Chapter 6 COUNTERCURRENT MULTISTAGE EXTRACTION II More Applications













![Flooding Correlation of the flooding points according to Wallis [10]: G. B Wallis, (1969), Flooding Correlation of the flooding points according to Wallis [10]: G. B Wallis, (1969),](https://slidetodoc.com/presentation_image_h2/d808e4967f6bc1f62c3787aaca6b7d7d/image-62.jpg)

- Slides: 63

Chapter 6 COUNTERCURRENT MULTISTAGE EXTRACTION II More Applications HETP, HTU, Capacity

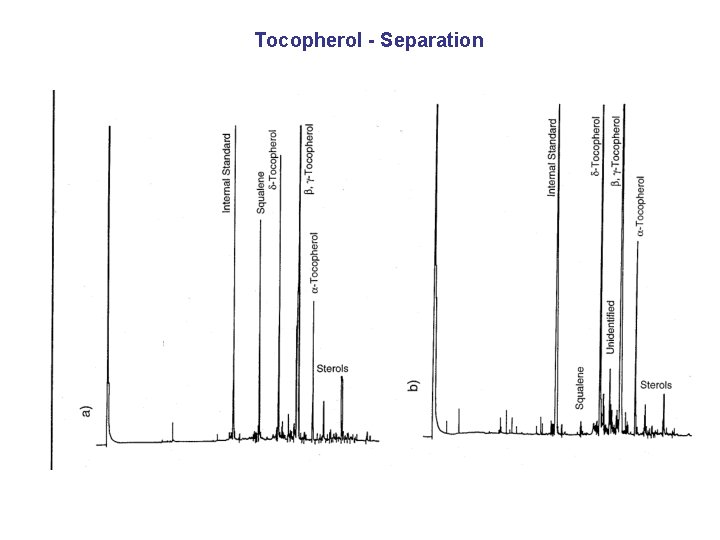
Tocopherol - Separation

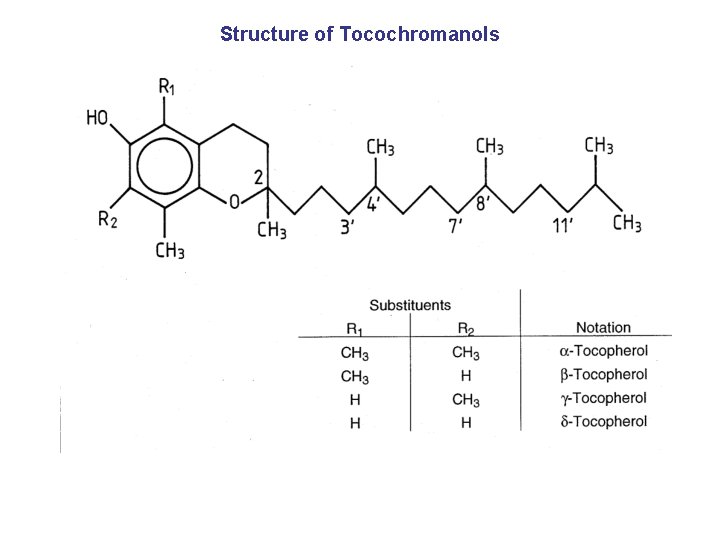
Structure of Tocochromanols

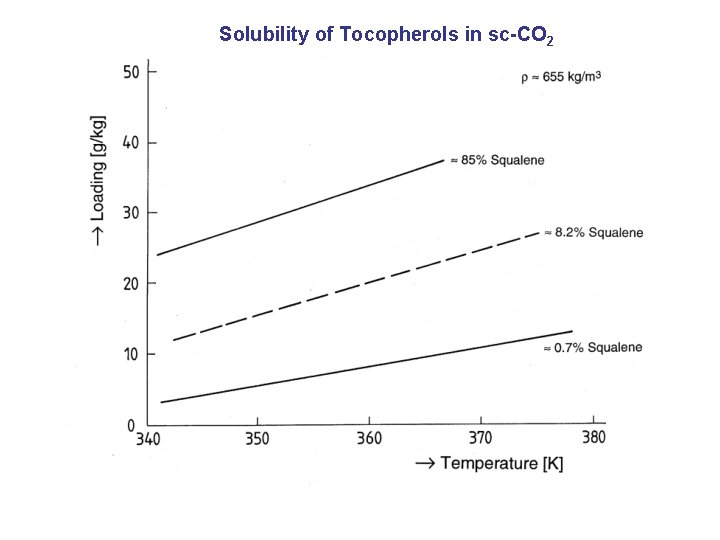
Solubility of Tocopherols in sc-CO 2

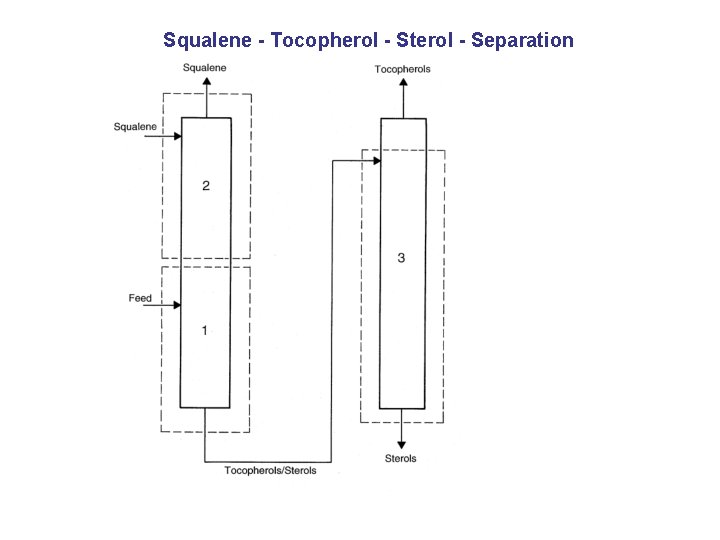
Squalene - Tocopherol - Sterol - Separation

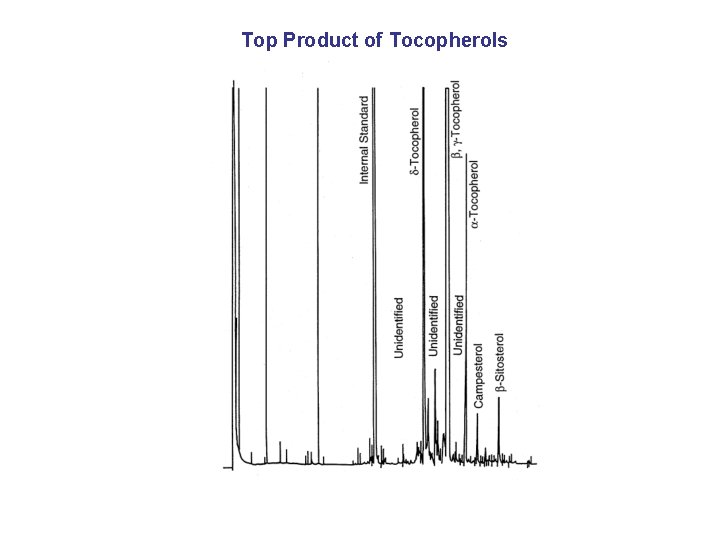
Top Product of Tocopherols

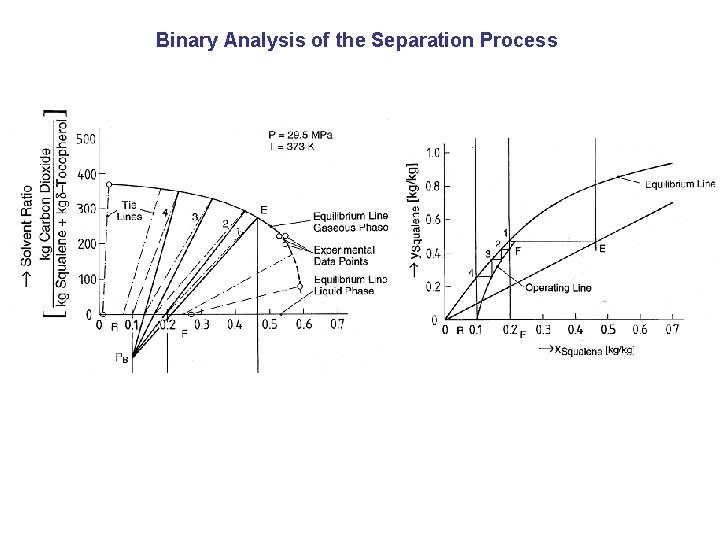
Binary Analysis of the Separation Process

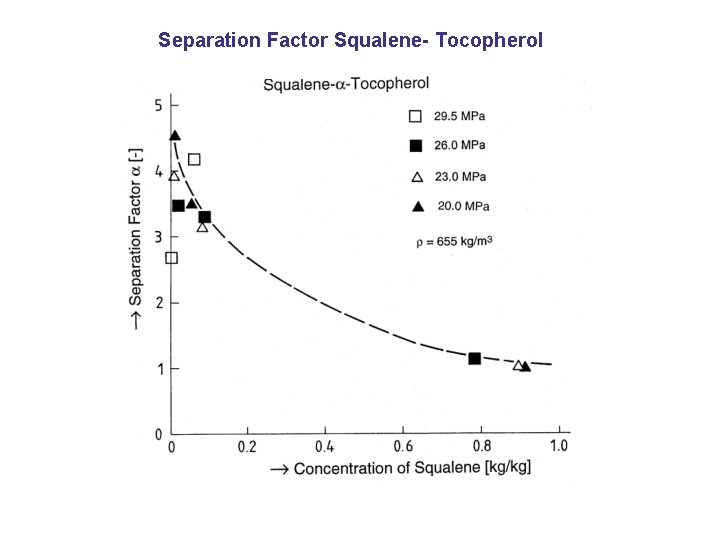
Separation Factor Squalene- Tocopherol

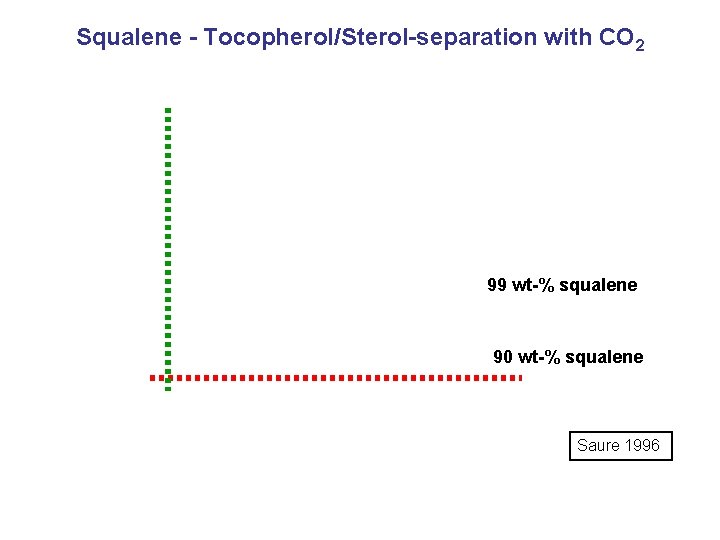
Squalene - Tocopherol/Sterol-separation with CO 2 99 wt-% squalene 90 wt-% squalene Saure 1996

Squalene/Tocopherols From Distillates Equilibrium stages Gas, liquid Squalene Tocopherols Sterols Feed Saure 1996

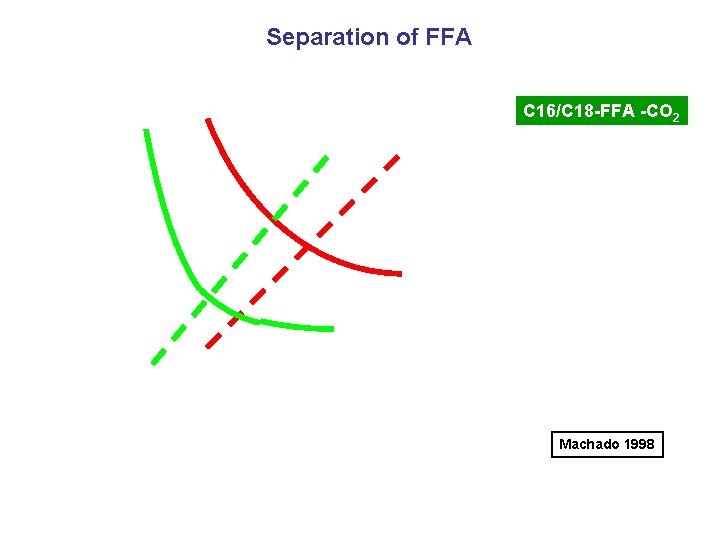
Separation of FFA C 16/C 18 -FFA -CO 2 Machado 1998

Purification of Synthetic Tocopherolacetate Calculation of number of theoretical stages (Jänecke). 333 K, 24 MPa CO 2. U. Fleck. Tocopherolacetate.

Purification of Synthetic Tocopherolacetate Determination of nth in dependence on reflux ratio for different purities. (Mc. Cabe-Thiele and Jänecke); 333 K, 16 MPa CO 2. . U. Fleck.

Purification of Synthetic Tocopherolacetate Concentration profiles along column length; 333 K, 20 MPa CO 2. U. Fleck.

Free fatty acids (FFA) -/- Squalene- FA-esters Buß 1999

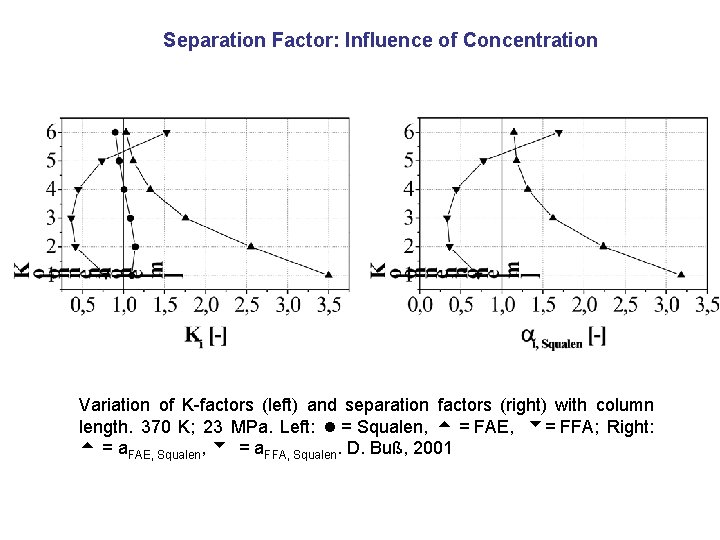
Separation Factor: Influence of Concentration Variation of K-factors (left) and separation factors (right) with column length. 370 K; 23 MPa. Left: = Squalen, = FAE, = FFA; Right: = a. FAE, Squalen, = a. FFA, Squalen. D. Buß, 2001

Separation Analysis: FFA, Toco - Triglycerides, Carotene / (kg reflux / kg extract Solvent ratio

Separation Analysis: FAME - Carotenes

Orange Peel Oil Representation of an improved separation factor model at 333 K. M. Budich

Orange Peel Oil: Removal of Terpenes Budich 1998

No Aceotrope in Ethanol - Water Budich, 1998

Ethanol - Water Calculation of theoretical number of stages. M. Budich.

Mixer-Settler (5 Stages) Flow Scheme of Mixer-Settler. M. Jungfer, 2000. Design: Trepp, ETH-Zürich

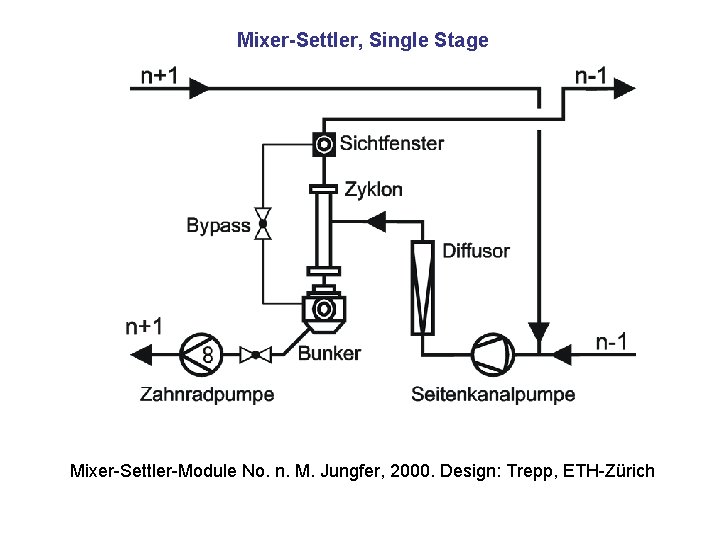
Mixer-Settler, Single Stage Mixer-Settler-Module No. n. M. Jungfer, 2000. Design: Trepp, ETH-Zürich

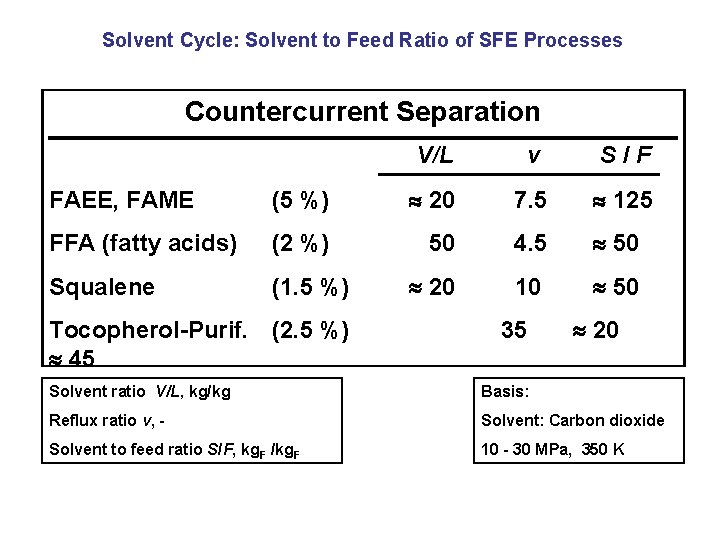
Solvent Cycle: Solvent to Feed Ratio of SFE Processes Countercurrent Separation V/L v S/F FAEE, FAME (5 %) 20 7. 5 125 FFA (fatty acids) (2 %) 50 4. 5 50 Squalene (1. 5 %) 20 10 50 Tocopherol-Purif. (2. 5 %) 45 35 20 Solvent ratio V/L, kg/kg Basis: Reflux ratio v, - Solvent: Carbon dioxide Solvent to feed ratio S/F, kg. F /kg. F 10 - 30 MPa, 350 K

Solubility and Solvent to Feed Ratio Relationship between loading and solvent-to-feed ratio. M. Budich. Orange peel oil.

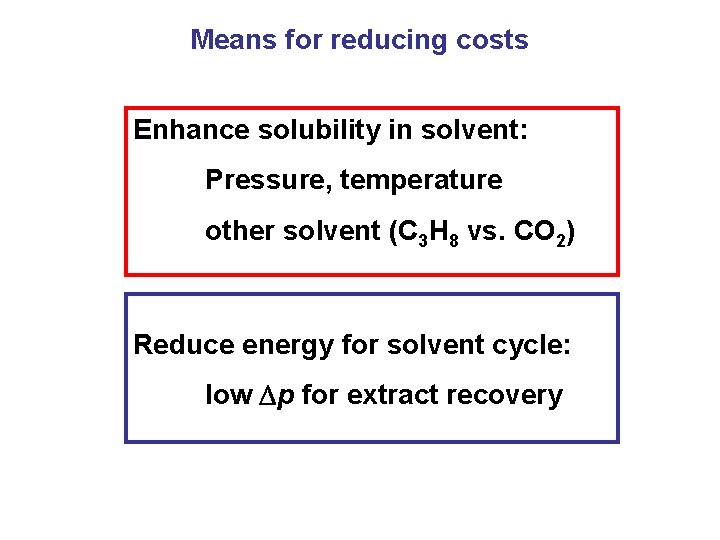
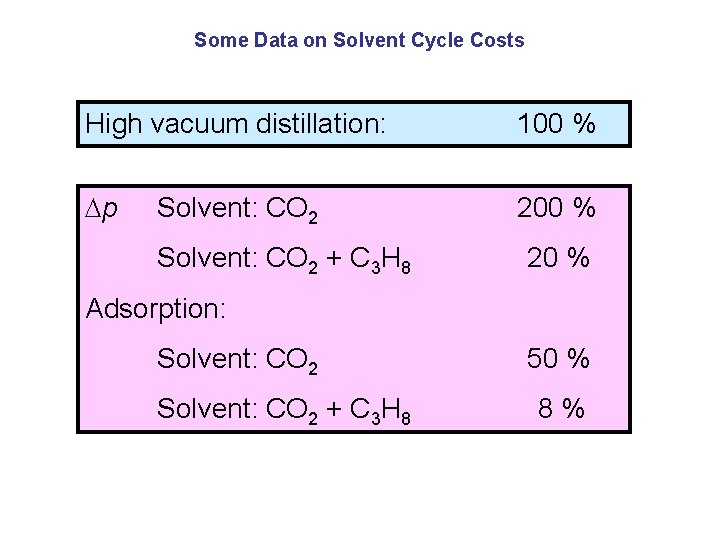
Means for reducing costs Enhance solubility in solvent: Pressure, temperature other solvent (C 3 H 8 vs. CO 2) Reduce energy for solvent cycle: low p for extract recovery

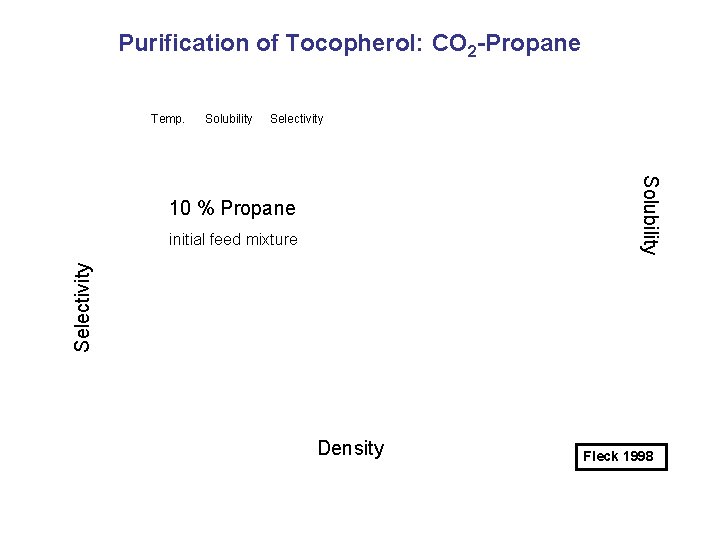
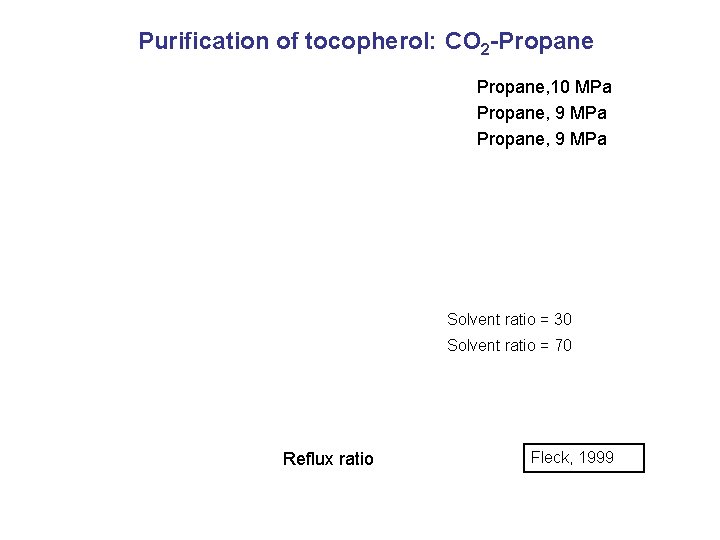
Purification of Tocopherol: CO 2 -Propane Temp. Solubility Selectivity Solubility 10 % Propane Selectivity initial feed mixture Density Fleck 1998

Purification of tocopherol: CO 2 -Propane, 10 MPa Propane, 9 MPa Solvent ratio = 30 Solvent ratio = 70 Reflux ratio Fleck, 1999

Some Data on Solvent Cycle Costs High vacuum distillation: 100 % p 200 % Solvent: CO 2 + C 3 H 8 20 % Adsorption: Solvent: CO 2 + C 3 H 8 50 % 8%

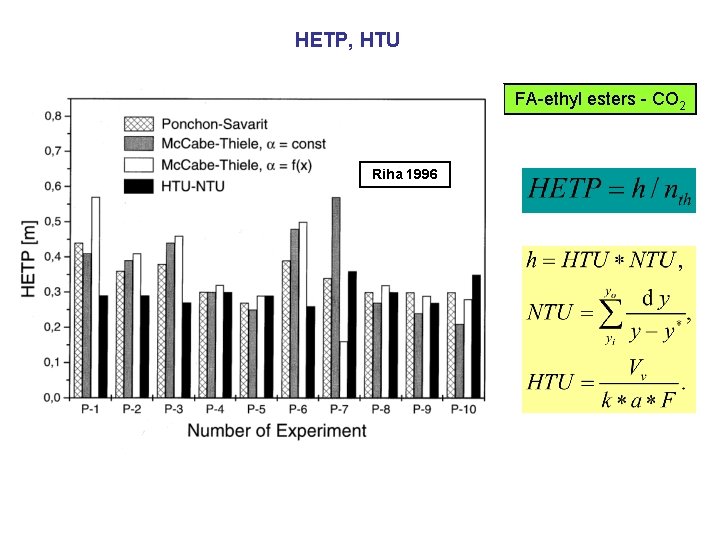
HETP, HTU FA-ethyl esters - CO 2 Riha 1996

HETP: Tocopherol HETP (Jänecke) vs solvent ratio in stripping section. Saure, 1996

HETS for aqueous mixtures M. Budich

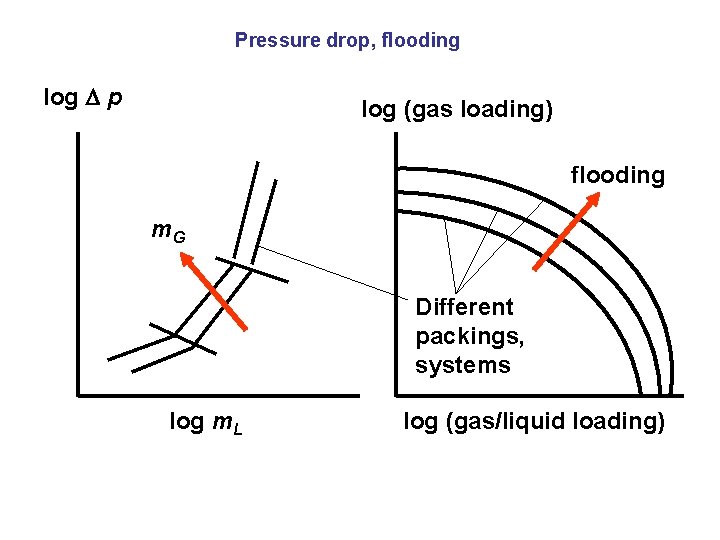
Pressure drop, flooding log p log (gas loading) flooding m. G Different packings, systems log m. L log (gas/liquid loading)

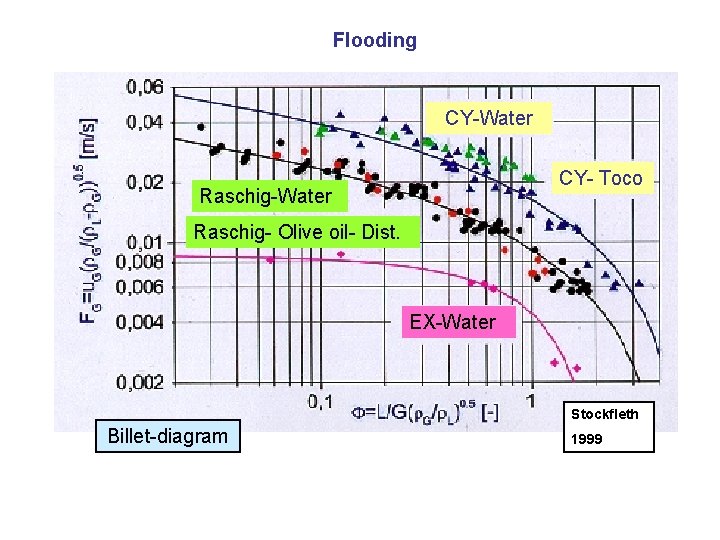
Flooding CY-Water CY- Toco Raschig-Water Raschig- Olive oil- Dist. EX-Water Stockfleth Billet-diagram 1999

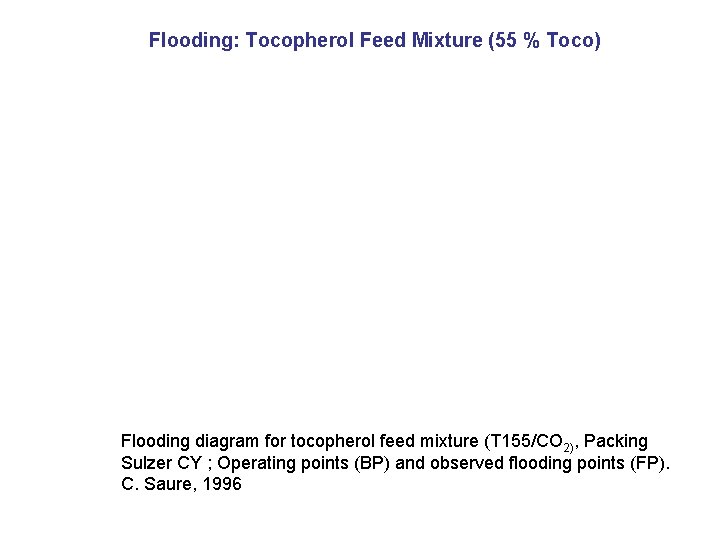
Flooding: Tocopherol Feed Mixture (55 % Toco) Flooding diagram for tocopherol feed mixture (T 155/CO 2), Packing Sulzer CY ; Operating points (BP) and observed flooding points (FP). C. Saure, 1996

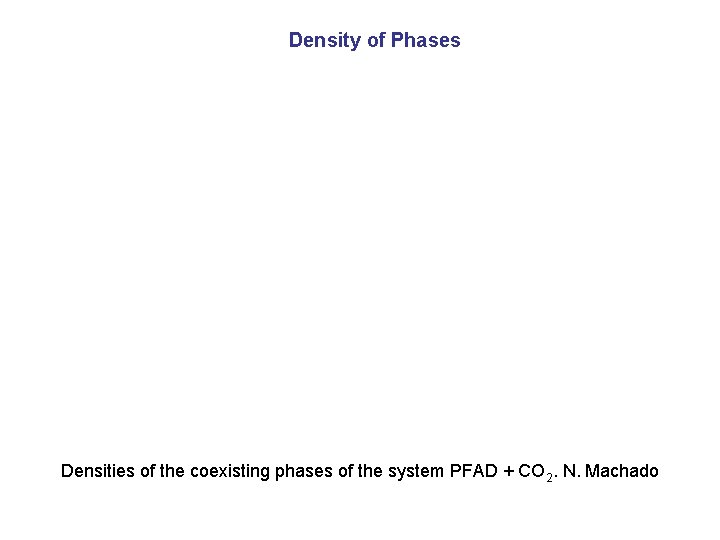
Density of Phases Densities of the coexisting phases of the system PFAD + CO 2. N. Machado

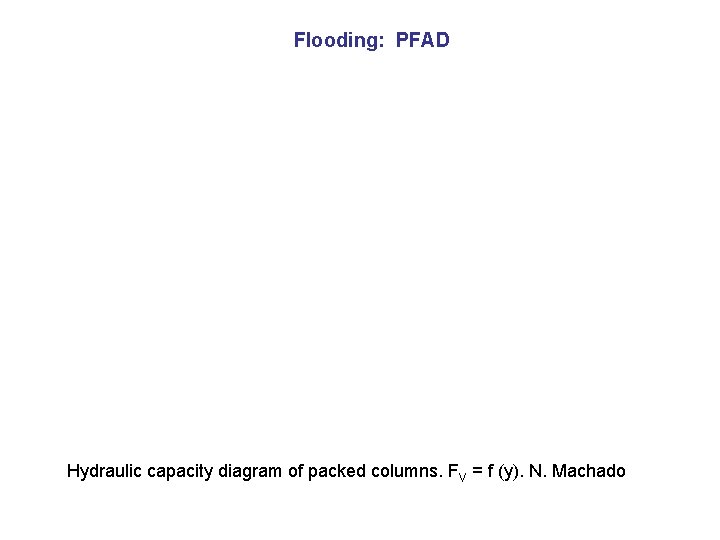
Flooding: PFAD Hydraulic capacity diagram of packed columns. FV = f (y). N. Machado

Density of Phases Density of coexisting phases: CO 2–Squalene. Flüssig-/Gasphase: /�= 333, 15 K, /�= 353, 15 K ▲/▲ = 373, 15 K. D. Buß

Flooding: Squalene Hydraulic capacity diagram of packed columns: Squalene - CO 2. N. Machado

Flooding: CPO Flooding Diagram, Crude Palm Oil - Carbon Dioxide, M. Jungfer, 2000

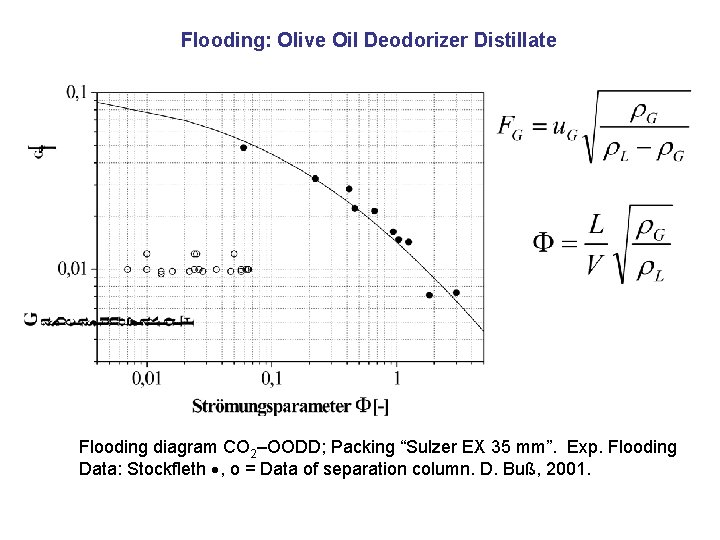
Flooding: Olive Oil Deodorizer Distillate Flooding diagram CO 2–OODD; Packing “Sulzer EX 35 mm”. Exp. Flooding Data: Stockfleth , o = Data of separation column. D. Buß, 2001.

Purification of Synthetic Tocopherolacetate Loading limits for a 35 and 50 mm column. CO 2. U. Fleck.

Pressure Drop Pressure-drop curves. M. Budich. Orange peel oil.

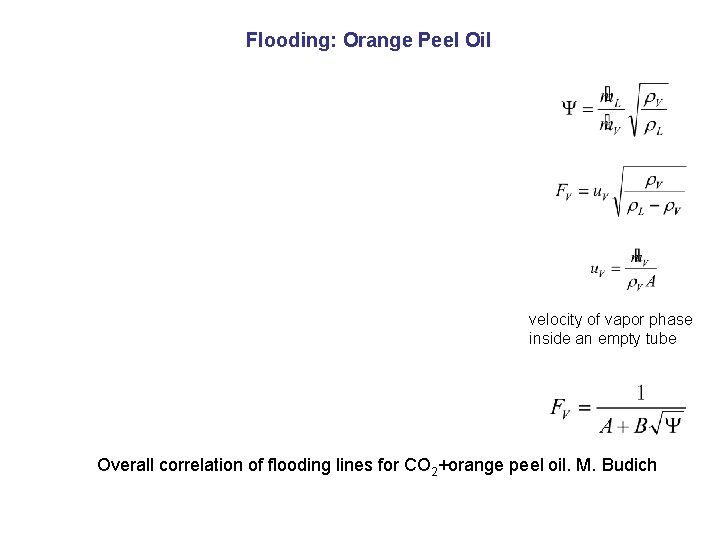
Flooding: Orange Peel Oil velocity of vapor phase inside an empty tube Overall correlation of flooding lines for CO 2+orange peel oil. M. Budich

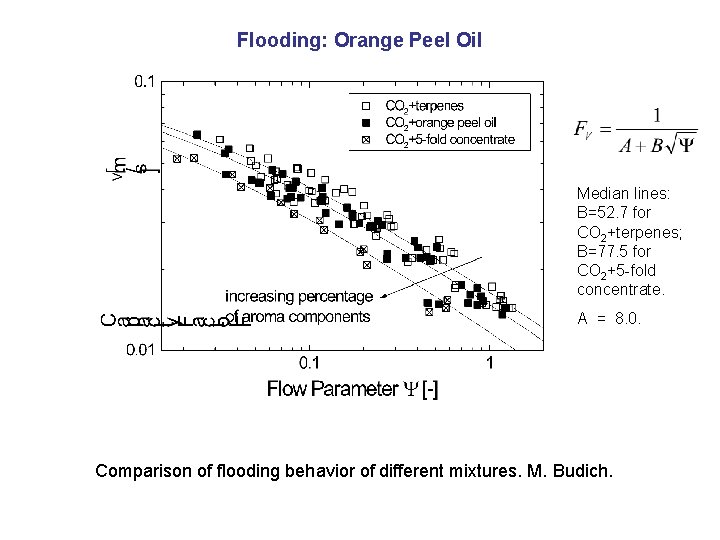
Flooding: Orange Peel Oil Median lines: B=52. 7 for CO 2+terpenes; B=77. 5 for CO 2+5 -fold concentrate. A = 8. 0. Comparison of flooding behavior of different mixtures. M. Budich.

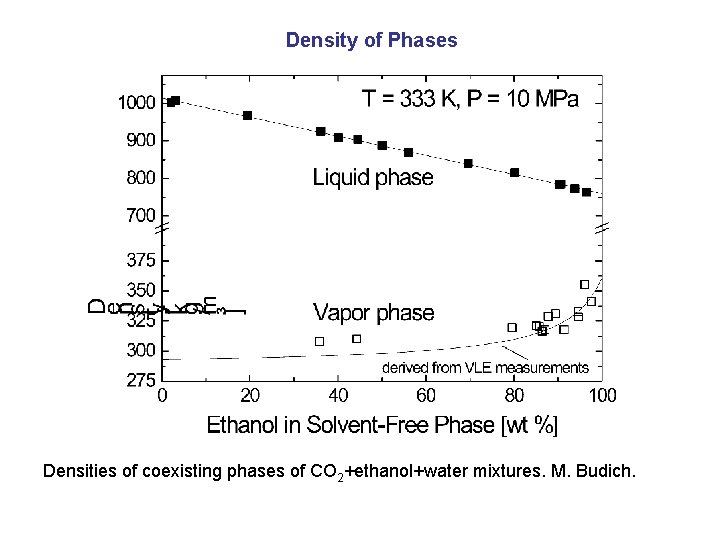
Density of Phases Densities of coexisting phases of CO 2+ethanol+water mixtures. M. Budich.

Flooding point data for CO 2+ethanol+water M. Budich, 1999

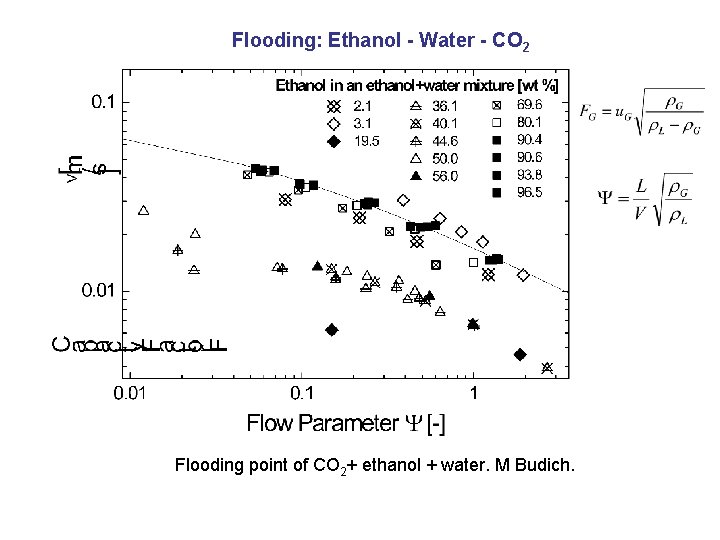
Flooding: Ethanol - Water - CO 2 Flooding point of CO 2+ ethanol + water. M Budich.

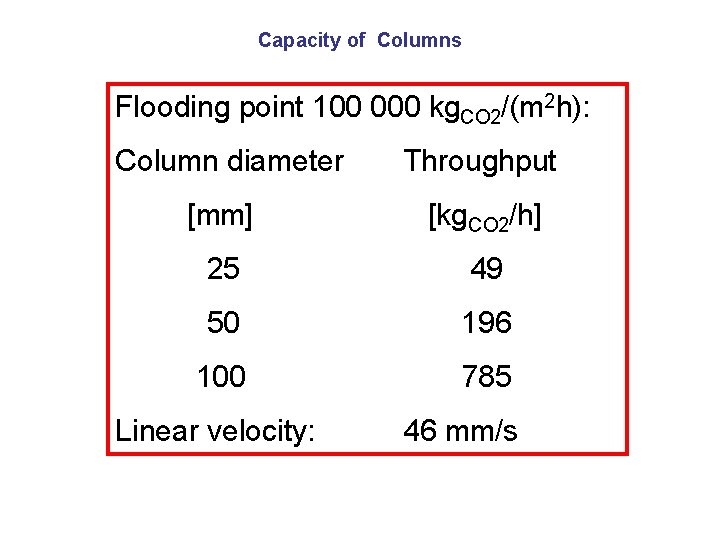
Capacity of Columns Flooding point 100 000 kg. CO 2/(m 2 h): Column diameter Throughput [mm] [kg. CO 2/h] 25 49 50 196 100 785 Linear velocity: 46 mm/s

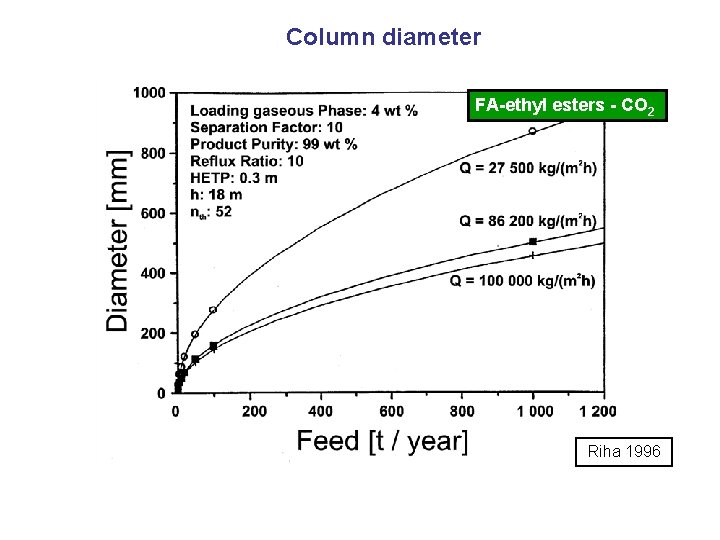
Column diameter FA-ethyl esters - CO 2 Riha 1996

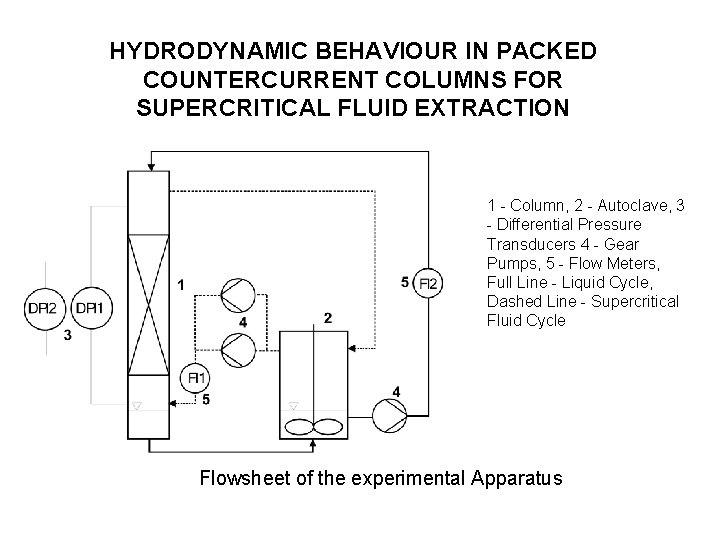
HYDRODYNAMIC BEHAVIOUR IN PACKED COUNTERCURRENT COLUMNS FOR SUPERCRITICAL FLUID EXTRACTION 1 - Column, 2 - Autoclave, 3 - Differential Pressure Transducers 4 - Gear Pumps, 5 - Flow Meters, Full Line - Liquid Cycle, Dashed Line - Supercritical Fluid Cycle Flowsheet of the experimental Apparatus

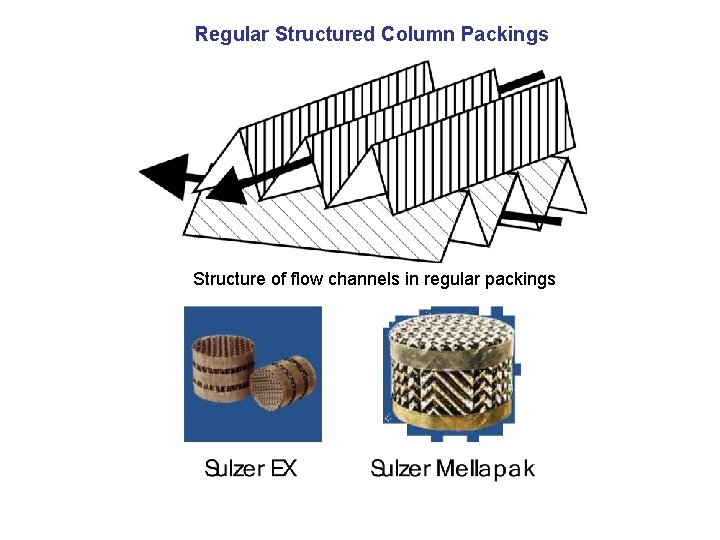
Regular Structured Column Packings Structure of flow channels in regular packings

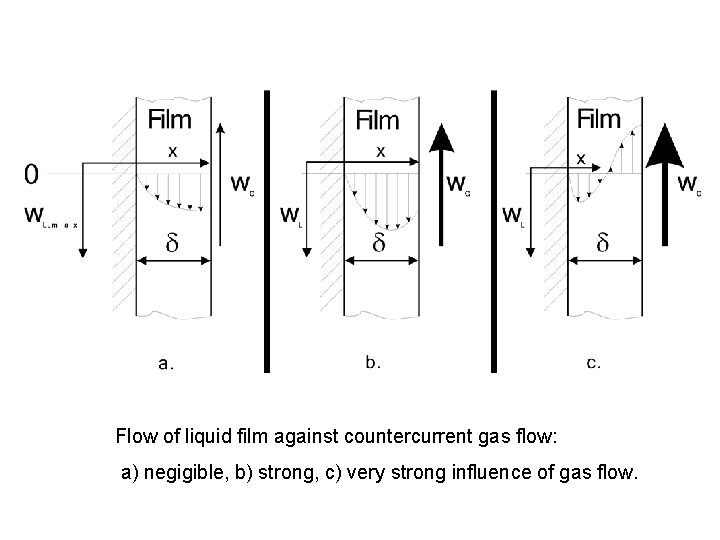
Flow of liquid film against countercurrent gas flow: a) negigible, b) strong, c) very strong influence of gas flow.

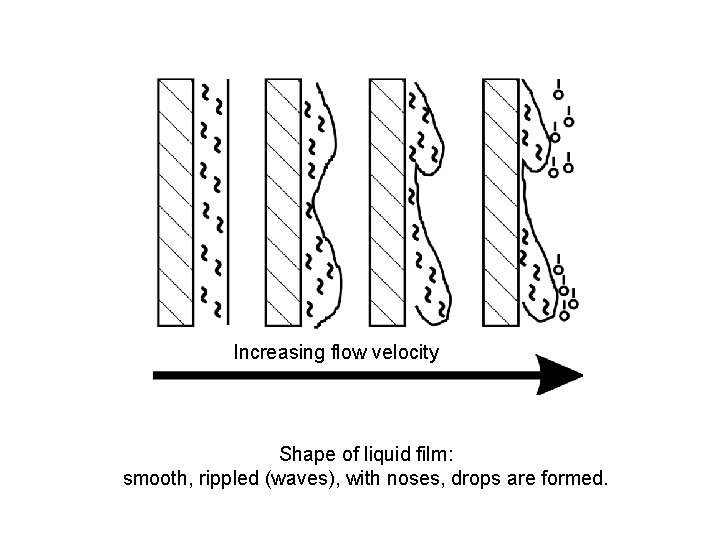
Increasing flow velocity Shape of liquid film: smooth, rippled (waves), with noses, drops are formed.

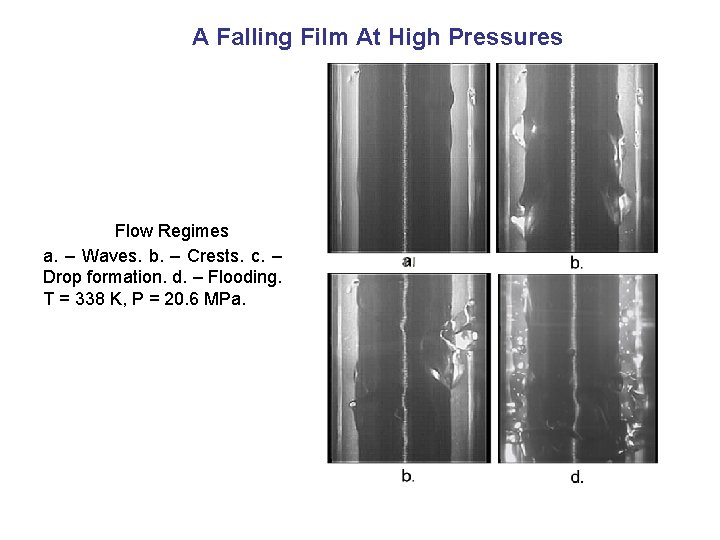
A Falling Film At High Pressures Flow Regimes a. – Waves. b. – Crests. c. – Drop formation. d. – Flooding. T = 338 K, P = 20. 6 MPa.

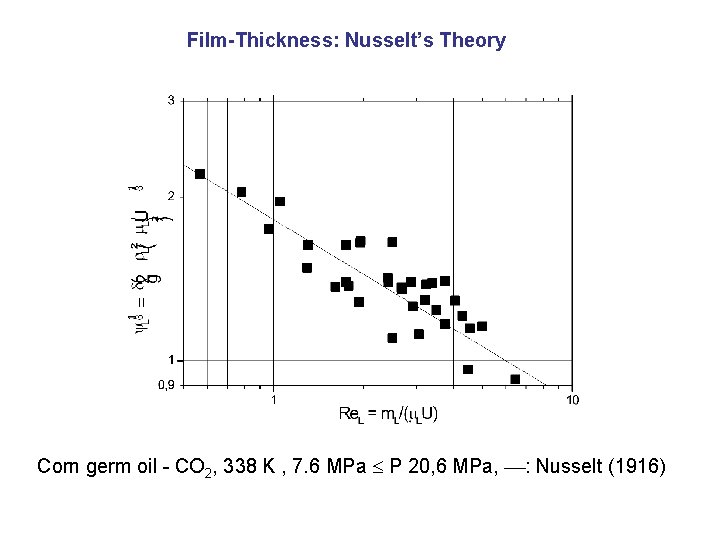
Film-Thickness: Nusselt’s Theory Corn germ oil - CO 2, 338 K , 7. 6 MPa P 20, 6 MPa, : Nusselt (1916)

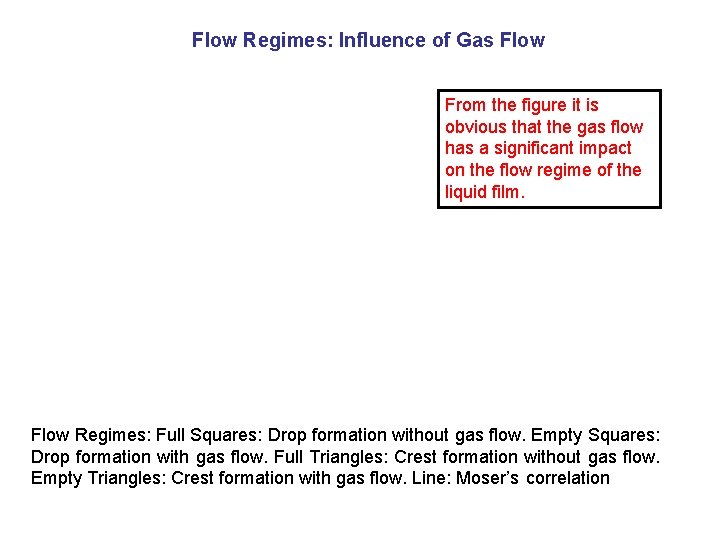
Flow Regimes: Influence of Gas Flow From the figure it is obvious that the gas flow has a significant impact on the flow regime of the liquid film. Flow Regimes: Full Squares: Drop formation without gas flow. Empty Squares: Drop formation with gas flow. Full Triangles: Crest formation without gas flow. Empty Triangles: Crest formation with gas flow. Line: Moser’s correlation

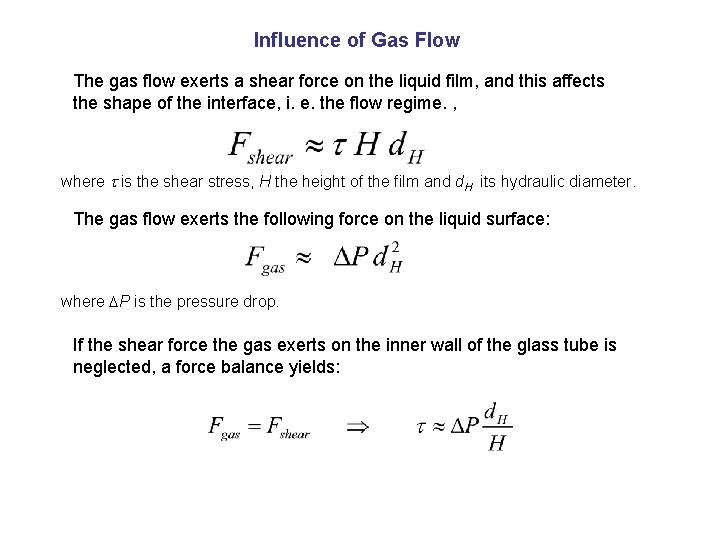
Influence of Gas Flow The gas flow exerts a shear force on the liquid film, and this affects the shape of the interface, i. e. the flow regime. , where is the shear stress, H the height of the film and d. H its hydraulic diameter. The gas flow exerts the following force on the liquid surface: where P is the pressure drop. If the shear force the gas exerts on the inner wall of the glass tube is neglected, a force balance yields:

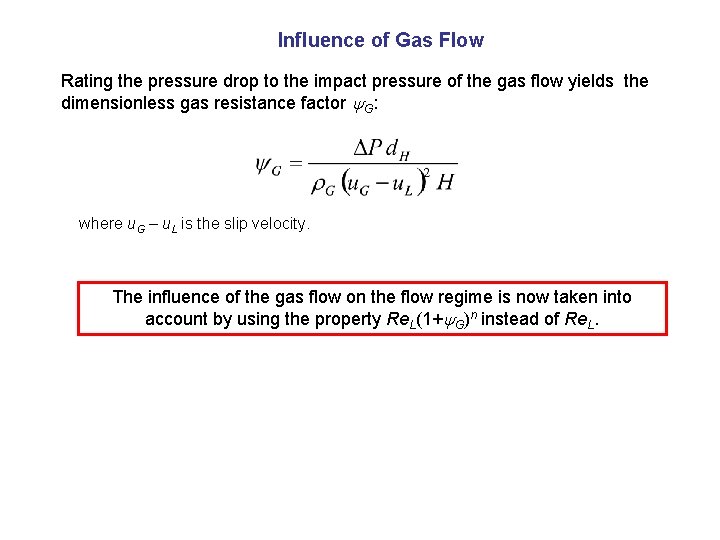
Influence of Gas Flow Rating the pressure drop to the impact pressure of the gas flow yields the dimensionless gas resistance factor G: where u. G – u. L is the slip velocity. The influence of the gas flow on the flow regime is now taken into account by using the property Re. L(1+ G)n instead of Re. L.

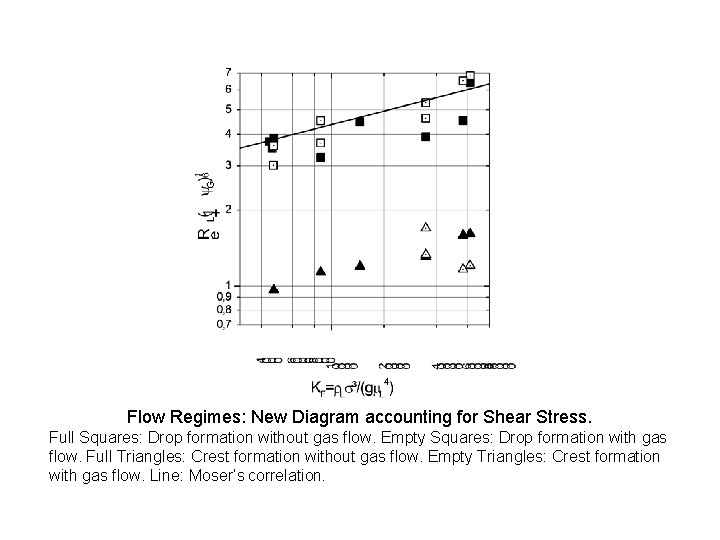
Flow Regimes: New Diagram accounting for Shear Stress. Full Squares: Drop formation without gas flow. Empty Squares: Drop formation with gas flow. Full Triangles: Crest formation without gas flow. Empty Triangles: Crest formation with gas flow. Line: Moser’s correlation.
![Flooding Correlation of the flooding points according to Wallis 10 G B Wallis 1969 Flooding Correlation of the flooding points according to Wallis [10]: G. B Wallis, (1969),](https://slidetodoc.com/presentation_image_h2/d808e4967f6bc1f62c3787aaca6b7d7d/image-62.jpg)
Flooding Correlation of the flooding points according to Wallis [10]: G. B Wallis, (1969), One-Dimensional Two-Phase Flow, Mc. Graw-Hill, New York With u. L for the superficial liquid velocity and the fractional void volume which is unity for a falling film column but smaller than unity for packed columns. j. G* and j. L* are modified Froude-Numbers rating the respective impact pressure to the difference between liquid head and buoyancy. For the correlation of the data displayed, the values K 1=0, 4222 and K 2=1, 1457 with a standard deviation of 19%.

General Flooding Diagram Packings: Sulzer CY, Sulzer EX, Sulzer Mellapak, 5 x 5 x 0. 5 mm Raschig rings, and 4 mm Berl saddles. Substances: water, air, carbon dioxide, olive oil deodorizer distillate, soybean oil deodorizer distillate, fatty acid methyl esters, and tocopherols. Very similar to: T. K. Sherwood, G. H. Shipley and F. A. L. Holloway (1938), Ind. Eng. Chem. , 7, 765 - 769, Flooding Diagram. Thick line: Correlation. Dashed lines: 30% interval. Empty triangles: Structured and random packings at high pressures. Circles: Structured packings at high pressures. Full diamond: Mellapak. TM at normal pressure. Full triangles: Falling film flooding at high pressures.