CHAPTER 6 CHEMICAL REACTIONS EVIDENCE FOR CHEMICAL CHANGE






















- Slides: 28

CHAPTER 6 – CHEMICAL REACTIONS EVIDENCE FOR CHEMICAL CHANGE 1) Color change 2) A solid forms 3) Bubbles are formed 4) A flame is produces 5) Heat is absorbed or released 5 A-1 (of 34) (15 – 26 -32 + 1 -13) (16 – 14 -23) (17 – 24 -27 + 1 -19)

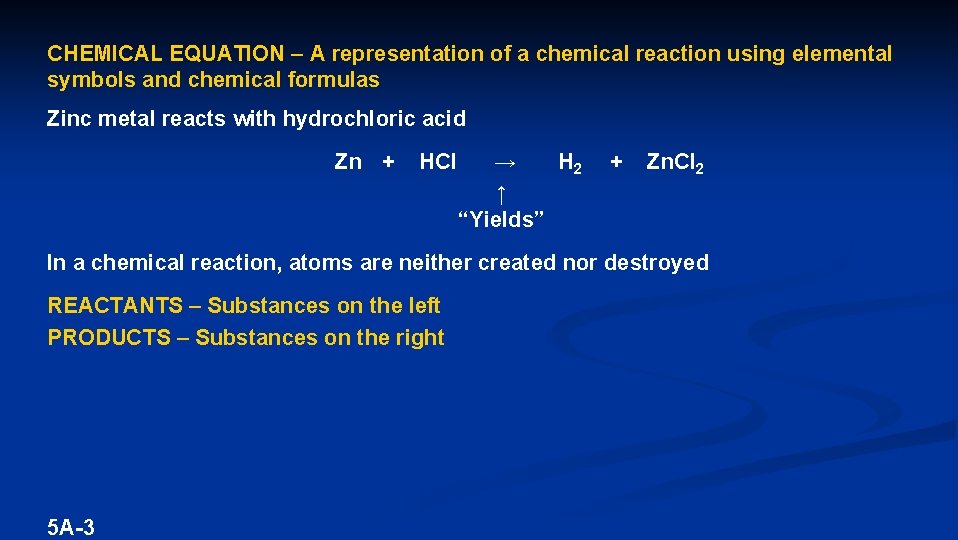
CHEMICAL EQUATION – A representation of a chemical reaction using elemental symbols and chemical formulas Zinc metal reacts with hydrochloric acid Zn + 5 A-2 HCl → H 2 + ?

CHEMICAL EQUATION – A representation of a chemical reaction using elemental symbols and chemical formulas Zinc metal reacts with hydrochloric acid Zn + HCl → H 2 ↑ “Yields” + Zn. Cl 2 In a chemical reaction, atoms are neither created nor destroyed REACTANTS – Substances on the left PRODUCTS – Substances on the right 5 A-3

Hydrogen gas and oxygen gas produce water when sparked H 2 + O 2 → H 2 O 2 - H - 2 2 - O - 1 All atoms of the reactants must be accounted for in the products Satisfying this is called BALANCING THE EQUATION 5 A-4

Hydrogen gas and oxygen gas produce water when sparked H 2 + O 2 → H 2 O 2 2 - H - 2 2 - O - 1 All atoms of the reactants must be accounted for in the products Satisfying this is called BALANCING THE EQUATION 5 A-5

Hydrogen gas and oxygen gas produce water when sparked 2 H 2 + O 2 → 2 H 2 O 4 2 - H - 2 4 2 - O - 1 2 All atoms of the reactants must be accounted for in the products Satisfying this is called BALANCING THE EQUATION Chemical equations are balanced by adjusting the COEFFICIENTS, not by changing the formulas of any reactant or product 5 A-6

BALANCING CHEMICAL EQUATIONS 2 KCl. O 3 → 2 KCl + 3 O 2 2 1 - K - 1 2 2 1 - Cl - 1 2 6 3 - O - 2 6 HINT: When the atoms of an element are odd and even, adjust each to a common multiple 5 A-7

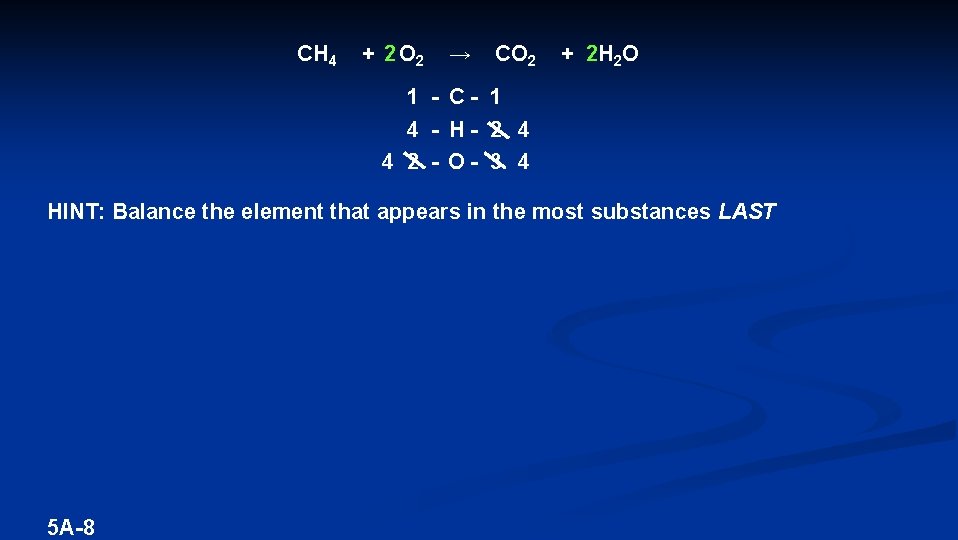
CH 4 + 2 O 2 → CO 2 + 2 H 2 O 1 - C- 1 4 - H- 2 4 4 2 - O- 3 4 HINT: Balance the element that appears in the most substances LAST 5 A-8

2 NH 3 + 2½ O 2 → 2 NO + 3 H 2 O 2 1 - N- 1 2 6 3 - H- 2 6 5 2 - O- 2 4 5 4 NH 3 + 5 O 2 → 4 NO + 6 H 2 O HINT: To remove fractional coefficients, multiply all coefficients by the denominator of the fraction 5 A-9

Fe(NO 3)3 + 3 Na. OH 1 3 3 1 → - Fe - NO 3 - Na - OH Fe(OH)3 - + 3 Na. NO 3 1 1 3 3 HINT: If a polyatomic ion is a reactant AND a product, balance it as a single element 5 A-10

H 3 PO 4 + Li. OH → Li 3 PO 4 + H 2 O HINT: Water can be written as H(OH) to balance the hydrogens and hydroxides separately 5 A-11

H 3 PO 4 + 3 Li. OH OH 3 1 3 1 → Li 3 PO 4 + 3 H(OH) OH - 1 3 - PO 4 - 1 - Li - 3 - OH - 1 3 HINT: Water can be written as H(OH) to balance the hydrogens and hydroxides separately 5 A-12

Physical states of reactants and products can be represented by (s) (l) (g) (aq) solid liquid gas dissolved in water 2 Na (s) + 2 H 2 O (l) → 2 Na. OH (aq) + H 2 (g) 5 A-13

CHAPTER 7 b – CLASSIFICATIONS OF CHEMICAL REACTIONS (A) Oxidation-Reduction Reactions (1) Composition (2) Decomposition (3) Replacement (4) Combustion (B) Exchange Reactions (5) Precipitation (6) Acid-Base 5 A-14

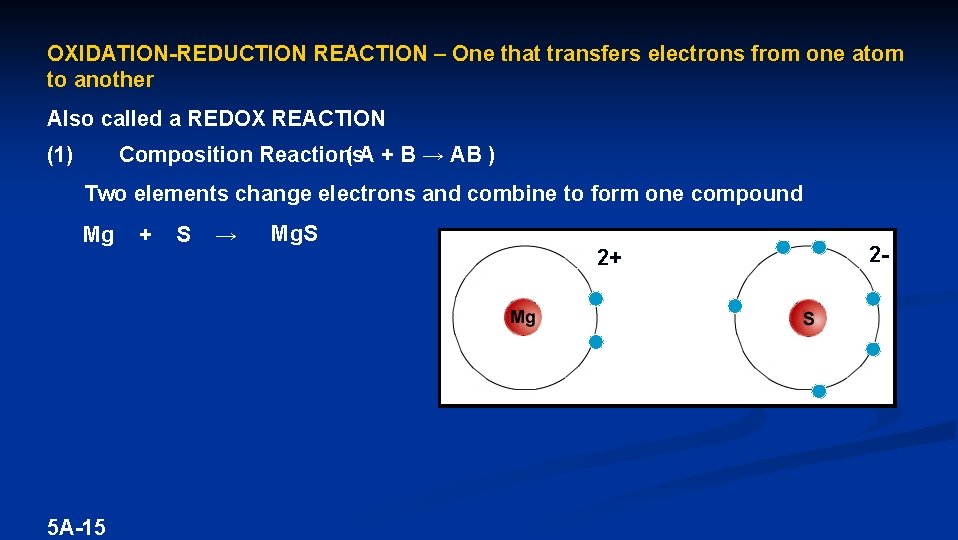
OXIDATION-REDUCTION REACTION – One that transfers electrons from one atom to another Also called a REDOX REACTION ( A + B → AB ) Composition Reactions (1) Two elements change electrons and combine to form one compound Mg 5 A-15 + S → Mg. S 2+ 2 -

Mg → Mg 2+ + 2 e- OXIDATION – Losing electrons 2 e- + S → S 2 - REDUCTION – Gaining electrons Metals and nonmetals react to form ionic compounds by transferring e-s from metal to nonmetal 5 A-16 LEO says GER

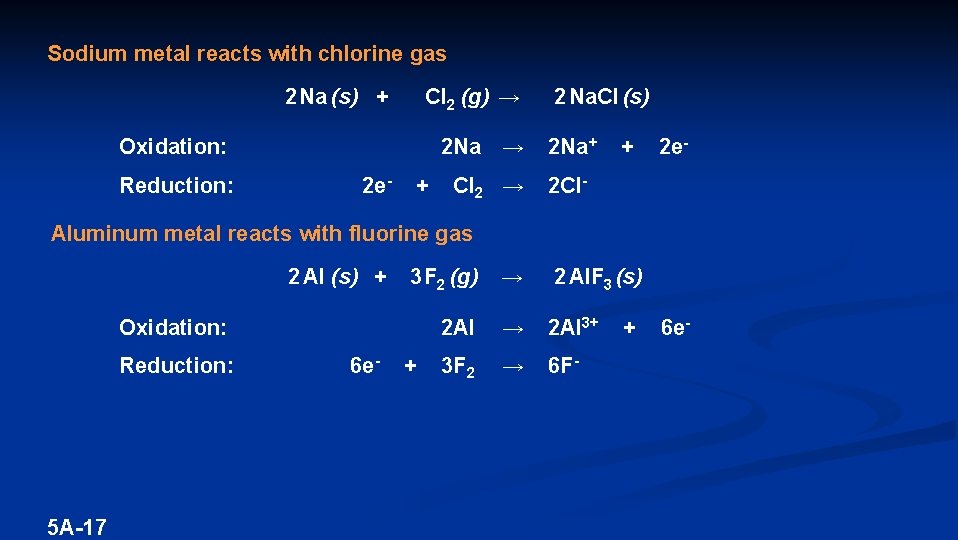
Sodium metal reacts with chlorine gas 2 Na (s) + Cl 2 (g) → Oxidation: Reduction: 2 e- + 2 Na. Cl (s) 2 Na → 2 Na+ Cl 2 → 2 Cl- + 2 e- Aluminum metal reacts with fluorine gas 2 Al (s) + 3 F 2 (g) → 2 Al. F 3 (s) 2 Al → 2 Al 3+ 3 F 2 → 6 F- Oxidation: Reduction: 5 A-17 6 e- + + 6 e-

(2) Decomposition Reactions ( AB → A + B ) One compound breaks down into elements when they exchange electrons Mercury (II) oxide is heated 2 Hg. O (s) → 2 Hg (l) + O 2 (g) Electricity is passed through molten sodium chloride 2 Na. Cl (l) 5 A-18 → 2 Na (s) + Cl 2 (g)

(3) Replacement Reactions ( A + BX → AX + B ) Replacement reactions occur when a more active element (A) changes electrons with a less active ion (B) in a compound (a) Active elemental metals react with less active metal ions Magnesium metal is heated with iron (III) oxide 3 Mg (s) + Fe 2 O 3 (s) → 3 Mg. O (s) + 2 Fe (s) Iron is heated with magnesium oxide Fe 5 A-19 + Mg. O → No Reaction

(b) Active elemental nonmetals react with less active nonmetal ions Chlorine gas is bubbled through a sodium bromide solution Cl 2 (g) + 5 A-20 2 Na. Br (aq) → 2 Na. Cl (aq) + Br 2 (l)

(c) Most metals (except the Noble Metals) will react with the hydrogen ions from acids Zinc metal reacts with hydrochloric acid Zn (s) + 2 HCl (aq) → Zn. Cl 2 (aq) + Copper metal reacts with hydrochloric acid Cu 5 A-21 + HCl → No Reaction H 2 (g)

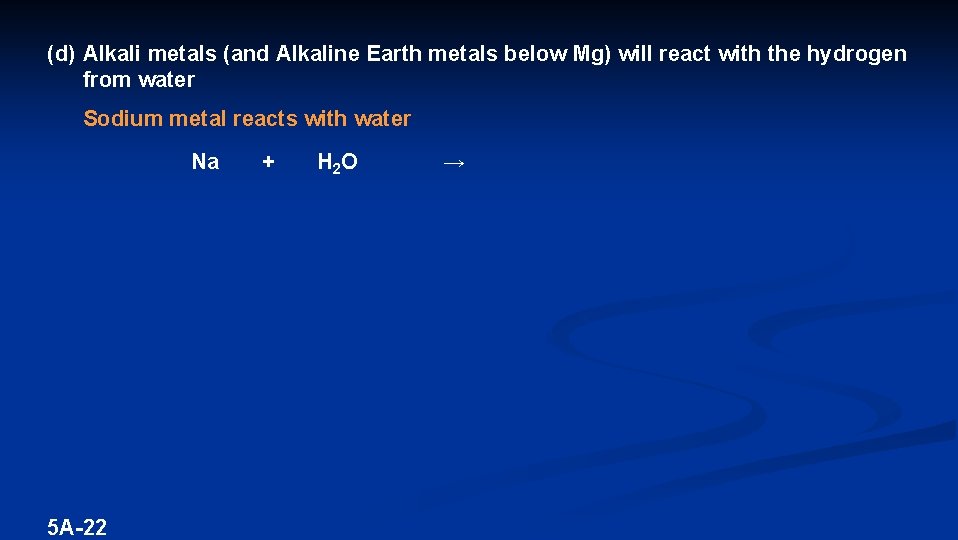
(d) Alkali metals (and Alkaline Earth metals below Mg) will react with the hydrogen from water Sodium metal reacts with water Na 5 A-22 + H 2 O →

(d) Alkali metals (and Alkaline Earth metals below Mg) will react with the hydrogen from water Sodium metal reacts with water 2 Na (s) + 5 A-23 2 H(OH) (l) → 2 Na. OH (aq) + H 2 (g)

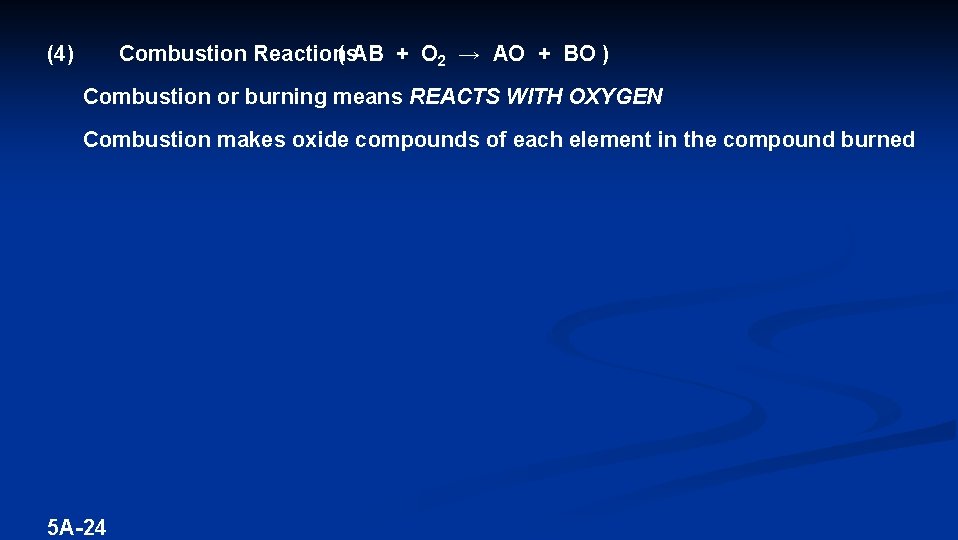
(4) Combustion Reactions ( AB + O 2 → AO + BO ) Combustion or burning means REACTS WITH OXYGEN Combustion makes oxide compounds of each element in the compound burned 5 A-24

Octane (C 8 H 18) burns in air C 8 H 18 + 12½ O 2 2 C 8 H 18 (l) + 5 A-25 → 25 O 2 (g) → 8 CO 2 + 9 H 2 O 16 CO 2 (g) + 18 H 2 O (g)

EXCHANGE REACTION – One in which ions in two compounds rearrange their bonding (5) Precipitation Reactions ( AX + BY → AY + BX ) Ions in solution rearrange to produce an insoluble product PRECIPITATE – A solid formed when two solutions are mixed 5 A-26

Solutions of lead (II) nitrate and sodium iodide are mixed Pb(NO 3)2 (aq) + 5 A-27 2 Na. I (aq) → Pb. I 2 (s) + 2 Na. NO 3 (aq)

(6) Acid-Base Reactions ( HX + AOH → HOH + AX ) Ions in solution rearrange to produce water Solutions of hydrochloric acid and sodium hydroxide are mixed HCl (aq) + Na. OH (aq) → H(OH) (l) + Na. Cl (aq) Solutions of sulfuric acid and potassium hydroxide are mixed H 2 SO 4 (aq) + 5 A-27 2 KOH (aq) → 2 H(OH) (l) + K 2 SO 4 (aq)
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Are kc and kp equal
Are kc and kp equal Chapter 10 chemical reactions
Chapter 10 chemical reactions Chapter 9 chapter assessment chemical reactions
Chapter 9 chapter assessment chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Examples of physical change
Examples of physical change 5 examples of redox reaction
5 examples of redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Chapter 9 chemical reactions
Chapter 9 chemical reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems Chapter 19 chemical reactions answer key
Chapter 19 chemical reactions answer key What is the type of reaction
What is the type of reaction What are active metals
What are active metals Is mashing potatoes a physical or chemical change
Is mashing potatoes a physical or chemical change Whats the difference between chemical and physical change
Whats the difference between chemical and physical change Physical vs chemical change examples
Physical vs chemical change examples Spare change physical versus chemical change
Spare change physical versus chemical change Whats the difference between chemical and physical change
Whats the difference between chemical and physical change When does a physical change occur study jams
When does a physical change occur study jams Study jams physical and chemical changes
Study jams physical and chemical changes How is chopping wood a physical change
How is chopping wood a physical change Lesson 90 solid evidence precipitation reactions
Lesson 90 solid evidence precipitation reactions