Chapter 6 Chemical Reactions Section 5 Double Displacement

Chapter 6: Chemical Reactions Section 5: Double Displacement – Precipitation Reactions

Learning Goals �Predict and write equations for precipitation reactions. �Write molecular, complete ionic, and net ionic equations.
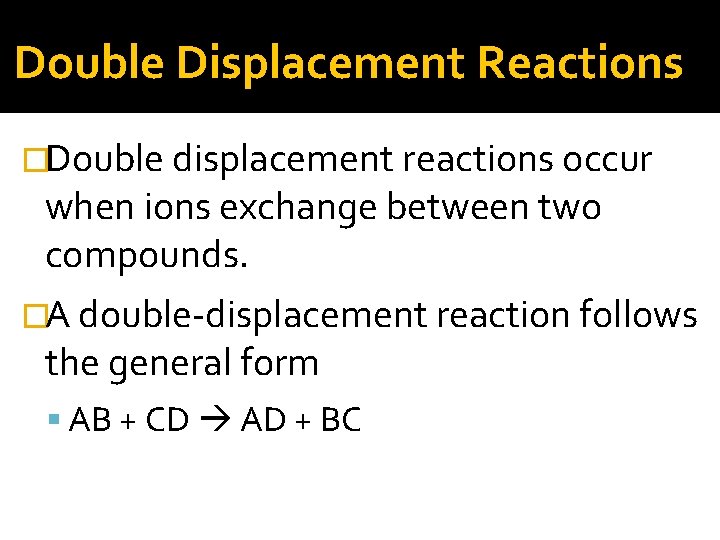
Double Displacement Reactions �Double displacement reactions occur when ions exchange between two compounds. �A double-displacement reaction follows the general form AB + CD AD + BC

Precipitation Reactions �Reactions that form a solid, called a precipitate, upon mixing two aqueous solutions are known as precipitation reactions.

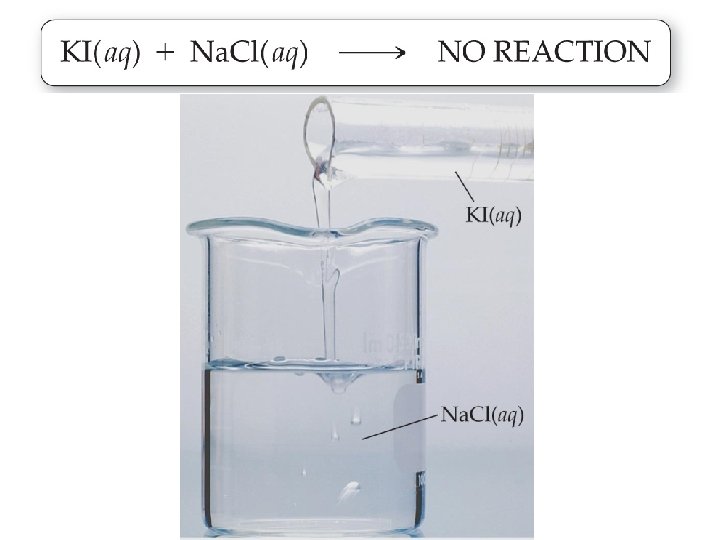
Precipitation Reactions �Precipitation reactions do not always occur when mixing two aqueous solutions.
Precipitation Reactions �The key to predicting precipitation reactions is understanding that only insoluble compounds form precipitates.
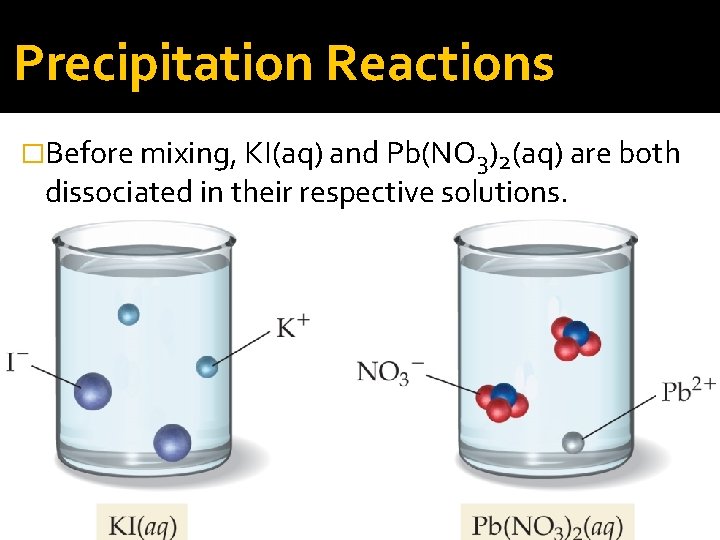
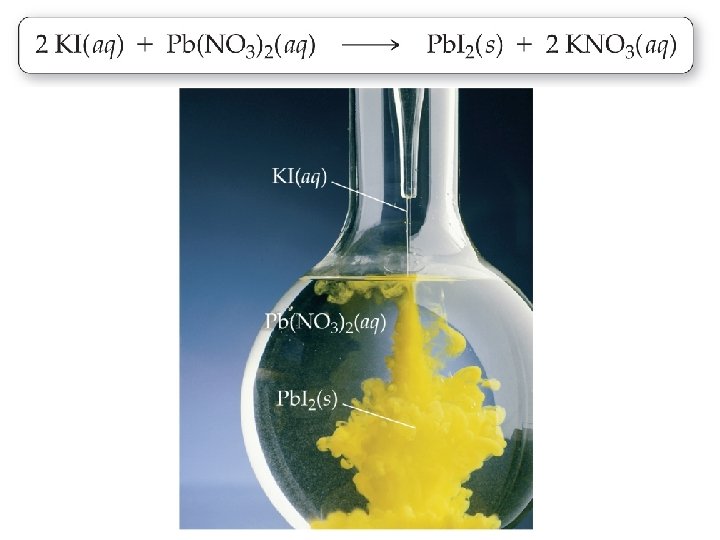
Precipitation Reactions �Before mixing, KI(aq) and Pb(NO 3)2(aq) are both dissociated in their respective solutions.
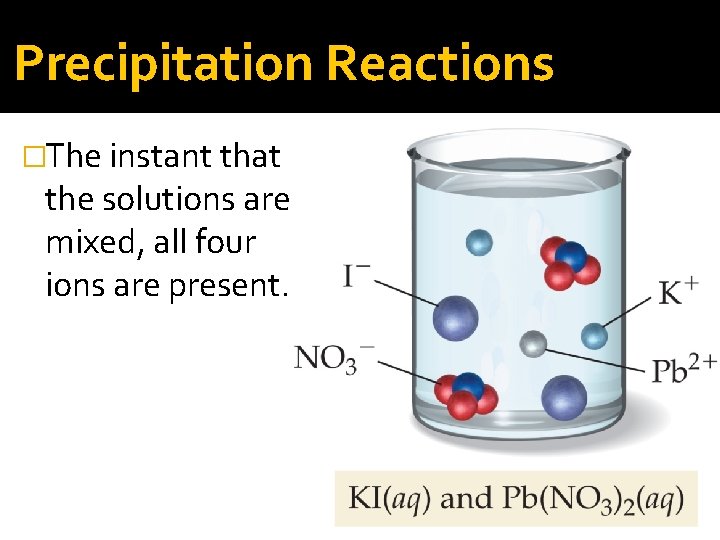
Precipitation Reactions �The instant that the solutions are mixed, all four ions are present.
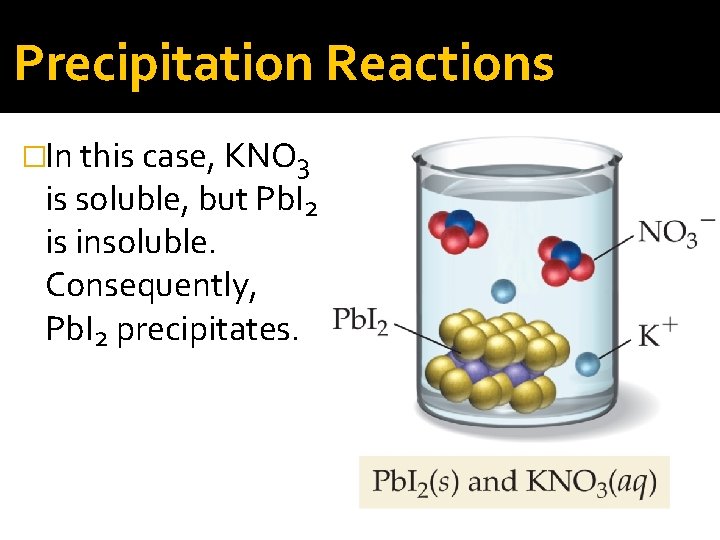
Precipitation Reactions �In this case, KNO 3 is soluble, but Pb. I 2 is insoluble. Consequently, Pb. I 2 precipitates.
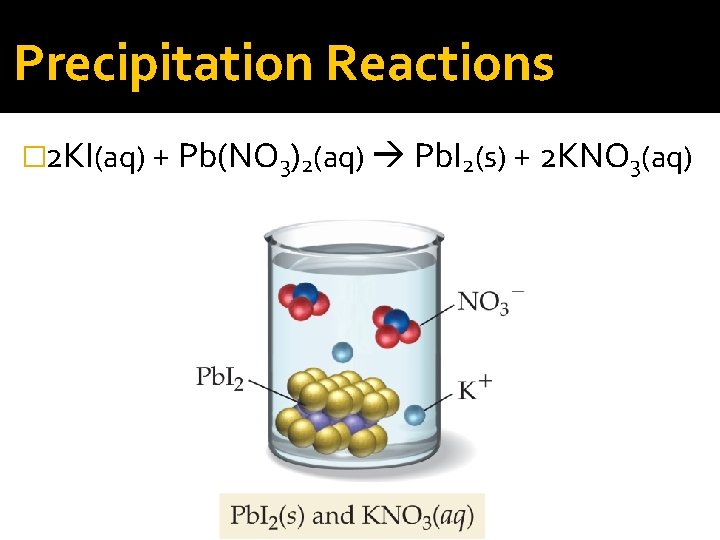
Precipitation Reactions � 2 KI(aq) + Pb(NO 3)2(aq) Pb. I 2(s) + 2 KNO 3(aq)

Predicting Reactions 1. Write an equation for the precipitation reaction that occurs (if any) when solutions of sodium carbonate and copper(II) chloride are mixed.
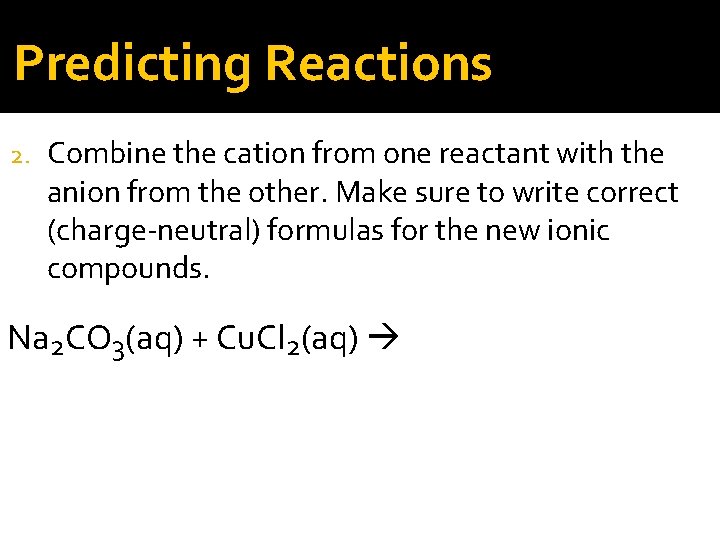
Predicting Reactions 2. Combine the cation from one reactant with the anion from the other. Make sure to write correct (charge-neutral) formulas for the new ionic compounds. Na 2 CO 3(aq) + Cu. Cl 2(aq)
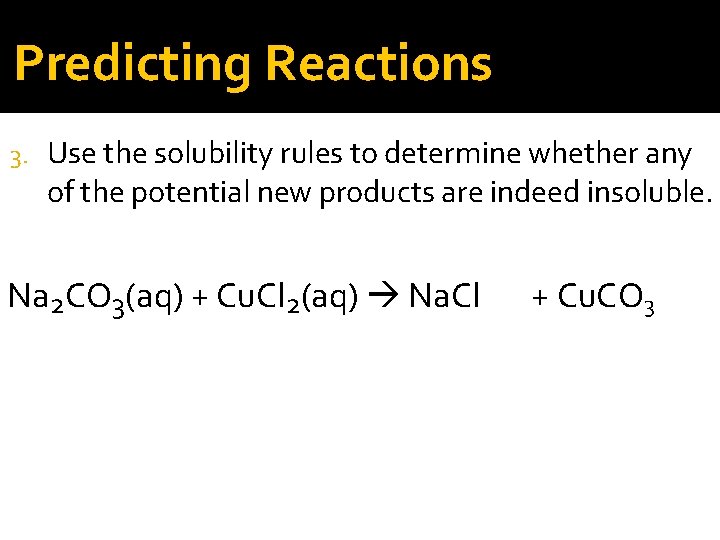
Predicting Reactions 3. Use the solubility rules to determine whether any of the potential new products are indeed insoluble. Na 2 CO 3(aq) + Cu. Cl 2(aq) Na. Cl + Cu. CO 3

Predicting Reactions 4. If all of the potentially insoluble products are soluble, there will be no precipitate. Write NO REACTION next to the arrow.
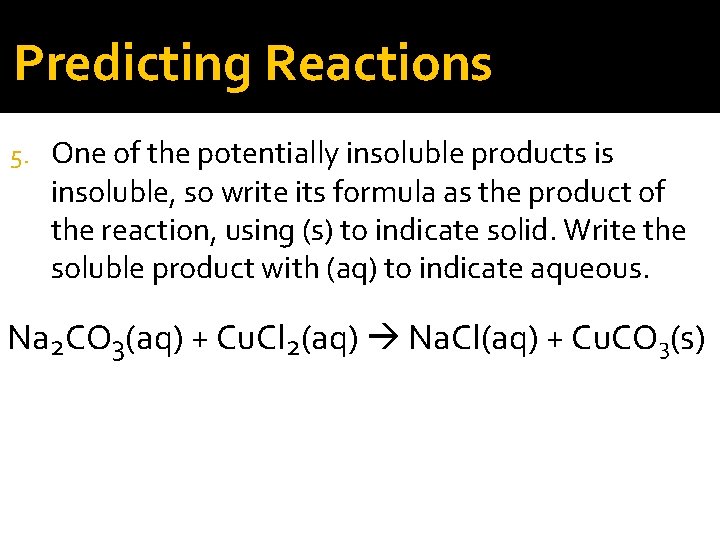
Predicting Reactions 5. One of the potentially insoluble products is insoluble, so write its formula as the product of the reaction, using (s) to indicate solid. Write the soluble product with (aq) to indicate aqueous. Na 2 CO 3(aq) + Cu. Cl 2(aq) Na. Cl(aq) + Cu. CO 3(s)
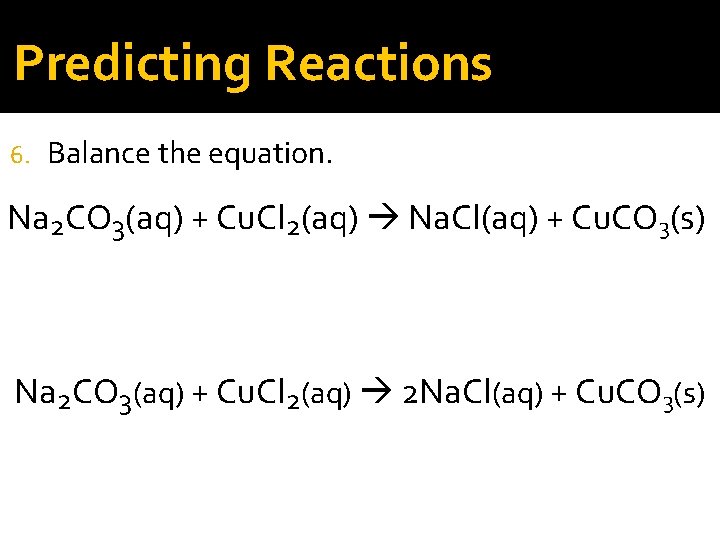
Predicting Reactions 6. Balance the equation. Na 2 CO 3(aq) + Cu. Cl 2(aq) Na. Cl(aq) + Cu. CO 3(s) Na 2 CO 3(aq) + Cu. Cl 2(aq) 2 Na. Cl(aq) + Cu. CO 3(s)

Practice �Use the solubility rules to predict what will happen when the following solutions are mixed. Write the balanced equation for any reaction that occurs. Na 2 SO 4(aq) and Pb(NO 3)2(aq)

Practice KNO 3 (aq) and Ba. Cl 2(aq)

Practice Potassium hydroxide(aq) and Iron (III) nitrate(aq)

Ionic Equations �Ionic equations that show all of the particles in a solution as they actually exist are called complete ionic equations.
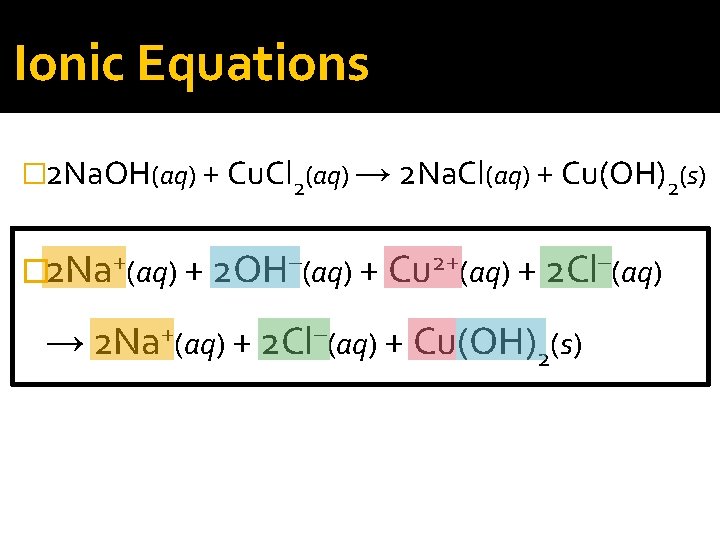
Ionic Equations � 2 Na. OH(aq) + Cu. Cl 2(aq) → 2 Na. Cl(aq) + Cu(OH)2(s) � 2 Na+(aq) + 2 OH–(aq) + Cu 2+(aq) + 2 Cl–(aq) → 2 Na+(aq) + 2 Cl–(aq) + Cu(OH)2(s)
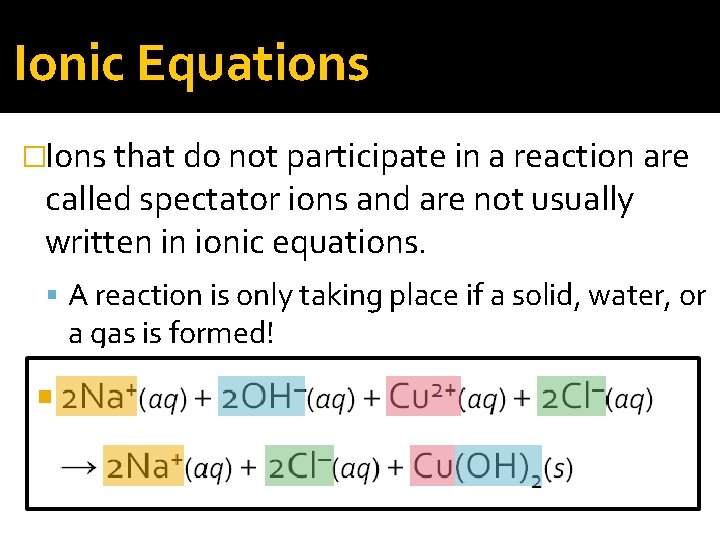
Ionic Equations �Ions that do not participate in a reaction are called spectator ions and are not usually written in ionic equations. A reaction is only taking place if a solid, water, or a gas is formed!

Ionic Equations �Formulas that include only the particles that participate in reactions are called net ionic equations.
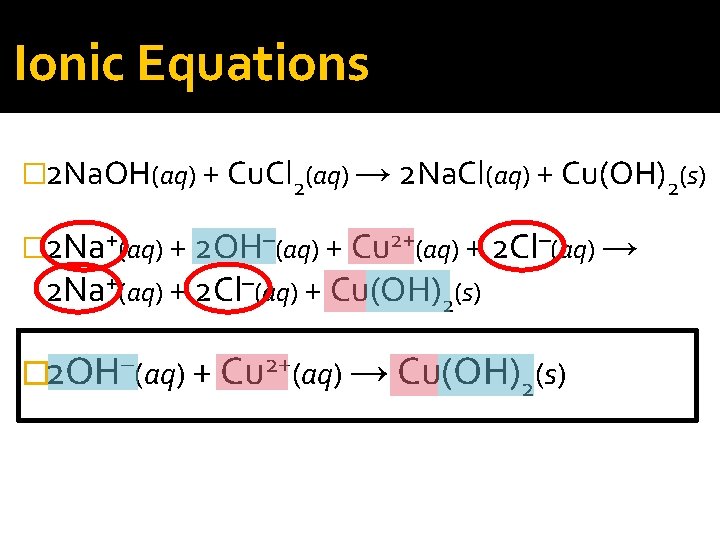
Ionic Equations � 2 Na. OH(aq) + Cu. Cl 2(aq) → 2 Na. Cl(aq) + Cu(OH)2(s) � 2 Na+(aq) + 2 OH–(aq) + Cu 2+(aq) + 2 Cl–(aq) → 2 Na+(aq) + 2 Cl–(aq) + Cu(OH)2(s) � 2 OH–(aq) + Cu 2+(aq) → Cu(OH)2(s)

Practice �Write chemical, complete ionic, and net ionic equations for each of the following reactions that produce a precipitate. Aqueous solutions of potassium iodide and silver nitrate are mixed, forming the precipitate silver iodide.

Practice Aqueous solutions of aluminum chloride and sodium hydroxide are mixed, forming the precipitate aluminum hydroxide.

Practice Aqueous solutions of sodium carbonate and manganese (V) chloride are mixed.
- Slides: 29