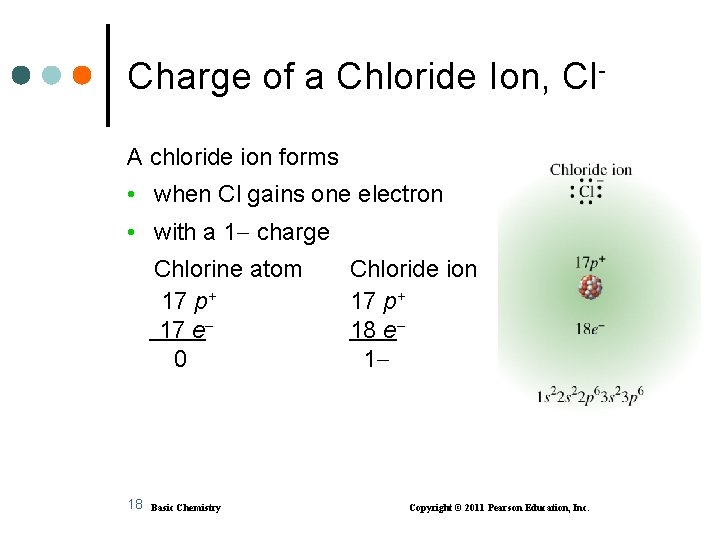
Charge of a Chloride Ion, Cl. A chloride ion forms • when Cl gains one electron • with a 1 charge Chlorine atom 17 p+ 17 e 0 18 Basic Chemistry Chloride ion 17 p+ 18 e 1 Copyright © 2011 Pearson Education, Inc.

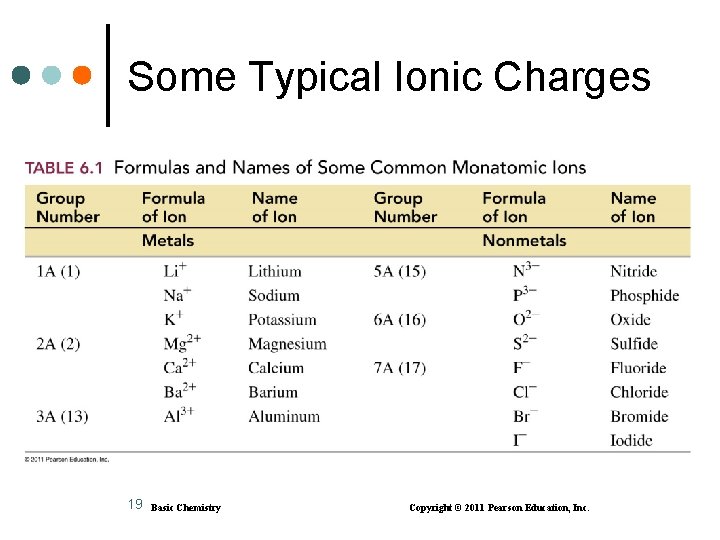
Some Typical Ionic Charges 19 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

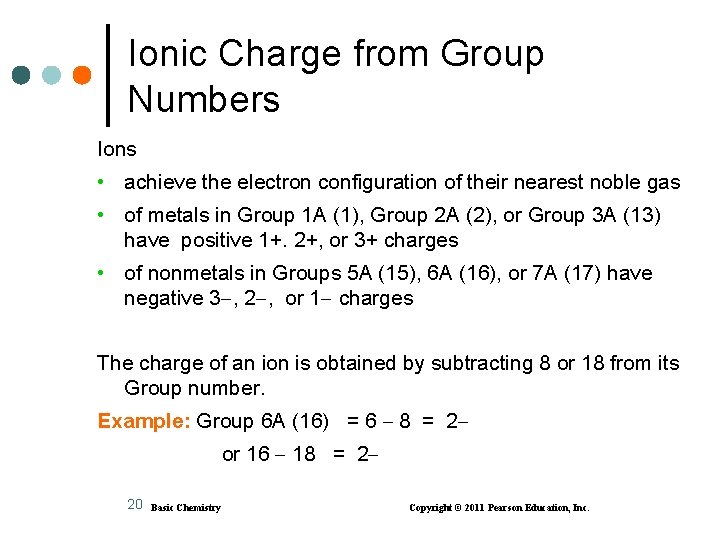
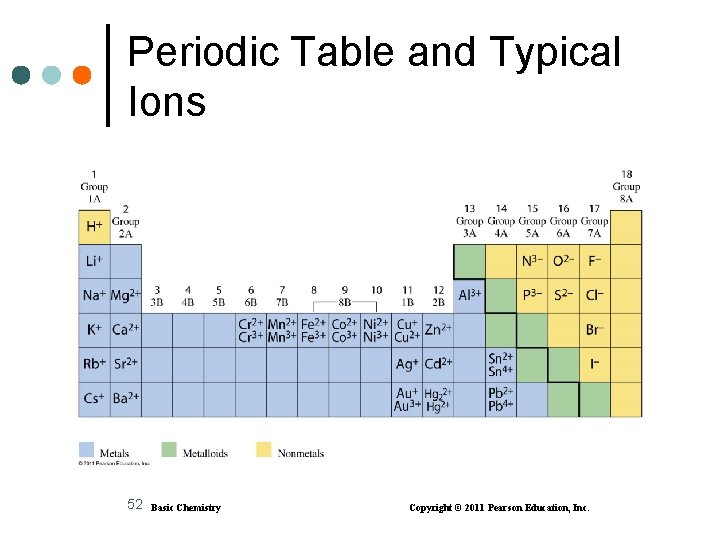
Ionic Charge from Group Numbers Ions • achieve the electron configuration of their nearest noble gas • of metals in Group 1 A (1), Group 2 A (2), or Group 3 A (13) have positive 1+. 2+, or 3+ charges • of nonmetals in Groups 5 A (15), 6 A (16), or 7 A (17) have negative 3 , 2 , or 1 charges The charge of an ion is obtained by subtracting 8 or 18 from its Group number. Example: Group 6 A (16) = 6 8 = 2 or 16 18 = 2 20 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

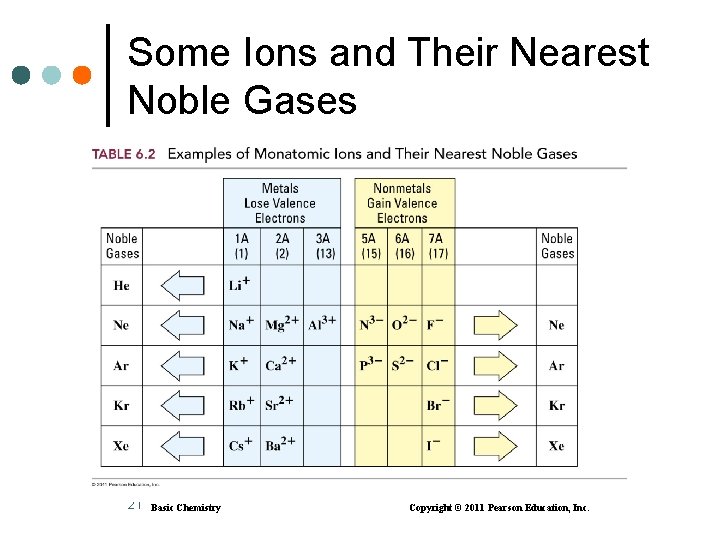
Some Ions and Their Nearest Noble Gases 21 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Learning Check Select the correct answer for sulfur. A. The group number for sulfur is _____. B. The number of valence electrons in sulfur is ____. 1) 4 e 2) 6 e 3) 8 e. C. The change in electrons for an octet requires a 1) loss of 2 e 2) gain of 2 e 3) gain of 4 e D. The ionic charge of a sulfide ion is _____. 1) 2+ 2) 2 3) 4 22 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution A. The group number for sulfur is 3) 6 A (16) B. The number of valence electrons in sulfur is 2) 6 e C. The change in electrons for an octet requires a 2) gain of 2 e D. The ionic charge of a sulfide ion 2) 2 23 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Chapter 6 Inorganic and Organic Compounds: Names and Formulas 6. 2 Ionic Compounds 24 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Ionic Compounds Ionic compounds • consist of positive and negative ions • have attractions called ionic bonds between positively and negatively charged ions • have high melting and boiling points • are solids at room temperature 25 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Salt is an Ionic Compound Sodium chloride (table salt) is an example of an ionic compound. 26 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Ionic Formulas An ionic formula • consists of positively and negatively charged ions • is neutral • has charge balance (net charge of zero) total positive charge = total negative charge • uses subscripts to indicate the number of ions needed to give charge balance 27 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

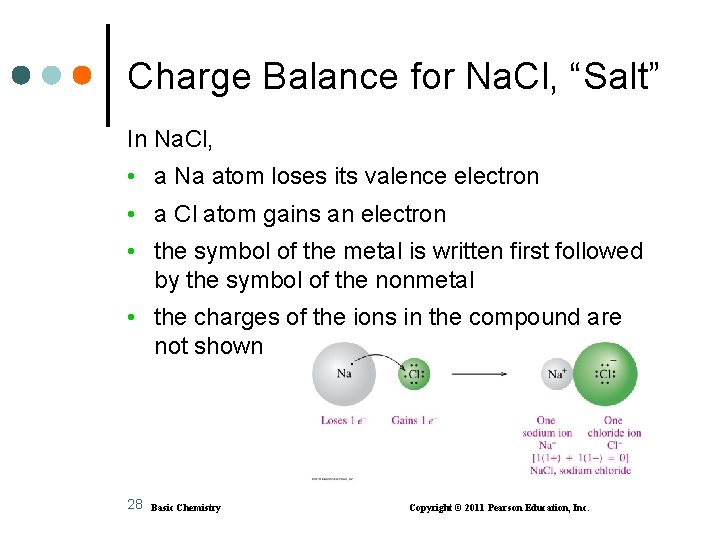
Charge Balance for Na. Cl, “Salt” In Na. Cl, • a Na atom loses its valence electron • a Cl atom gains an electron • the symbol of the metal is written first followed by the symbol of the nonmetal • the charges of the ions in the compound are not shown 28 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

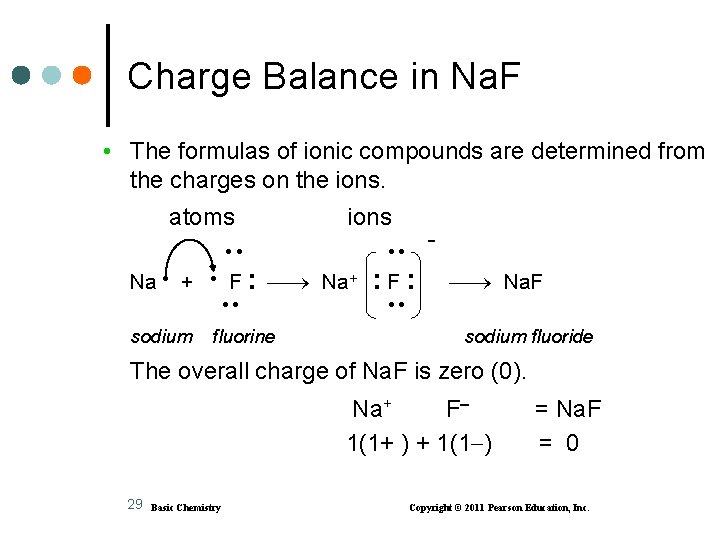
Charge Balance in Na. F • The formulas of ionic compounds are determined from the charges on the ions. atoms ions Na + F : Na+ sodium fluorine : F: Na. F sodium fluoride The overall charge of Na. F is zero (0). Na+ F 1(1+ ) + 1(1 ) 29 Basic Chemistry = Na. F = 0 Copyright © 2011 Pearson Education, Inc.

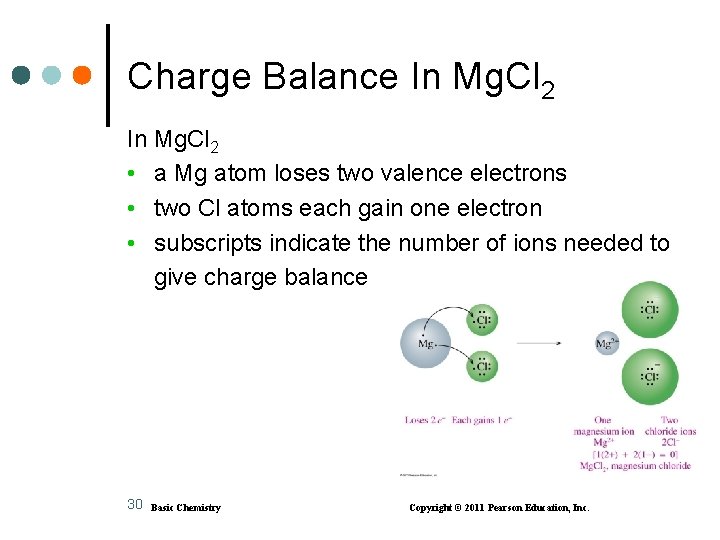
Charge Balance In Mg. Cl 2 • a Mg atom loses two valence electrons • two Cl atoms each gain one electron • subscripts indicate the number of ions needed to give charge balance 30 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

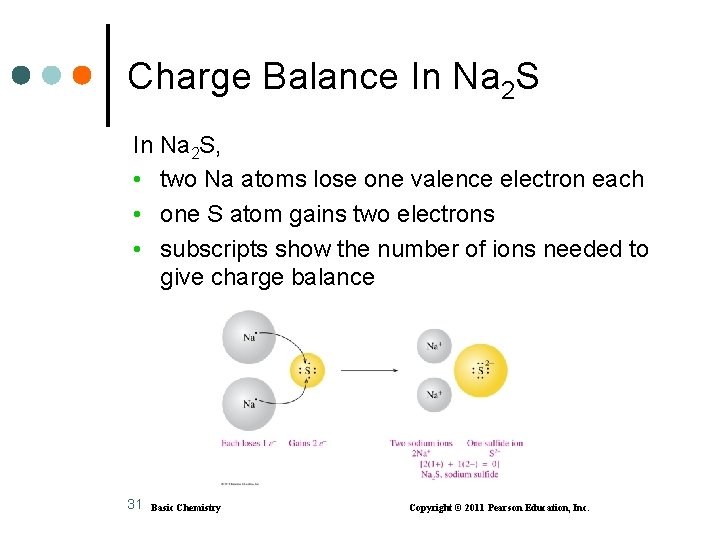
Charge Balance In Na 2 S, • two Na atoms lose one valence electron each • one S atom gains two electrons • subscripts show the number of ions needed to give charge balance 31 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

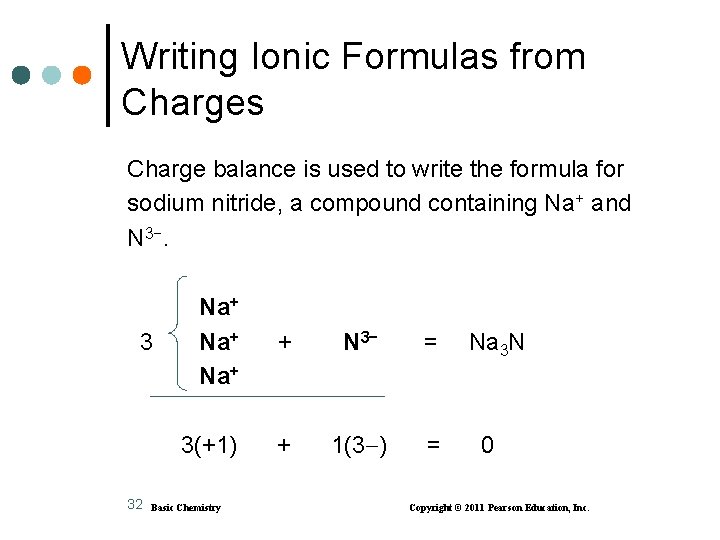
Writing Ionic Formulas from Charges Charge balance is used to write the formula for sodium nitride, a compound containing Na+ and N 3−. 3 32 Na+ Na+ + N 3− = 3(+1) + 1(3 ) = Basic Chemistry Na 3 N 0 Copyright © 2011 Pearson Education, Inc.

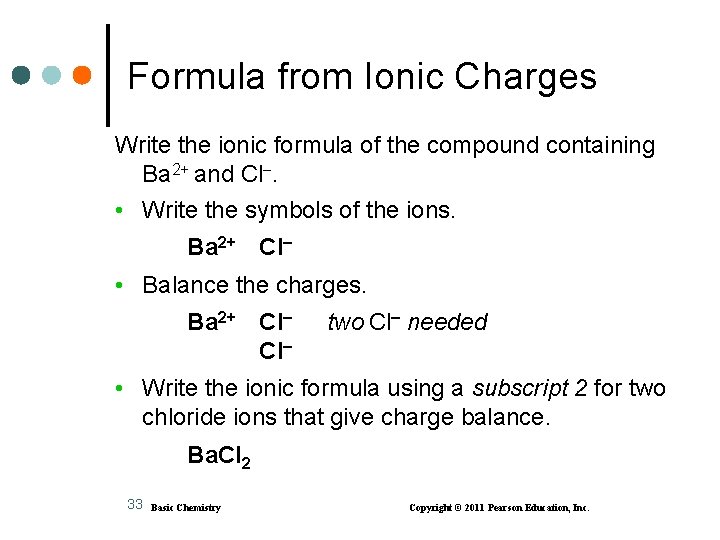
Formula from Ionic Charges Write the ionic formula of the compound containing Ba 2+ and Cl. • Write the symbols of the ions. Ba 2+ Cl • Balance the charges. Ba 2+ Cl Cl two Cl needed • Write the ionic formula using a subscript 2 for two chloride ions that give charge balance. Ba. Cl 2 33 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

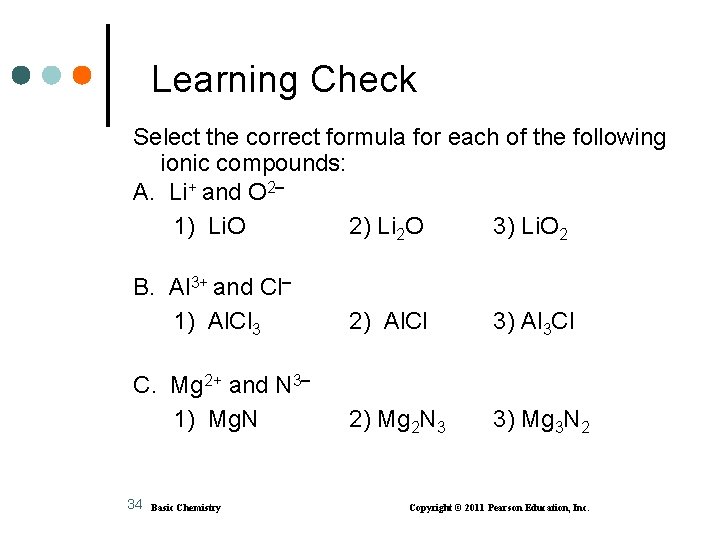
Learning Check Select the correct formula for each of the following ionic compounds: A. Li+ and O 2 1) Li. O 2) Li 2 O 3) Li. O 2 B. Al 3+ and Cl 1) Al. Cl 3 2) Al. Cl 3) Al 3 Cl C. Mg 2+ and N 3 1) Mg. N 2) Mg 2 N 3 3) Mg 3 N 2 34 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

What is the chemical formula for 1. 2. 3. Li. O Li 2 O Li. O 2 35 + Li and Basic Chemistry 2 O Copyright © 2011 Pearson Education, Inc.

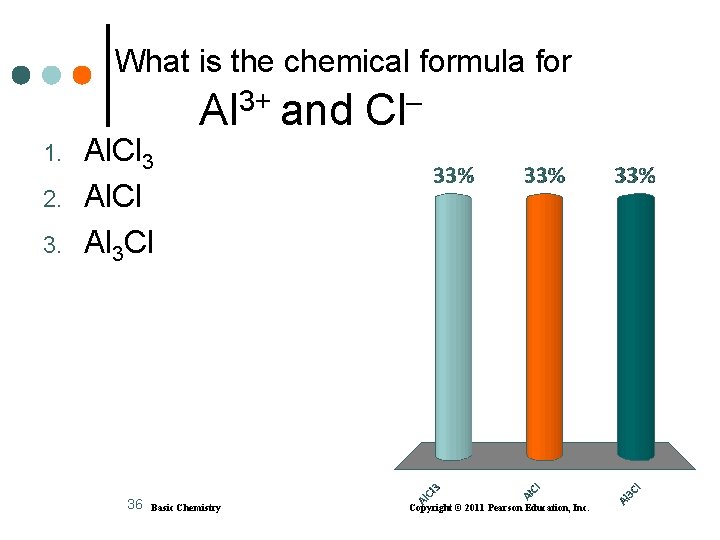
What is the chemical formula for 1. 2. 3. Al. Cl 3 Al. Cl Al 3 Cl 36 3+ Al and Basic Chemistry Cl Copyright © 2011 Pearson Education, Inc.

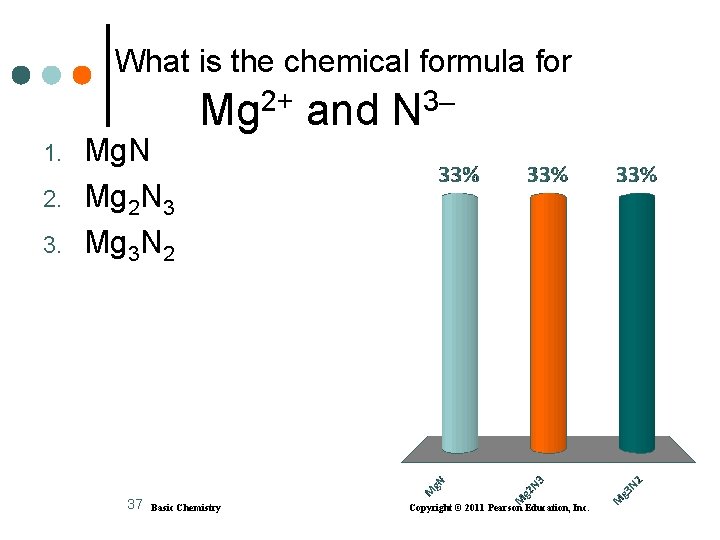
What is the chemical formula for 1. 2. 3. Mg. N Mg 2 N 3 Mg 3 N 2 37 2+ Mg Basic Chemistry and 3 N Copyright © 2011 Pearson Education, Inc.

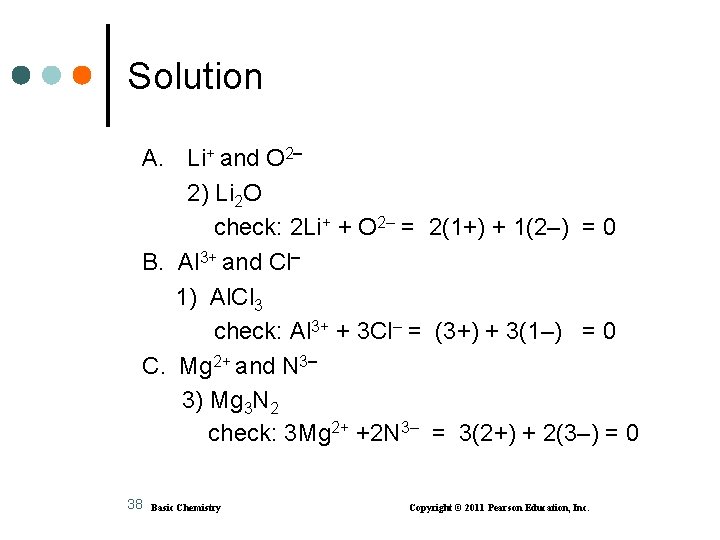
Solution A. Li+ and O 2 2) Li 2 O check: 2 Li+ + O 2– = 2(1+) + 1(2–) = 0 B. Al 3+ and Cl 1) Al. Cl 3 check: Al 3+ + 3 Cl– = (3+) + 3(1–) = 0 C. Mg 2+ and N 3 3) Mg 3 N 2 check: 3 Mg 2+ +2 N 3– = 3(2+) + 2(3–) = 0 38 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Chapter 6 Inorganic and Organic Compounds: Names and Formulas 6. 3 Naming and Writing Ionic Formulas 39 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Naming of Ionic Compounds In the name of an ionic compound, • the positive ion (first ion) is named as the element • the negative ion (second ion) is named by changing the end of the element name to –ide 40 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

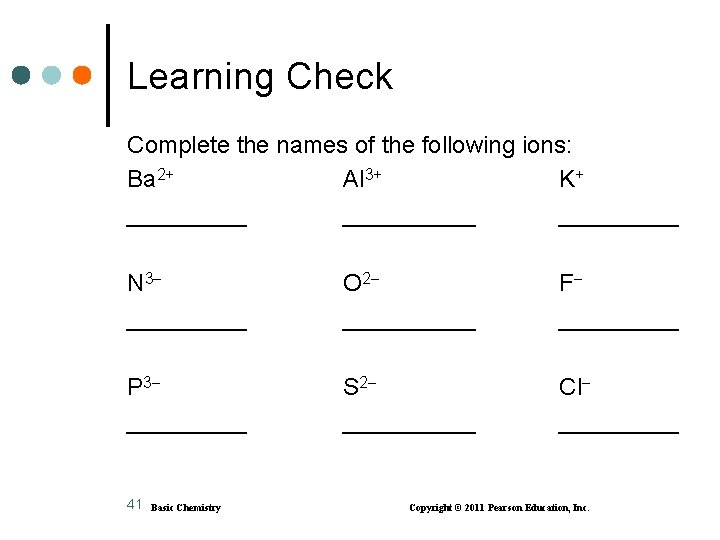
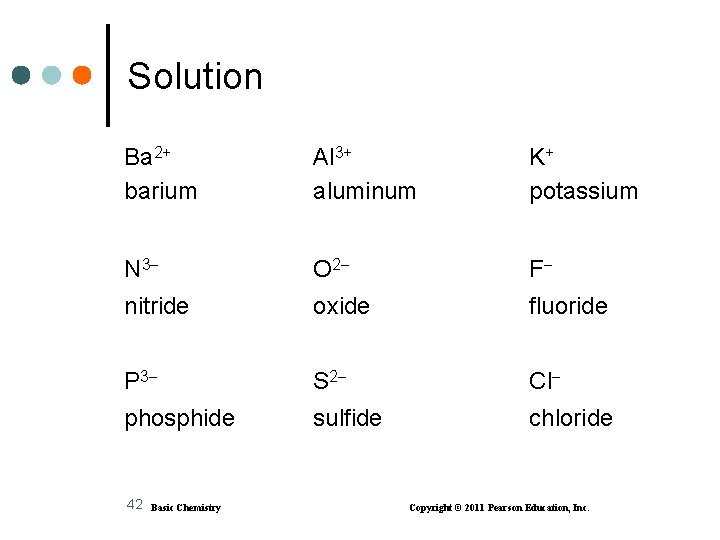
Learning Check Complete the names of the following ions: Ba 2+ Al 3+ K+ __________ N 3 _____ O 2 _____ F _____ P 3 _____ S 2 _____ Cl _____ 41 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Ba 2+ barium Al 3+ aluminum K+ potassium N 3 O 2 F nitride oxide fluoride P 3 S 2 Cl phosphide sulfide chloride 42 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Naming Ionic Compounds with Two Elements 43 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

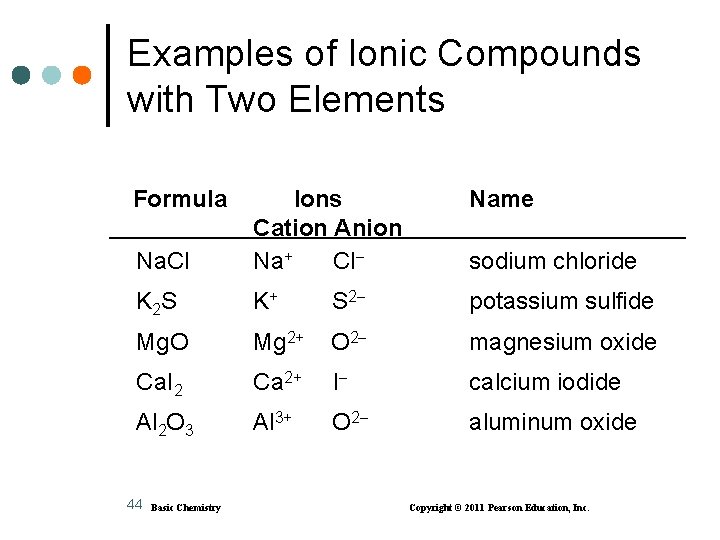
Examples of Ionic Compounds with Two Elements Formula Name Na. Cl Ions Cation Anion Na+ Cl K 2 S K+ S 2 potassium sulfide Mg. O Mg 2+ O 2 magnesium oxide Ca. I 2 Ca 2+ I calcium iodide Al 2 O 3 Al 3+ O 2 aluminum oxide 44 Basic Chemistry sodium chloride Copyright © 2011 Pearson Education, Inc.

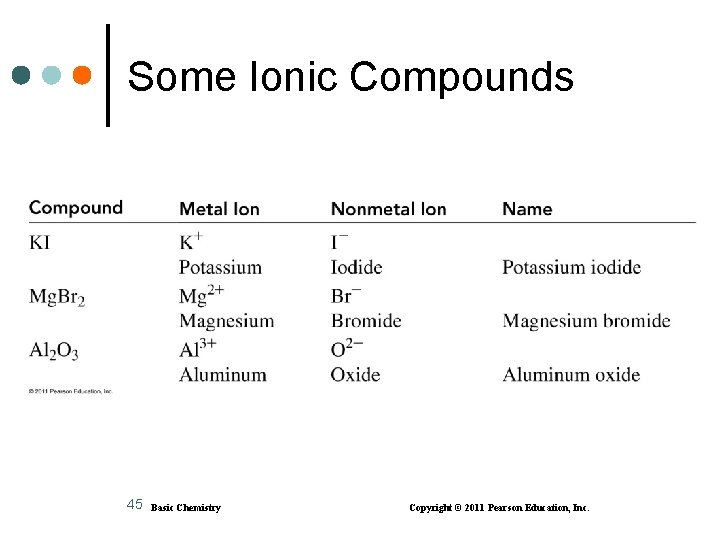
Some Ionic Compounds 45 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

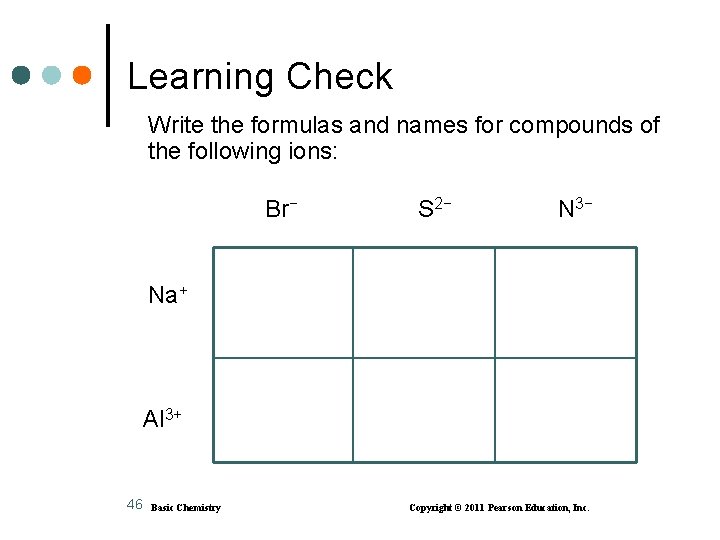
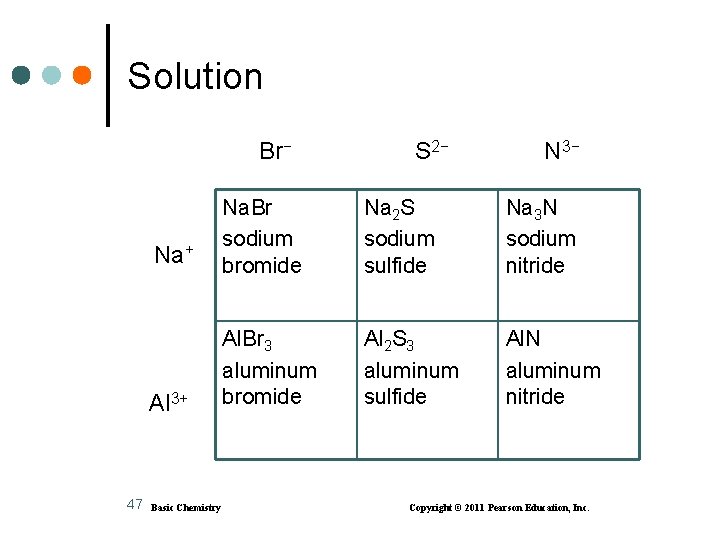
Learning Check Write the formulas and names for compounds of the following ions: Br− S 2− N 3− Na+ Al 3+ 46 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Br− 47 S 2− N 3− Na+ Na. Br sodium bromide Na 2 S sodium sulfide Na 3 N sodium nitride Al 3+ Al. Br 3 aluminum bromide Al 2 S 3 aluminum sulfide Al. N aluminum nitride Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Learning Check Write the names of each of the following compounds: 1) Ca. O ______ 2) KBr ______ 3) Al 2 O 3 ______ 4) Mg. Cl 2 ______ 48 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Write the names of each of the following compounds: 1) Ca. O calcium oxide 2) KBr potassium bromide 3) Al 2 O 3 aluminum oxide 4) Mg. Cl 2 magnesium chloride 49 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

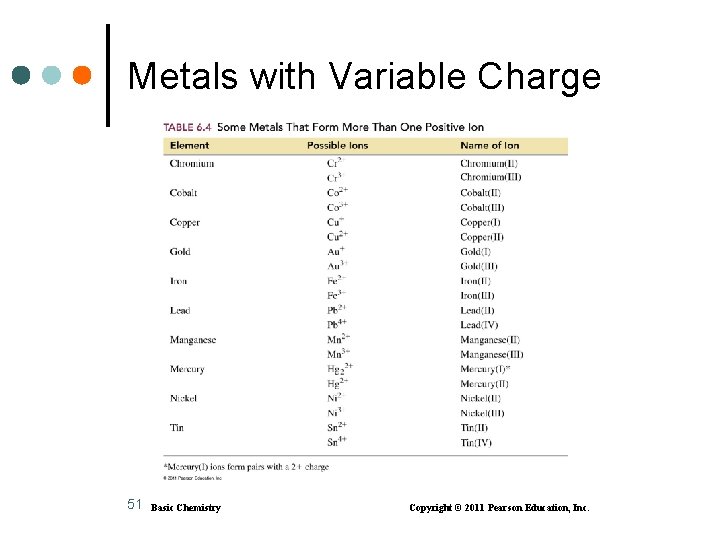
Transition Metals That Form Two or More Positive Ions Most transition metals and Group 4 (14) metals • form two or more positive ions • Zn 2+, Ag+, and Cd 2+ form only one ion Examples: Copper forms Cu+ and Cu 2+ Iron forms Fe 2+ and Fe 3+ Gold forms Au+ and Au 3+ 50 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Metals with Variable Charge 51 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Periodic Table and Typical Ions 52 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

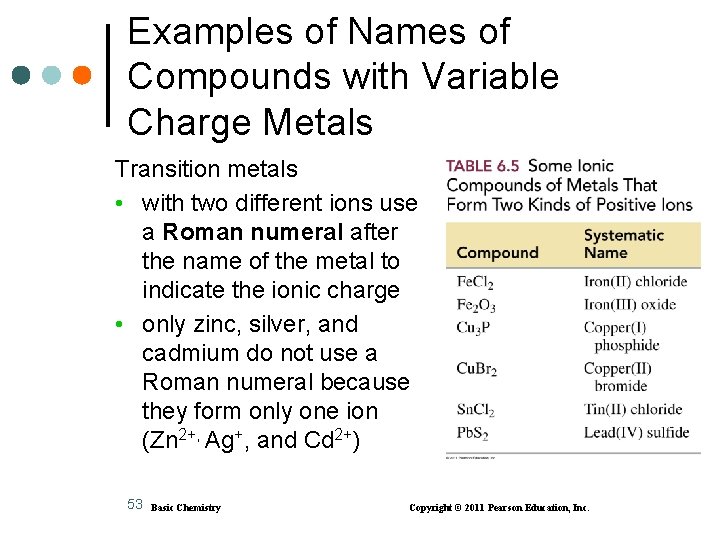
Examples of Names of Compounds with Variable Charge Metals Transition metals • with two different ions use a Roman numeral after the name of the metal to indicate the ionic charge • only zinc, silver, and cadmium do not use a Roman numeral because they form only one ion (Zn 2+, Ag+, and Cd 2+) 53 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

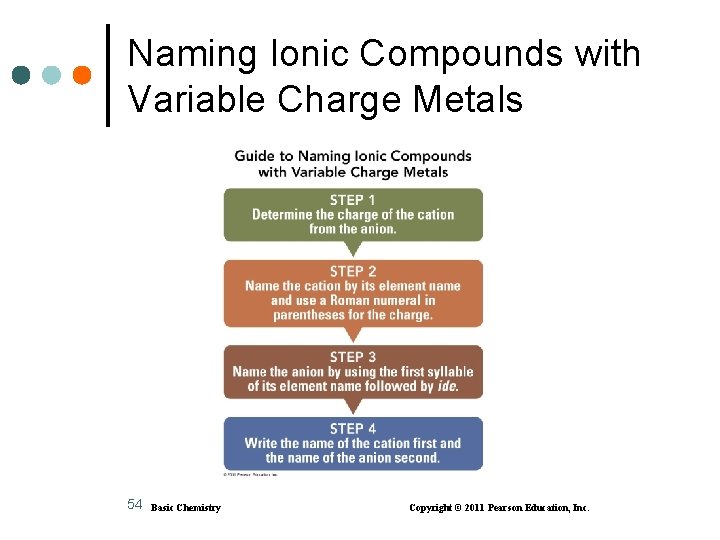
Naming Ionic Compounds with Variable Charge Metals 54 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

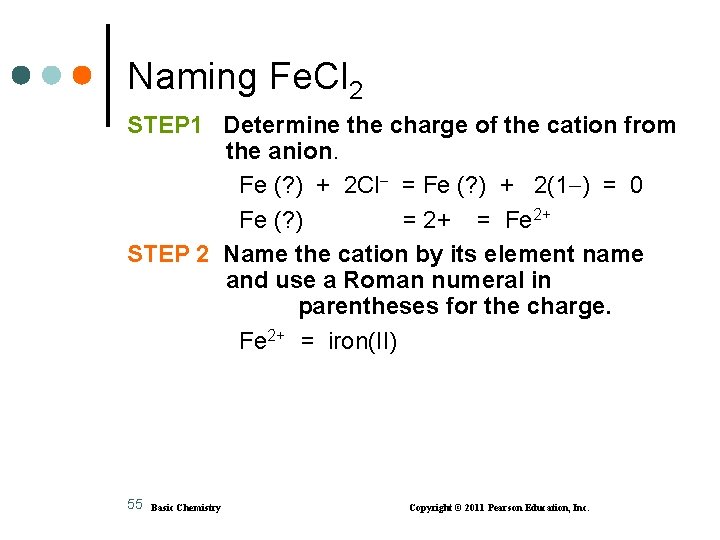
Naming Fe. Cl 2 STEP 1 Determine the charge of the cation from the anion. Fe (? ) + 2 Cl = Fe (? ) + 2(1 ) = 0 Fe (? ) = 2+ = Fe 2+ STEP 2 Name the cation by its element name and use a Roman numeral in parentheses for the charge. Fe 2+ = iron(II) 55 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

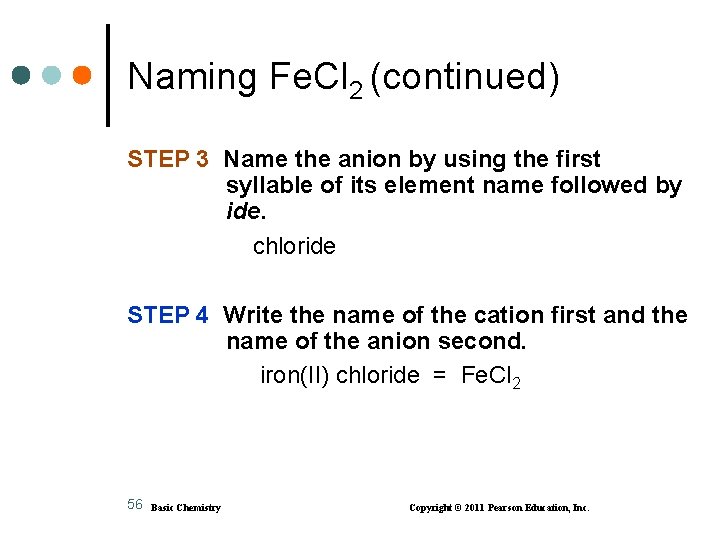
Naming Fe. Cl 2 (continued) STEP 3 Name the anion by using the first syllable of its element name followed by ide. chloride STEP 4 Write the name of the cation first and the name of the anion second. iron(II) chloride = Fe. Cl 2 56 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

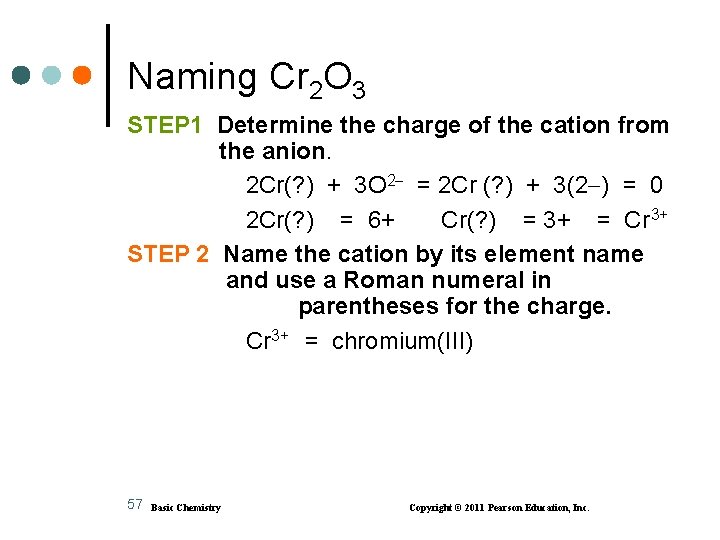
Naming Cr 2 O 3 STEP 1 Determine the charge of the cation from the anion. 2 Cr(? ) + 3 O 2 = 2 Cr (? ) + 3(2 ) = 0 2 Cr(? ) = 6+ Cr(? ) = 3+ = Cr 3+ STEP 2 Name the cation by its element name and use a Roman numeral in parentheses for the charge. Cr 3+ = chromium(III) 57 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

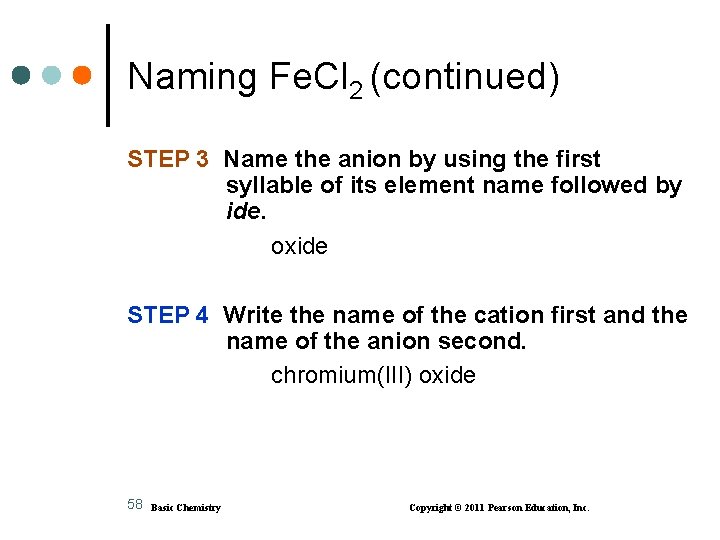
Naming Fe. Cl 2 (continued) STEP 3 Name the anion by using the first syllable of its element name followed by ide. oxide STEP 4 Write the name of the cation first and the name of the anion second. chromium(III) oxide 58 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

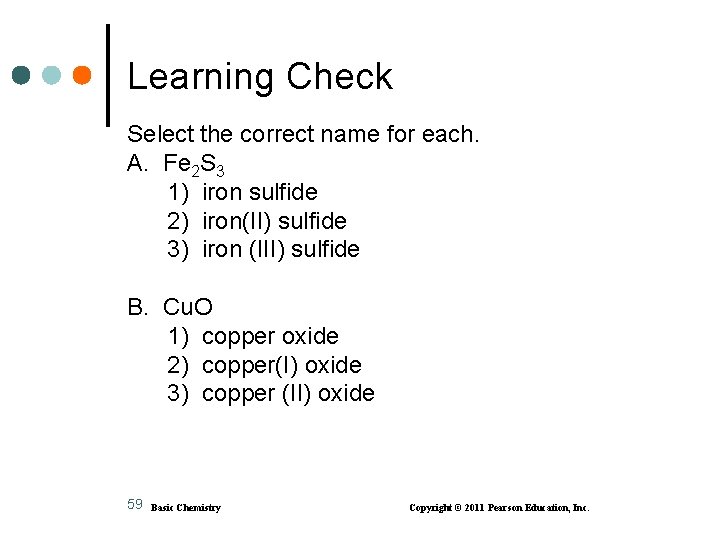
Learning Check Select the correct name for each. A. Fe 2 S 3 1) iron sulfide 2) iron(II) sulfide 3) iron (III) sulfide B. Cu. O 1) copper oxide 2) copper(I) oxide 3) copper (II) oxide 59 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Select the correct name for each. A. Fe 2 S 3 3) iron (III) sulfide Fe 3+ S 2 B. Cu. O 3) copper (II) oxide 60 Basic Chemistry Cu 2+ O 2 Copyright © 2011 Pearson Education, Inc.

Guide to Writing Formulas from the Name 61 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

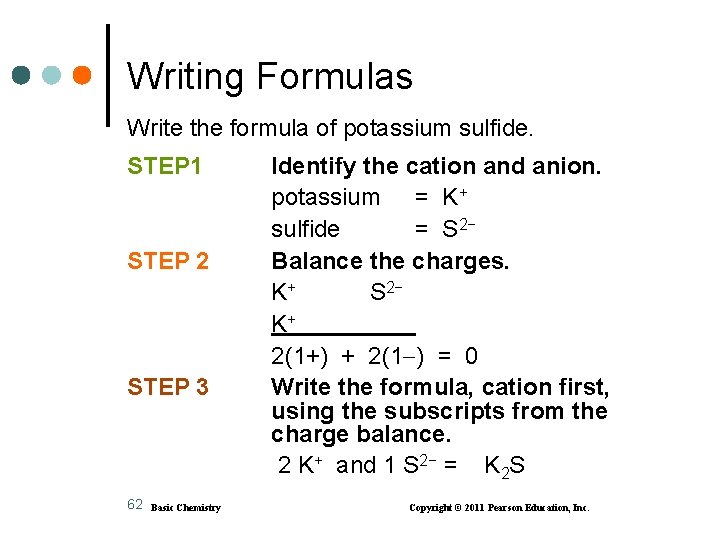
Writing Formulas Write the formula of potassium sulfide. STEP 1 STEP 2 STEP 3 62 Basic Chemistry Identify the cation and anion. potassium = K+ sulfide = S 2− Balance the charges. K+ S 2− K+ 2(1+) + 2(1 ) = 0 Write the formula, cation first, using the subscripts from the charge balance. 2 K+ and 1 S 2− = K 2 S Copyright © 2011 Pearson Education, Inc.

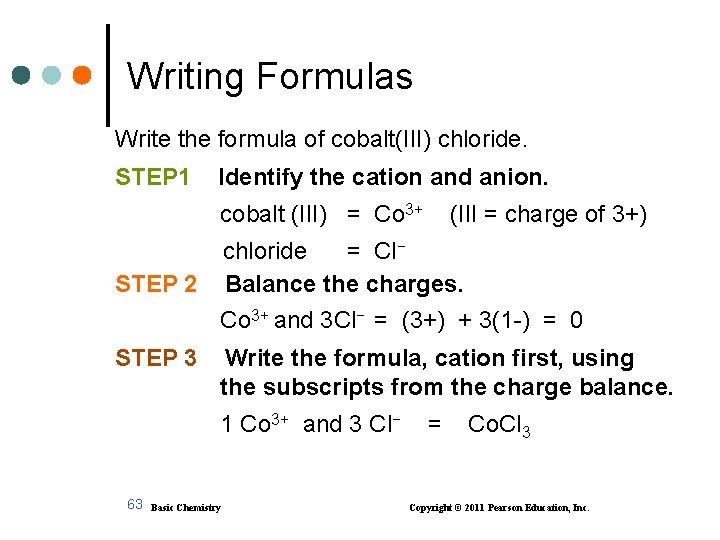
Writing Formulas Write the formula of cobalt(III) chloride. STEP 1 Identify the cation and anion. cobalt (III) = Co 3+ (III = charge of 3+) chloride = Cl− Balance the charges. STEP 2 Co 3+ and 3 Cl− = (3+) + 3(1 -) = 0 STEP 3 Write the formula, cation first, using the subscripts from the charge balance. 1 Co 3+ and 3 Cl− 63 Basic Chemistry = Co. Cl 3 Copyright © 2011 Pearson Education, Inc.

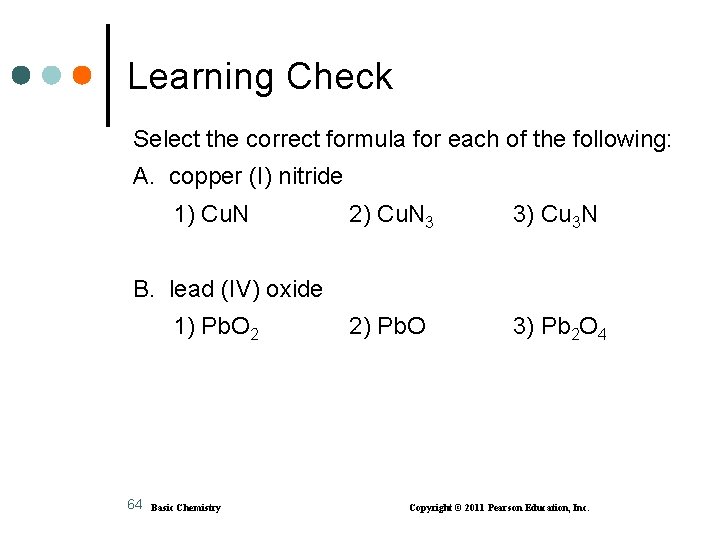
Learning Check Select the correct formula for each of the following: A. copper (I) nitride 1) Cu. N 2) Cu. N 3 3) Cu 3 N 2) Pb. O 3) Pb 2 O 4 B. lead (IV) oxide 1) Pb. O 2 64 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

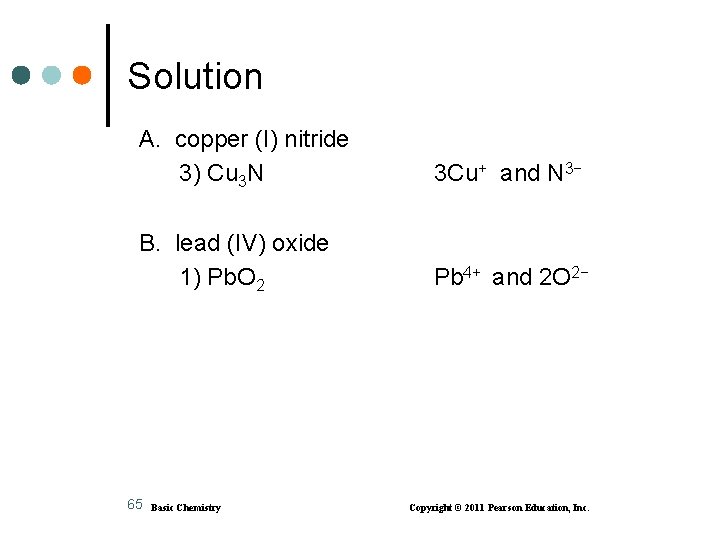
Solution A. copper (I) nitride 3) Cu 3 N 3 Cu+ and N 3− B. lead (IV) oxide 1) Pb. O 2 Pb 4+ and 2 O 2− 65 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Chapter 6 Inorganic and Organic Compounds: Names and Formulas 6. 4 Polyatomic Ions 66 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

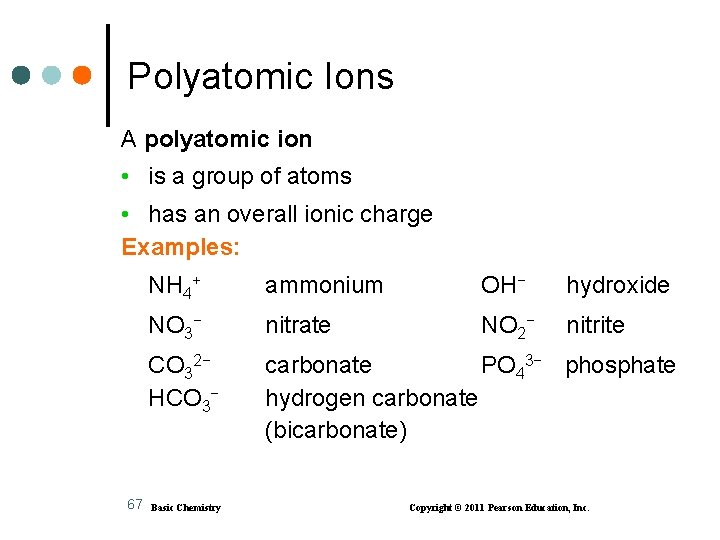
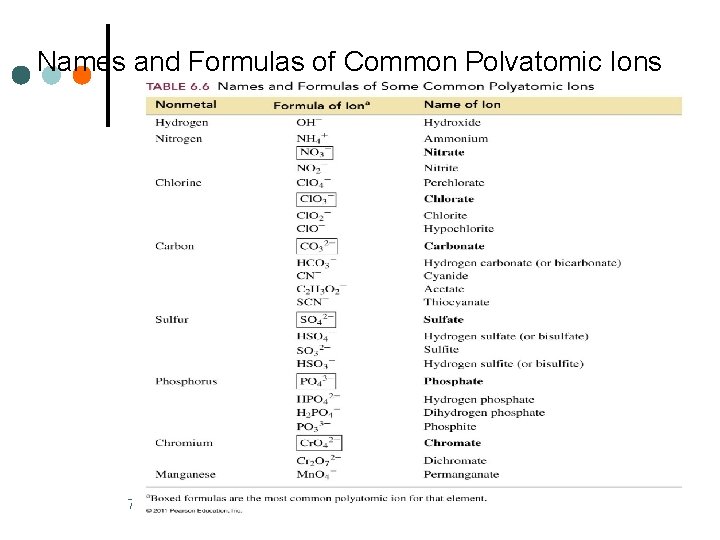
Polyatomic Ions A polyatomic ion • is a group of atoms • has an overall ionic charge Examples: 67 NH 4+ ammonium OH− hydroxide NO 3− nitrate NO 2− nitrite CO 32− HCO 3− carbonate PO 43− phosphate hydrogen carbonate (bicarbonate) Basic Chemistry Copyright © 2011 Pearson Education, Inc.

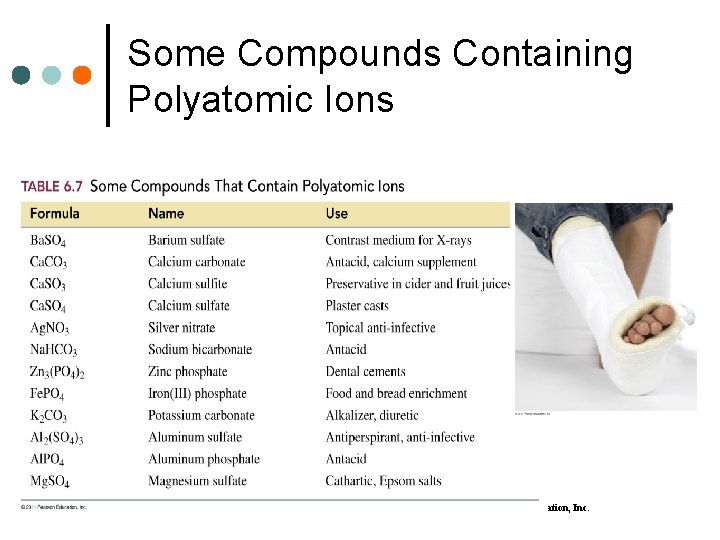
Some Compounds with Polyatomic Ions 68 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

More Names of Polyatomic Ions The names of the common polyatomic anions • end in ate NO 3− nitrate PO 43− phosphate • with one oxygen less end in ite NO 2− • nitrite PO 33− phosphite with hydrogen use prefix hydrogen (or bi) HCO 3− hydrogen carbonate (bicarbonate) HSO 3− hydrogen sulfite (bisulfite) 69 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Names and Formulas of Common Polyatomic Ions 70 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Some Compounds Containing Polyatomic Ions 71 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Prefixes for Names of Polyatomic Ions of Halogens Some polyatomic ions of the halogens require prefixes. Cl. O 4− perchlorate one oxygen more Cl. O 3− chlorate most common form Cl. O 2− chlorite one oxygen less Cl. O− hypochlorite two oxygens less 72 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Guide to Naming Compounds with Polyatomic Ions 73 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

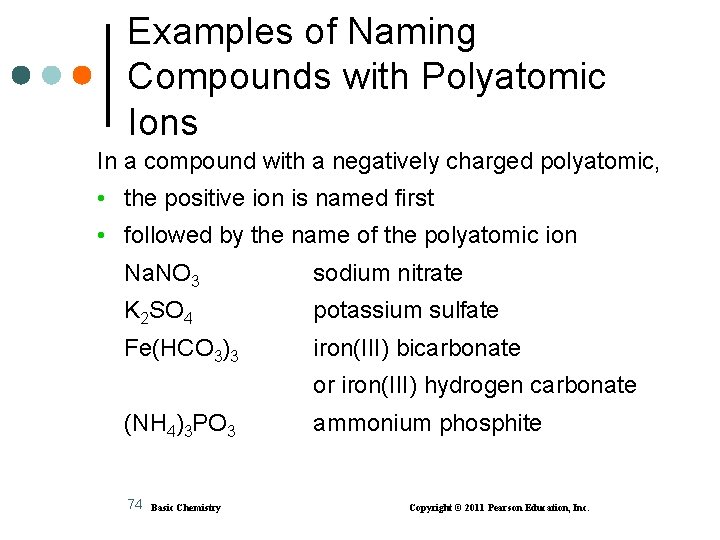
Examples of Naming Compounds with Polyatomic Ions In a compound with a negatively charged polyatomic, • the positive ion is named first • followed by the name of the polyatomic ion Na. NO 3 sodium nitrate K 2 SO 4 potassium sulfate Fe(HCO 3)3 iron(III) bicarbonate or iron(III) hydrogen carbonate (NH 4)3 PO 3 74 Basic Chemistry ammonium phosphite Copyright © 2011 Pearson Education, Inc.

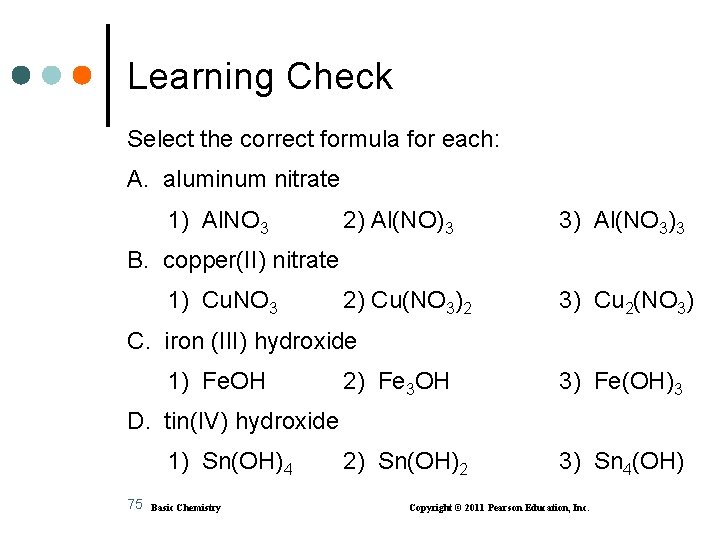
Learning Check Select the correct formula for each: A. aluminum nitrate 1) Al. NO 3 2) Al(NO)3 3) Al(NO 3)3 2) Cu(NO 3)2 3) Cu 2(NO 3) B. copper(II) nitrate 1) Cu. NO 3 C. iron (III) hydroxide 1) Fe. OH 2) Fe 3 OH 3) Fe(OH)3 2) Sn(OH)2 3) Sn 4(OH) D. tin(IV) hydroxide 1) Sn(OH)4 75 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Select the correct formula for each: A. aluminum nitrate 3) Al(NO 3)3 B. copper(II) nitrate 2) Cu(NO 3)2 C. iron(III) hydroxide 3) Fe(OH)3 D. tin(IV) hydroxide 1) Sn(OH)4 76 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

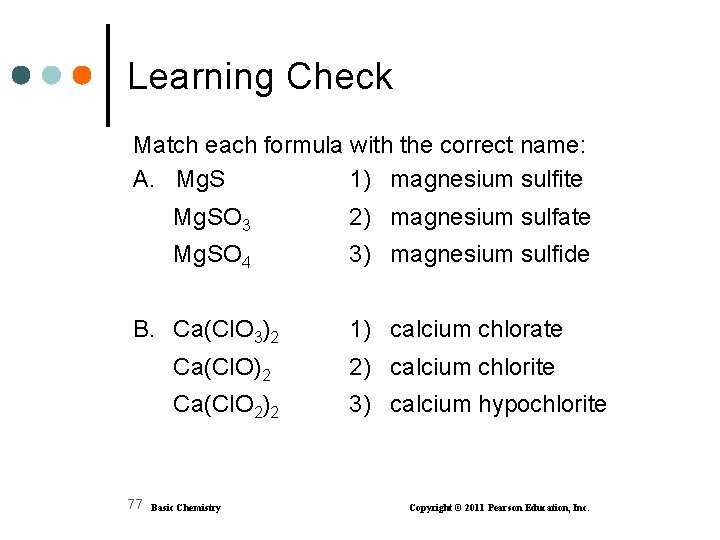
Learning Check Match each formula with the correct name: A. Mg. S 1) magnesium sulfite Mg. SO 3 2) magnesium sulfate Mg. SO 4 3) magnesium sulfide B. Ca(Cl. O 3)2 77 1) calcium chlorate Ca(Cl. O)2 2) calcium chlorite Ca(Cl. O 2)2 3) calcium hypochlorite Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Match each formula with the correct name: A. Mg. S 3) magnesium sulfide Mg. SO 3 1) magnesium sulfite Mg. SO 4 2) magnesium sulfate B. Ca(Cl. O 3)2 78 1) calcium chlorate Ca(Cl. O)2 3) calcium hypochlorite Ca(Cl. O 2)2 2) calcium chlorite Basic Chemistry Copyright © 2011 Pearson Education, Inc.

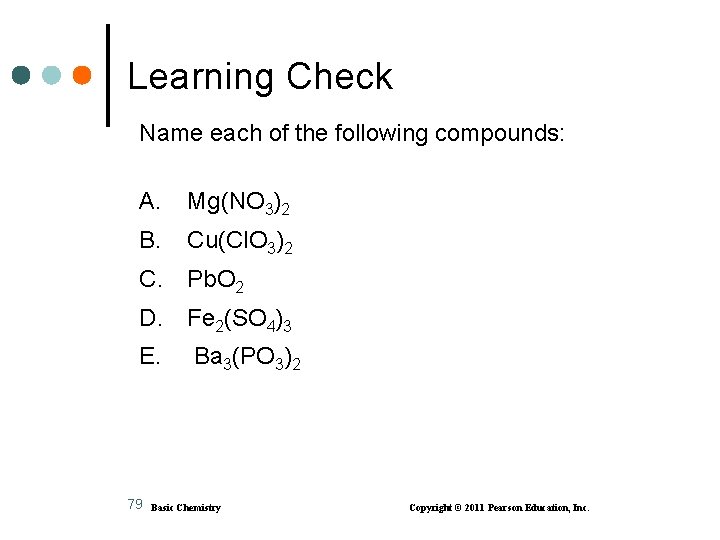
Learning Check Name each of the following compounds: A. Mg(NO 3)2 B. Cu(Cl. O 3)2 C. Pb. O 2 D. Fe 2(SO 4)3 E. Ba 3(PO 3)2 79 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Name each of the following compounds: A. Mg(NO 3)2 magnesium nitrate B. Cu(Cl. O 3)2 copper(II) chlorate C. Pb. O 2 lead (IV) oxide D. Fe 2(SO 4)3 iron(III) sulfate E. Ba 3(PO 3)2 barium phosphite 80 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

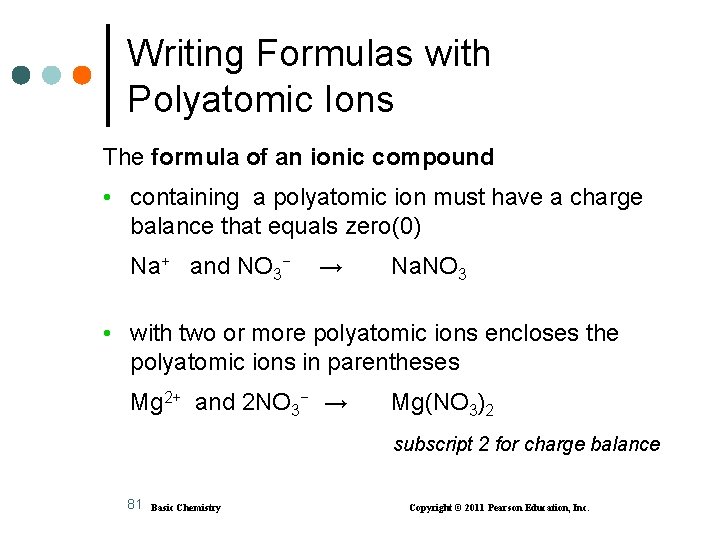
Writing Formulas with Polyatomic Ions The formula of an ionic compound • containing a polyatomic ion must have a charge balance that equals zero(0) Na+ and NO 3− → Na. NO 3 • with two or more polyatomic ions encloses the polyatomic ions in parentheses Mg 2+ and 2 NO 3− → Mg(NO 3)2 subscript 2 for charge balance 81 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

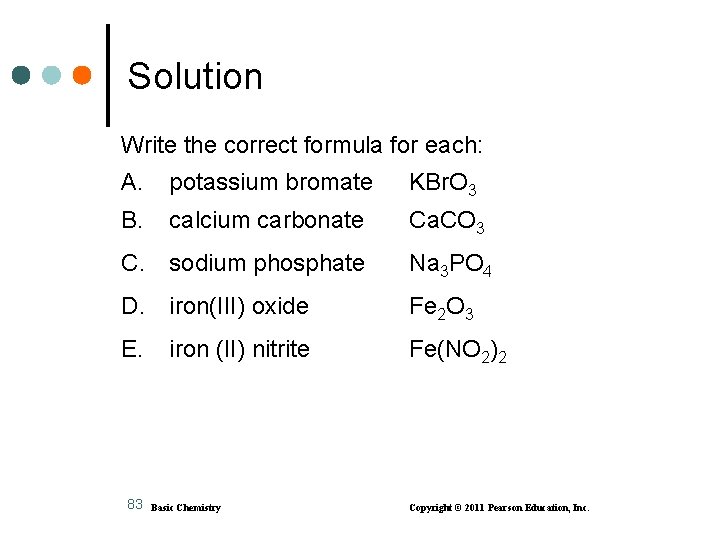
Learning Check Write the correct formula for each: A. potassium bromate B. calcium carbonate C. sodium phosphate D. iron(III) oxide E. iron (II) nitrite 82 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Write the correct formula for each: A. potassium bromate KBr. O 3 B. calcium carbonate Ca. CO 3 C. sodium phosphate Na 3 PO 4 D. iron(III) oxide Fe 2 O 3 E. iron (II) nitrite Fe(NO 2)2 83 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

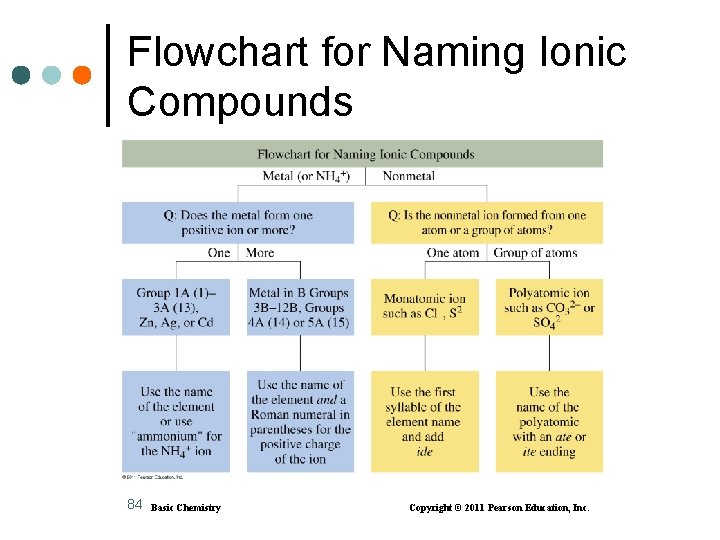
Flowchart for Naming Ionic Compounds 84 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

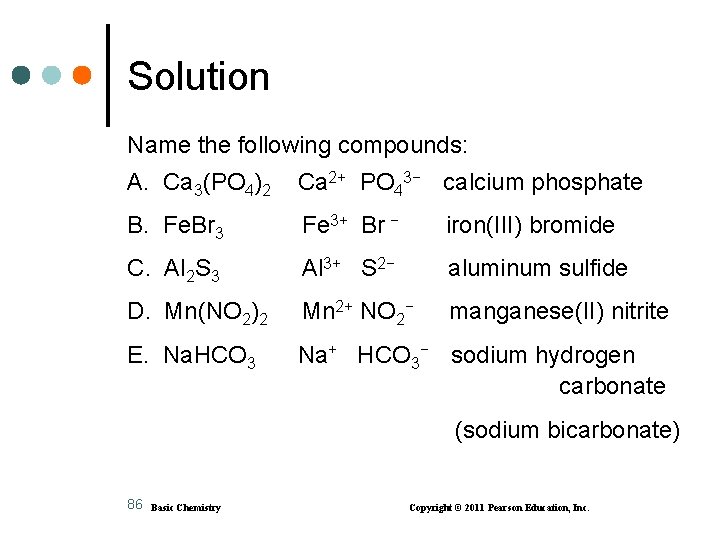
Learning Check Name the following compounds: A. Ca 3(PO 4)2 B. Fe. Br 3 C. Al 2 S 3 D. Mn(NO 2)2 E. Na. HCO 3 85 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Name the following compounds: A. Ca 3(PO 4)2 Ca 2+ PO 43− calcium phosphate B. Fe. Br 3 Fe 3+ Br − iron(III) bromide C. Al 2 S 3 Al 3+ S 2− aluminum sulfide D. Mn(NO 2)2 Mn 2+ NO 2− manganese(II) nitrite E. Na. HCO 3 Na+ HCO 3− sodium hydrogen carbonate (sodium bicarbonate) 86 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

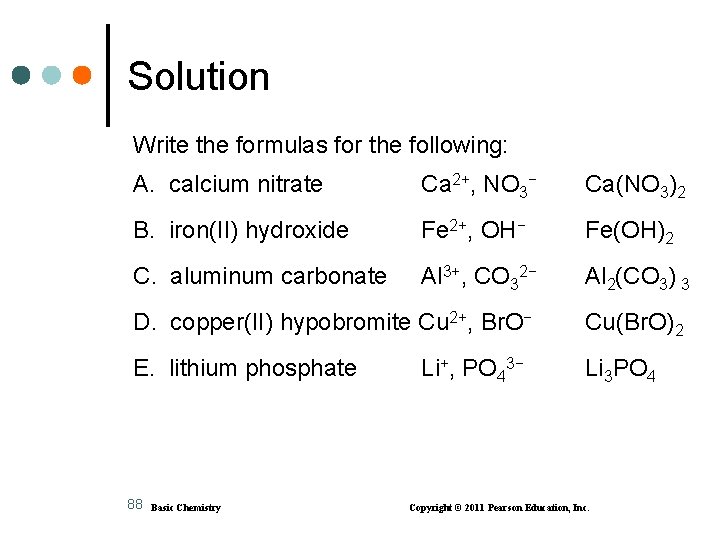
Learning Check Write the formulas for the following: A. calcium nitrate B. iron(II) hydroxide C. aluminum carbonate D. copper(II) hypobromite E. lithium phosphate 87 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Write the formulas for the following: A. calcium nitrate Ca 2+, NO 3− Ca(NO 3)2 B. iron(II) hydroxide Fe 2+, OH− Fe(OH)2 C. aluminum carbonate Al 3+, CO 32− Al 2(CO 3) 3 D. copper(II) hypobromite Cu 2+, Br. O− Cu(Br. O)2 E. lithium phosphate Li 3 PO 4 88 Basic Chemistry Li+, PO 43− Copyright © 2011 Pearson Education, Inc.