Chapter 6 Fluid Electrolyte and AcidBase Homeostasis Body

Chapter 6 Fluid, Electrolyte, and Acid-Base Homeostasis
Body Fluids • Water – Where metabolic reactions and cellular processes occur – Carries nutrients, waste products, enzymes, and blood cells – Facilitates movement of body parts

Fluid Compartments • Intracellular fluid – inside the cells • Extracellular fluid – fluid outside the cells – Interstitial fluid – between the cells – Intravascular fluid – inside the blood vessels – Transcellular fluid – third space

Fluid Movement • Osmosis – movement of water across a semi-permeable membrane
Fluid Movement • Tonicity – osmotic pressure of two solutions separated by a semipermeable membrane – Isotonic – equal solute concentrations, causes no fluid shifts – Hypotonic – lower solute concentrations, causing fluids to shift out – Hypertonic – higher solute concentrations, causing fluids to shift in

Fluid Sources • Oral intake • Intravenous solutions – Isotonic – 0. 9% saline, lactated ringers – Hypotonic – 0. 45% saline – Hypertonic – 5% dextrose in 0. 9% saline, 3% saline

Fluid Loses • Urine • Feces • Insensible losses
Fluid Balance Control • Thirst mechanism – Triggered by decreased blood volume and increased osmolarity • Antidiuretic hormone – Promotes reabsorption of water in the kidneys • Aldosterone – Increases reabsorption of sodium and water in the kidneys • Atrial natriuretic peptide – stimulates renal vasodilatation and suppresses aldosterone, increasing urinary output

Fluid Excess • Edema – Excess fluid in the interstitial space • Hypervolemia or fluid volume excess – Excess fluid in the intravascular space • Water intoxication – Excess fluid in the intracellular space

Causes of Fluid Excess • Excessive sodium or water intake – – – High-sodium diet Psychogenic polydipsia Hypertonic fluid administration Free water Enteral feedings • Inadequate sodium or water elimination – – – Hyperaldosteronism Cushing’s syndrome Syndrome of inappropriate antidiuretic hormone Renal failure Liver failure Heart failure

Fluid Excess • Manifestations: peripheral edema, periorbital edema, anasarca, cerebral edema, dyspnea, bounding pulse, tachycardia, jugular vein distension, hypertension, polyuria, rapid weight gain, crackles, and bulging fontanelles • Diagnosis: history, physical examination, daily weights, measurement of intake and output, blood chemistry, urine analysis, and complete blood count • Treatment: wearing compression stockings, administering diuretics, restricting sodium and fluids, maintaining high Fowler’s position, and hypertonic solutions

Fluid Deficit • Dehydration • Hypovolemia or fluid volume deficit – Decreased fluid in the intravascular space

Causes of Fluid Deficit • Inadequate fluid intake – Poor oral intake – Inadequate IV fluid replacement • Excessive fluid or sodium losses – – – – Gastrointestinal losses Excessive diaphoresis Prolonged hyperventilation Hemorrhage Nephrosis Diabetes mellitus Diabetes insipidus Burns Open wounds Ascites Effusions Excessive use of diuretics Osmotic diuresis

Fluid Deficit • Manifestations: thirst, altered level of consciousness, hypotension, tachycardia, weak and thready pulse, flat jugular veins, dry mucous membranes, decreased skin turgor, oliguria, weight loss, and sunken fontanelles • Diagnosis: history, physical examination, measurements of intake and output, daily weights, blood chemistry, urine analysis, and complete blood count • Treatment: indentify and manage underlying cause along with fluid replacement

Electrolyte Balance • Cations – Positively charged electrolytes • Anions – Negatively charged electrolytes • Play a role in – muscle and neural activity – acid-base and fluid balance

Sodium • Normal range 135 -145 m. Eq/L • Most significant cation and prevalent electrolyte of extracellular fluid • Controls serum osmolality and water balance • Plays a role in acid-base balance • Facilitates muscles and nerve impulses • Dietary intake main source • Excreted through the kidneys and gastrointestinal tract
Hypernatremia • Sodium > 145 m. Eq/L • Serum osmolarity increases • Results in fluid shifts
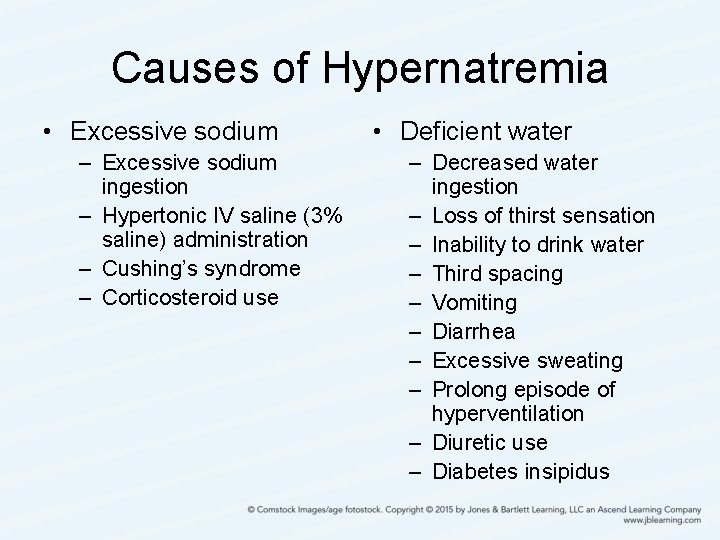
Causes of Hypernatremia • Excessive sodium – Excessive sodium ingestion – Hypertonic IV saline (3% saline) administration – Cushing’s syndrome – Corticosteroid use • Deficient water – Decreased water ingestion – Loss of thirst sensation – Inability to drink water – Third spacing – Vomiting – Diarrhea – Excessive sweating – Prolong episode of hyperventilation – Diuretic use – Diabetes insipidus
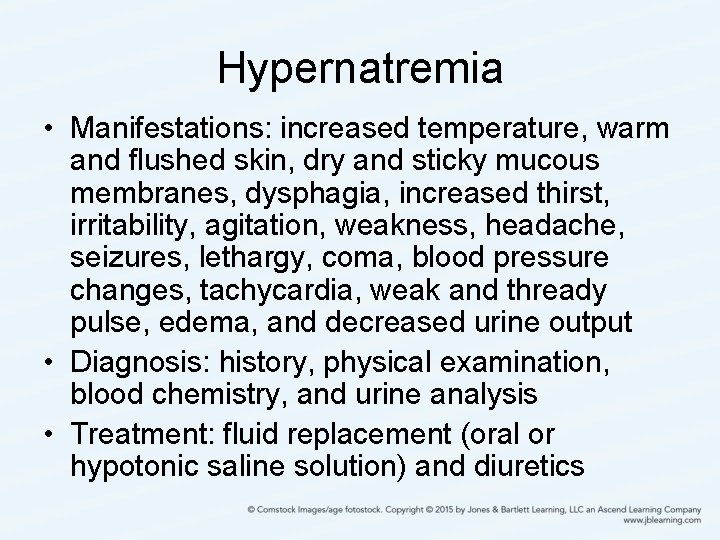
Hypernatremia • Manifestations: increased temperature, warm and flushed skin, dry and sticky mucous membranes, dysphagia, increased thirst, irritability, agitation, weakness, headache, seizures, lethargy, coma, blood pressure changes, tachycardia, weak and thready pulse, edema, and decreased urine output • Diagnosis: history, physical examination, blood chemistry, and urine analysis • Treatment: fluid replacement (oral or hypotonic saline solution) and diuretics
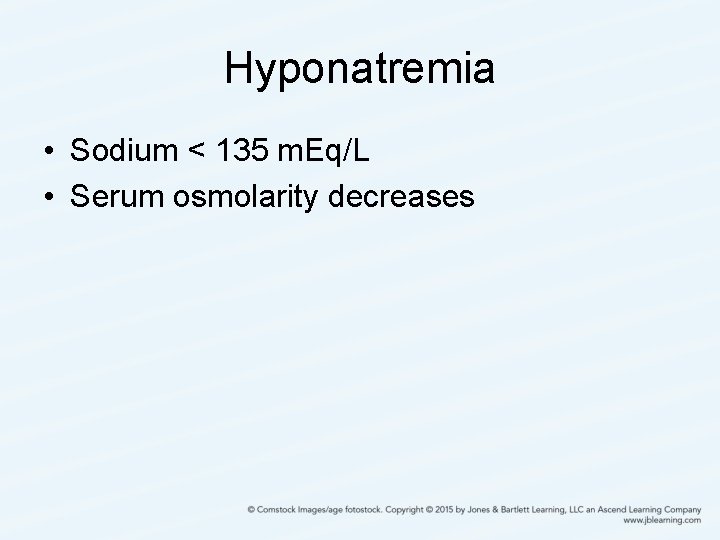
Hyponatremia • Sodium < 135 m. Eq/L • Serum osmolarity decreases

Causes of Hyponatremia • Deficient sodium – – Diuretic use Gastrointestinal losses Excessive sweating Insufficient aldosterone levels – Adrenal insufficiency – Dietary sodium restrictions • Excessive water – Hypotonic intravenous saline (0. 45% saline) – Hyperglycemia – Excessive water ingestion – Renal failure – Syndrome of inappropriate antidiuretic hormone – Heart failure

Hyponatremia • Manifestations: anorexia, gastrointestinal upset, poor skin turgor, dry mucous membranes, blood pressure changes, pulse changes, edema, headache, lethargy, confusion, diminished deep tendon reflexes, muscle weakness, seizures, and coma • Diagnosis: history, physical examination, blood chemistry, and urine analysis • Treatment: limit fluids and increase dietary sodium

Chloride • • Normal range 98 -108 m. Eq/L Mineral electrolyte Major extracellular anion Found in gastric secretions, pancreatic juices, bile, and cerebrospinal fluid • Plays a role in acid-base balance • Dietary intake main source • Excreted through the kidneys

Hyperchloremia • Chloride > 108 m. Eq/L • Causes – Increased chloride intake or exchange: hypernatremia, hypertonic intravenous solution, metabolic acidosis, and hyperkalemia – Decreased chloride excretion: hyperparathyroidism, hyperaldosteronism, and renal failure
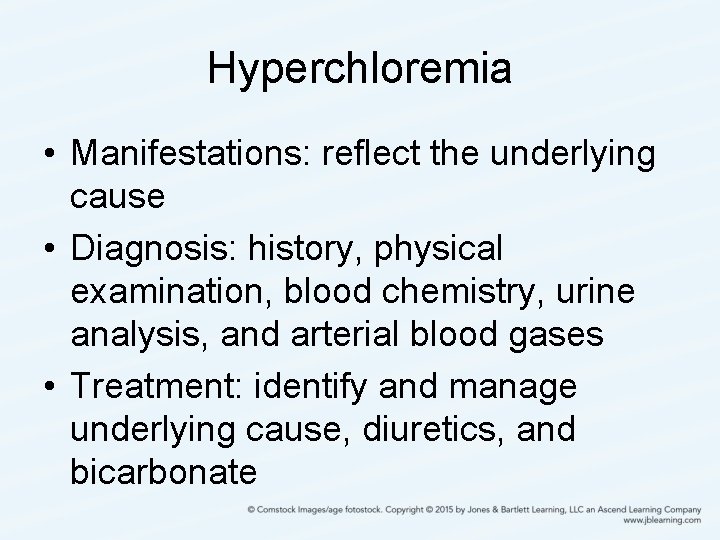
Hyperchloremia • Manifestations: reflect the underlying cause • Diagnosis: history, physical examination, blood chemistry, urine analysis, and arterial blood gases • Treatment: identify and manage underlying cause, diuretics, and bicarbonate

Hypochloremia • Chloride < 98 m. Eq/L • Causes – Decreased chloride intake or exchange: hyponatremia, 5% dextrose in water intravenous solution, water intoxication, and hypokalemia – Increased chloride excretion: diuretics, vomiting, metabolic alkalosis, and other gastrointestinal losses
Hypochloremia • Manifestations: reflect the underlying cause • Diagnosis: same as for hyperchloremia • Treatment: identify and manage underlying cause, sodium replacement (oral or intravenous), ammonium chloride, and saline irrigation of gastric tubes
Potassium • Normal range 3. 5 -5 m. Eq/L • The primary intracellular cation • Plays a role in electrical conduction, acid-base balance, and metabolism • Dietary intake main source • Excreted through the kidneys and gastrointestinal tract

Hyperkalemia • Potassium > 5 m. Eq/L • Causes – Deficient excretion: renal failure, Addison’s disease, certain medications, and Gordon’s syndrome – Excessive intake: oral potassium supplements, salt substitutes, and rapid intravenous administration of diluted potassium – Increased release from cells: acidosis, blood transfusions, and burns or any other cellular injuries

Hyperkalemia • Manifestions: paresthesia, flaccid paralysis, bradycardia, dysrhythmias, electrocardiogram changes, cardiac arrest, respiratory depression, abdominal cramping, nausea, and diarrhea • Diagnosis: history, physical examination, blood chemistry, 12 -lead electrocardiogram, and arterial blood gas

Hyperkalemia • Treatment: – Correct acidosis, usually with sodium bicarbonate – Calcium gluconate – Decrease dietary potassium intake – Dialysis – Kayexalate – Intravenous fluids – Potassium-losing diuretics – Insulin

Hypokalemia • Potassium < 3. 5 m. Eq/L • Causes – Excessive loss: vomiting, diarrhea, nasogastric suctioning, fistulas, laxatives, potassium-losing diuretics, Cushing’s syndrome, and corticosteroids – Deficient intake: malnutrition, extreme dieting, and alcoholism – Increased shift into the cell: alkalosis and insulin excess

Hypokalemia • Manifestations: muscle weakness, paresthesias, hyporeflexia, leg cramps, weak and irregular pulse, hypotension, dysrhythmias, electrocardiogram changes, decreased bowel sounds, abdominal distension, constipation, ileus, and cardiac arrest • Diagnosis: history, physical examination, blood chemistry, 12 -lead electrocardiogram, and arterial blood gas

Hypokalemia • Treatment: identify and manage underlying cause along with potassium replacement (oral or intravenous)
Calcium • Normal range 4 -5 m. Eq/L • Mostly found in the bone and teeth • Plays a role in blood clotting, hormone secretion, receptor functions, nerve transmission, and muscular contraction • Has inverse relationship with phosphorus • Has synergistic relationship with magnesium

Calcium • Dietary intake main source – Vitamin D aids absorption • Excreted through the gastrointestinal trace • Regulated by – Vitamin K – Parathyroid hormone – Calcitonin
Hypercalcemia • Calcium > 5 m. Eq/L • Causes: – Increased intake or release: calcium antacids, calcium supplements, cancer, immobilization, corticosteroids, vitamin D deficiency, and hypophosphatemia – Deficit excretion: renal failure, thiazide diuretics, and hyperparathyroidism
Hypercalcemia • Manifestations: dysrhythmias, electrocardiogram changes, personality changes, confusion, decreased memory, headache, lethargy, stupor, coma, muscle weakness, decreased deep tendon reflexes, anorexia, nausea, vomiting, constipation, abdominal pain, pancreatitis, renal calculi, polyuria, and dehydration • Diagnosis: history, physical examination, blood chemistry, and 12 -lead electrocardiogram
Hypercalcemia • Treatment: – Identify and manage underlying cause – Manage symptoms – Phosphate – Increase mobility – Calcitonin – Intravenous fluids – Diuretics

Hypocalcemia • Calcium < 4 m. Eq/L • Causes – Excessive losses: hypoparathyroidism, renal failure, hyperphosphatemia, alkalosis, pancreatitis, laxatives, diarrhea, and other medications – Deficient intake: decreased dietary intake, alcoholism, absorption disorders, and hypoalbuminemia

Hypocalcemia • Manifestations: dysrhythmias, electrocardiogram changes, increased bleeding tendencies, anxiety, confusion, depression, irritability, fatigue, lethargy, paresthesia, increased deep tendon reflexes, tremors, muscle spasms, seizures, laryngeal spasms, increased bowel sounds, abdominal cramping, and positive Trousseau’s and Chvostek’s signs
Hypocalcemia • Diagnosis: same as hypercalcemia • Treatment – Identify and manage underlying cause – Calcium replacement (oral or intravenous) – Vitamin D – Decrease phosphorus

Phosphorus • Normal range 2. 5 -4. 5 mg/d. L • Mostly found in the bones and small amoutns are in the bloodstream • Plays a role in bone and tooth mineralization, cellular metabolism, acidbase balance, and cell membrane formation • Dietary intake main source • Excreted through the kidneys
Hyperphosphatemia • Phosphorus > 4. 5 mg/d. L • Causes – Deficient excretion: renal failure, hypoparathyroidism, adrenal insufficiency, hypothyroidism, and laxatives – Excessive intake or cellular exchange: cellular damage, hypocalcemia, and acidosis

Hyperphosphatemia • Manifestations: rarely seen alone • Diagnosis: history, physical examination, and blood chemistry • Treatment: – Identify and manage underlying cause – Aluminum hydroxide or aluminum carbonate – Treat hypocalcemia

Hypophosphatemia • Phosphorus < 2. 5 mg/d. L • Causes – Excessive excretion or cellular exchange: renal failure, hyperparathyroidism, and alkalosis – Deficient intake: malabsorption, vitamin D deficiency, magnesium and aluminum antacids, alcoholism, and decreased dietary intake
Hypophosphatemia • Manifestations: similar to hypercalcemia • Diagnosis: history, physical examination, and blood chemistry • Treatment: – Identify and manage the underlying cause – Phosphorus replacement (oral or intravenous)

Magnesium • • Normal range 1. 8 -2. 5 m. Eq/L An intracellular cation Mostly stored in the bone and muscle Plays a role in muscle and nerve function, cardiac rhythm, immune function, bone strength, blood glucose managment, blood pressure, energy metabolism, and protein synthesis • Dietary intake main source • Excreted through the kidneys

Hypermagnesemia • Magnesium > 2. 5 m. Eq/L • Causes: renal failure, excessive laxative, and antacid use • Manifestations: similar to hypercalcemia • Diagnosis: history, physical examination, and blood chemistry • Treatment: diuretics, dialysis, and intravenous calcium

Hypomagnesemia • Magnesium < 1. 8 m. Eq/L • Causes: inadequate intake, chronic alcoholism, malnutrition, pregnancy, diarrhea, diuretics, and stress • Manifestations: similar to hypocalcemia • Diagnosis: similar to hypermagnesemia • Treatment: magnesium replacement (oral or intravenous)
Acid-Base Balance • Measured by p. H – Normal serum p. H 7. 35 -7. 45 • Body fluids, kidneys, and lungs maintain balance • Subtle changes can cause serious effects
p. H Regulation • p. H reflects hydrogen concentrations – Hydrogen is an acid – The more hydrogen, the lower the p. H • Acids are byproducts of metabolism – Volatile acids – Volatile gases – Nonvolatile gases • 3 systems work to maintain acid-base balance―the buffers, respiratory system, and renal system.
Buffers • Chemicals that combine with an acid or base to change p. H • Immediate reaction to counteract p. H variations until compensation is initiated • 4 major buffer mechanisms—the bicarbonate-carbonic acid system, the phosphate system, the hemoglobin system, and the protein system

Bicarbonate-Carbonic Acid System • Most significant in the extracellular fluid • Carbonic acid and bicarbonate are the key players • Carbonic acid forms from carbon dioxide reacting with water • Carbonic anhydrase causes carbonic acid to separate into hydrogen and bicarbonate • Carbonic anhydrase in the lungs allows for carbon dioxide excretion and in the kidneys allows for hydrogen excretion
Phosphate System • Similar to the bicarbonate-carbonic acid system • Phosphates are in high concentrations in the intracellular fluid • Some phosphates act as weak acids, and some act as weak bases • This system primarily occurs in the kidneys by accepting or donating hydrogen

Hemoglobin System • Primarily occurs in the capillaries • Acidity and hypoxia causes hemoglobin to release the oxygen • Hemoglobin then becomes a weaker acid, taking up extra hydrogen • Binding with oxygen makes hemoglobin more prone to release hydrogen • Hydrogen reacts with bicarbonate to form carbonic acid, which is converted to carbon dioxide and released into the alveoli
Protein System • Most abundant buffering system • Proteins can act as an acid or base by binding to or releasing hydrogen • Occurs in the intracellular and extracellular spaces • Hydrogen and carbon dioxide diffuse across the cell membrane to bind with protein inside the cell • Albumin and plasma are the primary buffers in the intravascular space
Potassium • Potassium and hydrogen move interchangeably in and out of the cell to balance p. H • With extracellular excess, hydrogen moves inside the cell for buffering, and, in exchange, potassium moves out • Potassium imbalances can lead to p. H imbalances

Respiratory Regulation • Alters carbon dioxide excretion • Speeding up respirations will excrete more carbon dioxide, decreasing acidity • Slowing down respirations will excrete less carbon dioxide, increasing acidity • Uses chemoreceptors • Responds quickly, but is short-lived
Renal Regulation • Alters the excretion or retention of hydrogen or bicarbonate • More effective by permanently removing hydrogen • Responds slowly, but longer lasting

Compensation • The body never overcompensates • The cause of the imbalance often determines the compensatory change – If the problem causing the p. H imbalance originates in the lungs, the kidneys initiates efforts to correct it – If the problem causing the p. H imbalance originates outside the lungs, the lungs initiate efforts to correct it

Metabolic Acidosis • Results from a deficiency of bicarbonate or an excess of hydrogen • Causes – Bicarbonate deficit: intestinal and renal losses – Acid excess: tissue hypoxia resulting in lactic acid accumulation, ketoacidosis, drugs, toxins, and renal retention

Metabolic Acidosis • Manifestations – Appear as regulatory systems fail to maintain p. H within normal range – Occur in combination with manifestations of underlying condition – Include: headache, malaise, weakness, fatigue, lethargy, coma, warm and flushed skin, nausea, vomiting, anorexia, hypotension, dysrhythmias, shock, Kussmaul’s respirations, and hyperkalemia

Metabolic Acidosis • Diagnosis: history, physical examination, arterial blood gases, blood chemistry, and complete blood count

Anion Gap • Helpful in determining the cause of metabolic acidosis • Identifies the anions that are not measured • Conditions that cause excess acid will increase the anion gap; otherwise, the anion gap is normal

Anion Gap • The sum of cations should be approximately equal to the sum of anions in the extracellular fluid • Sodium is the most plentiful cation in the extracellular fluid while bicarbonate and chloride are the most abundant anions • To determine the anion gap, the bicarbonate and chloride results are added together and subtracted from the sodium • Normal anion gap is 6– 9 m. Eq/L

Metabolic Acidosis • Treatment – Identifying and treating the causative condition – Strategies to correct the acidosis include: Intravenous bicarbonate, correction of electrolyte disturbances, improving oxygenation, and insulin

Metabolic Alkalosis • Results from excess bicarbonate or deficient acid or both • Causes – Excess bicarbonate: excessive antacid use, use of bicarbonate-containing fluids, and hypochloremia – Deficient acid: gastrointestinal loss, hypokalemia, renal loss, hypovolemia , and hyperaldosteronism

Metabolic Alkalosis • Manifestations – Appear as regulatory systems fail to maintain p. H within normal range – Occur in combination with manifestations of underlying condition – Include: mental confusion, hyperactive reflexes, paresthesia, tetany, seizures, respiratory depression, dysrhythmias, and coma

Metabolic Alkalosis • Diagnosis: history, physical examination, arterial blood gases, blood chemistry, and complete blood count • Treatment – Identifying and treating the causative condition – Strategies to correct the alkalosis include: adequate hydration, correcting electrolyte disturbances, Diamox, arginine hydrochloride, and administering a weak hydrochloric acid solution

Respiratory Acidosis • Results from carbon dioxide retention, which increases carbonic acid • Caused by conditions that result in hypoventilation or decreased gas exchange – Includes: acute asthma exacerbations, chronic obstructive pulmonary disease, airway obstructions, pulmonary edema, pneumonia, drug overdose, respiratory failure, and central nervous system depression

Respiratory Acidosis • Manifestations – Appear as regulatory systems fail to maintain p. H within normal range – Occur in combination with manifestations of underlying condition – Include: headache, blurred vision, tremors, muscle twitching, vertigo, irritability, disorientation, lethargy, coma, tachycardia leading to bradycardia, blood pressure fluctuations, and diaphoresis

Respiratory Acidosis • Diagnosis: history, physical examination, arterial blood gases, blood chemistry, complete blood count , and chest X-ray • Treatment: oxygen therapy, mechanical ventilation, positioning the patient for optimum ventilation, bronchial hygiene measures, bronchodilators, and manage causative conditions

Respiratory Alkalosis • Results from excess exhalation of carbon dioxide, which leads to carbonic acid deficits • Caused by conditions that result in hyperventilation – Includes: acute anxiety, pain, fever, hypoxia, gram-negative septicemia, aspirin overdose, excessive mechanical ventilation, and hypermetabolic states

Respiratory Alkalosis • Manifestations – Appear as regulatory systems fail to maintain p. H within normal range – Occur in combination with manifestations of underlying condition – Include: paresthesia, dizziness, vertigo, syncope, muscle irritability and twitching, tetany, inability to concentrate, seizures, tachycardia, dysrhythmias, dry mouth, anxiety, excessive diaphoresis, and coma

Respiratory Alkalosis • Diagnosis: history, physical examination, arterial blood gases, blood chemistry, complete blood count, and chest X-ray • Treatment: manage underlying conditions, breath into a paper bag, mechanical ventilation, and anxiety reduction strategies

Mixed Disorders • Respiratory and metabolic disorders resulting in an acidotic or alkalotic state • Both the respiratory and renal systems demonstrate an imbalance of acid or base • Causes: pulmonary edema, aspirin overdose, fever, and vomiting • Critical and complex condition
Arterial Blood Gas Interpretation • p. H – serum hydrogen concentration – Indicates acid-base status • Pa. CO 2 - partial pressure of carbon dioxide – Indicates the adequacy of pulmonary ventilation • HCO 3 – bicarbonate – Indicates the activity in the kidneys to retain or excrete bicarbonate
Arterial Blood Gas Interpretation • Pa. O 2 - partial pressure of oxygen – Indicates serum oxygen concentration • Base excess/deficit – Indicates serum buffer concentration, particularly bicarbonate – Positive values indicate an excess of base or a deficit of acid – Negative values indicate a deficit of base or an excess of acid
Steps in Arterial Blood Gas Interpretation 1. Is the p. H high, low, or normal? – If it is > 7. 4, write a B beside it for basic – If it is < 7. 4, write an A beside it for acidic – Make note if it within normal limits (7. 357. 45)
Steps in Arterial Blood Gas Interpretation 2. Is the Pa. CO 2 high, low, or normal? – If it is between 35 -45 mm. Hg, write an N beside it for normal – If it is > 45 mm Hg, write an A beside it for acidic – If it is < 35 mm Hg, write a B beside it for basic

Steps in Arterial Blood Gas Interpretation 3. Is the HCO 3 high, low, or normal? – If it is between 22 -26, write an N beside it for normal – If it is > 26 m. Eq/L, write a B beside it for basic – If it is < 22 m. Eq/L, write an A beside it for acidic

Steps in Arterial Blood Gas Interpretation 4. Look for patterns – Two As = acidosis • One of the As is CO 2 = respiratory disorder • One of the As is HCO 3 = metabolic disorder – Two Bs = alkalosis • One of the Bs is CO 2 = respiratory disorder • One of the Bs is HCO 3 = metabolic disorder – Three As or Bs = a mixed disorder • All As = mixed respiratory and metabolic acidosis • All Bs = mixed respiratory and metabolic alkalosis
Steps in Arterial Blood Gas Interpretation 5. Determine compensation: – The unpaired result is within normal range = uncompensated – The unpaired result is the opposite letter of the pairs but the p. H is still abnormal = partially compensated – The unpaired result is the opposite letter and the p. H has returned to normal range = fully compensated
- Slides: 84