Chapter 6 CHEMICAL KINETICS 6 1 Reaction Rates


![A B time D[A] rate = Dt D[B] rate = Dt A B time D[A] rate = Dt D[B] rate = Dt](https://slidetodoc.com/presentation_image_h2/1b74ac65eca5137130d1992e95a1ed12/image-6.jpg)








- Slides: 36

Chapter 6 CHEMICAL KINETICS

6. 1 Reaction Rates

Key Terms • Chemical kinetics • Reaction rate • Average reaction rate • Instantaneous reaction rate

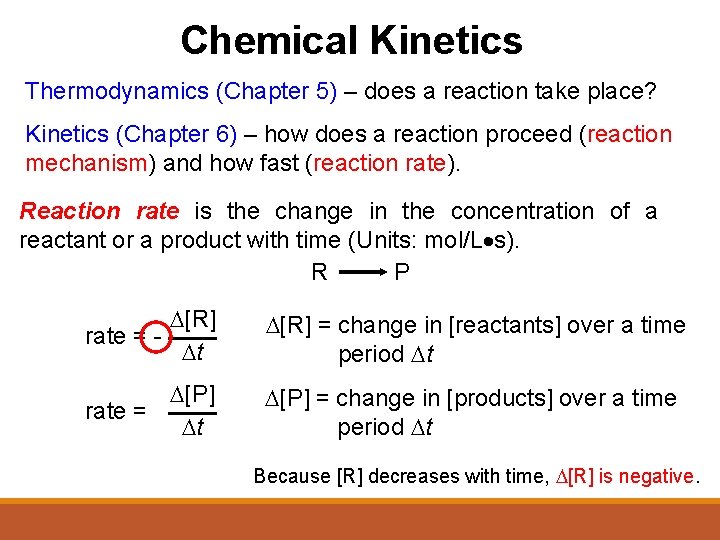
Chemical Kinetics Thermodynamics (Chapter 5) – does a reaction take place? Kinetics (Chapter 6) – how does a reaction proceed (reaction mechanism) and how fast (reaction rate). Reaction rate is the change in the concentration of a reactant or a product with time (Units: mol/L s). R P D[R] rate = Dt D[R] = change in [reactants] over a time period Dt D[P] rate = Dt D[P] = change in [products] over a time period Dt Because [R] decreases with time, D[R] is negative.

Describing Reaction Rates v. Reaction rate (r) is determined by measuring the rate at which a product is formed or the rate at which a reactant is consumed over a series of time intervals vproperties like mass, colour, conductivity, volume, pressure or concentration may be measured to determine the reaction rate vreaction rate is expressed mathematically in terms of a change in property of reactant or product per unit time
![A B time DA rate Dt DB rate Dt A B time D[A] rate = Dt D[B] rate = Dt](https://slidetodoc.com/presentation_image_h2/1b74ac65eca5137130d1992e95a1ed12/image-6.jpg)
A B time D[A] rate = Dt D[B] rate = Dt

TASK 1 • Copy down the example from the board. (Sample Problem 1 and 2) • Do Practice Problem 1 on page 350

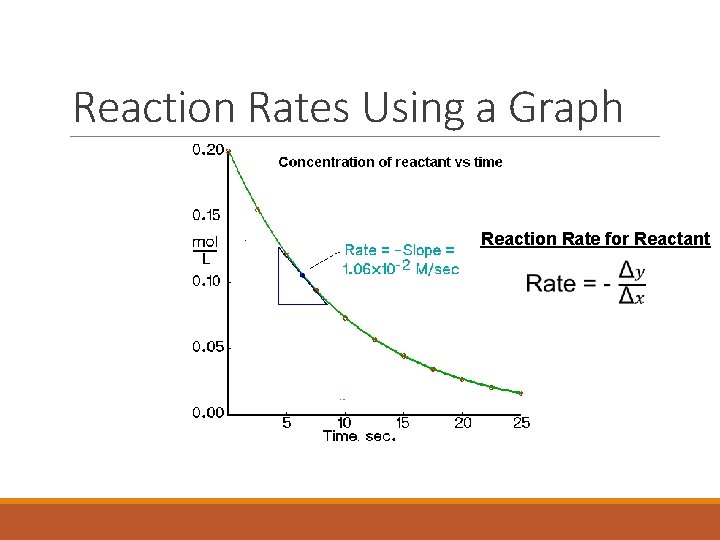
Reaction Rates Using a Graph Reaction Rate for Reactant

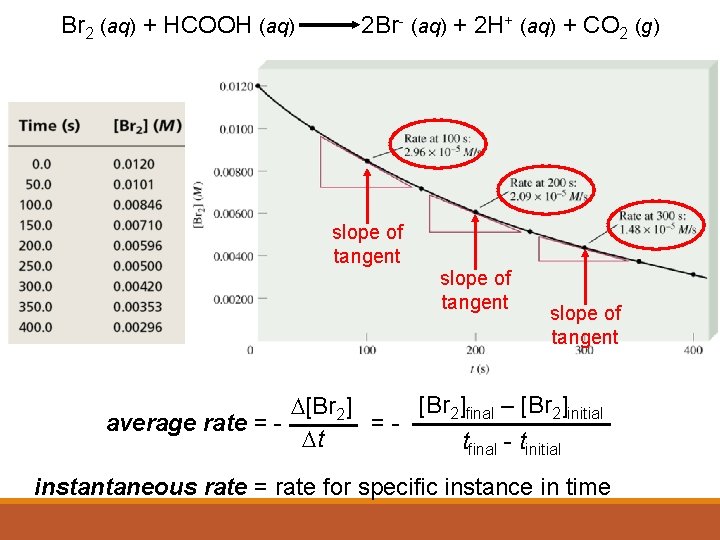
Br 2 (aq) + HCOOH (aq) 2 Br- (aq) + 2 H+ (aq) + CO 2 (g) slope of tangent [Br 2]final – [Br 2]initial D[Br 2] average rate = =Dt tfinal - tinitial instantaneous rate = rate for specific instance in time

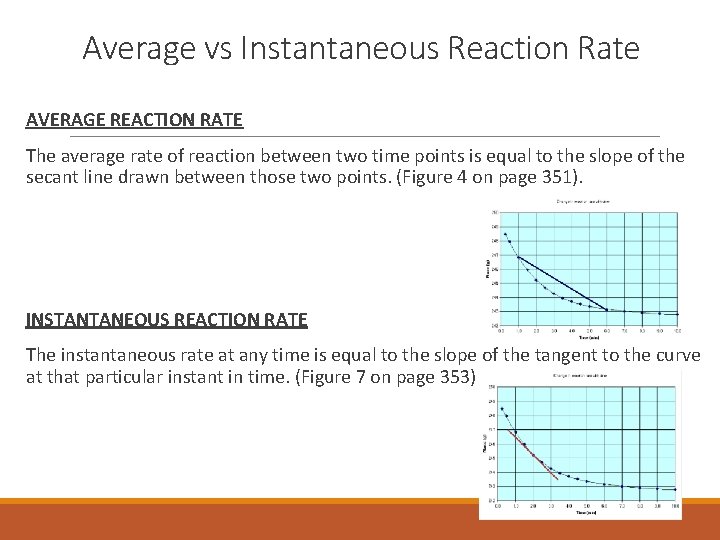
Average vs Instantaneous Reaction Rate AVERAGE REACTION RATE The average rate of reaction between two time points is equal to the slope of the secant line drawn between those two points. (Figure 4 on page 351). INSTANTANEOUS REACTION RATE The instantaneous rate at any time is equal to the slope of the tangent to the curve at that particular instant in time. (Figure 7 on page 353)

TASK 2 • Copy down the sample problems frompage 352 • Do practice problem 1 on page 352 • Do sample problem 1 and 2 on page 354 • Do Practice Problem 1 and 2 on page 356

6. 2 and 6. 3 Factors Affecting Reaction Rates

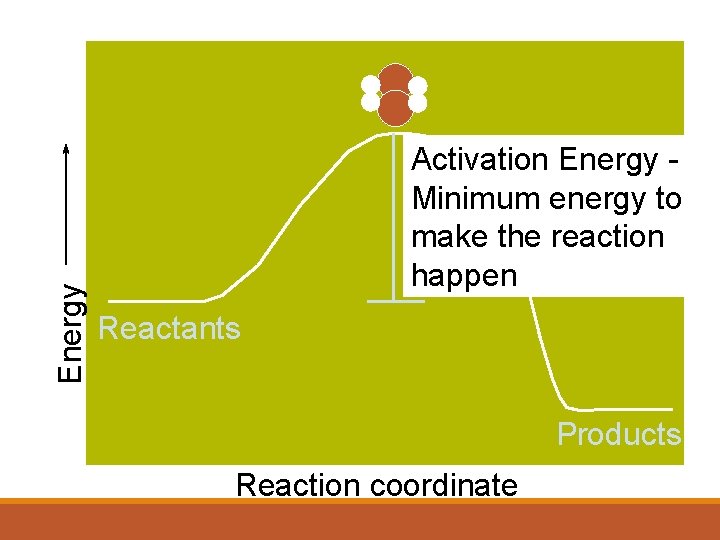
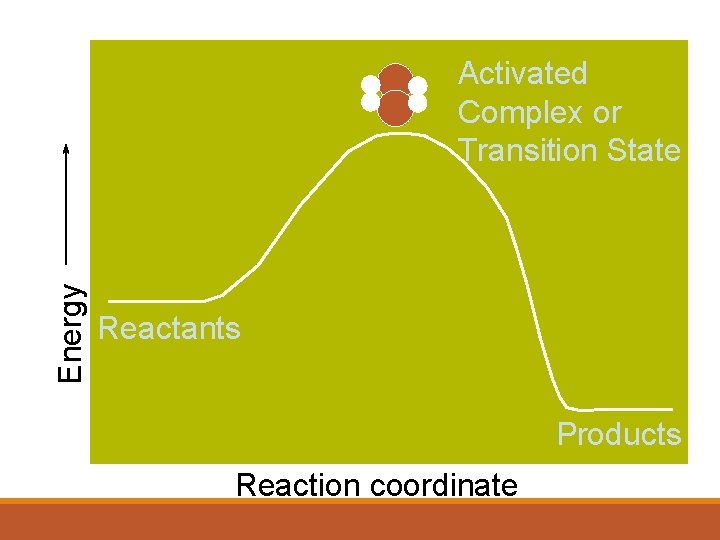
Collision Theory tells us that in order to react, molecules and atoms must touch each other. They must hit each other hard enough to react. Anything that increase these things will make the reaction faster. Activation Energy is the minimum energy that reactant molecules must possess for a reaction to be successful.

Activation Energy 1 Defined as: The minimum energy required to bring about a chemical reaction. If there were no such thing as ‘activation energy’ life would be very difficult: Gasoline for your car would ignite as soon as it came into contact with air. You would burst into flames. Trees would spontaneously combust. Activation energy is why these things do not happen, there is an energy barrier so most reactions need to be ‘started off’ by putting in some energy.

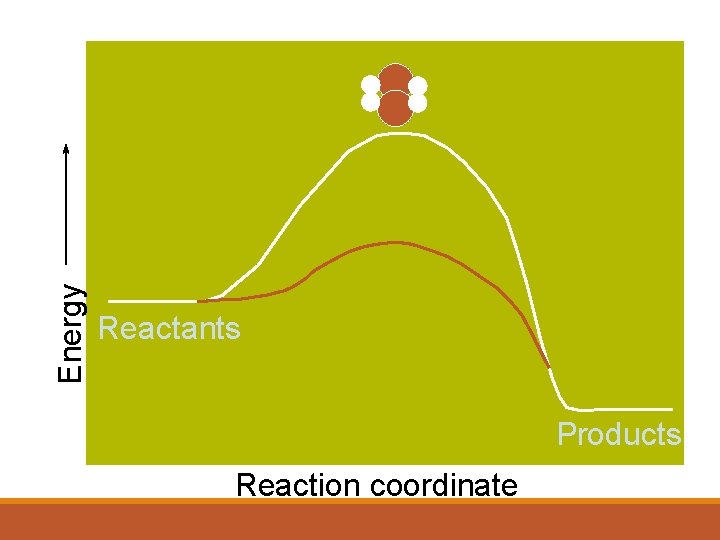
Energy Reactants Products Reaction coordinate

Energy Activation Energy Minimum energy to make the reaction happen Reactants Products Reaction coordinate

Energy Activated Complex or Transition State Reactants Products Reaction coordinate

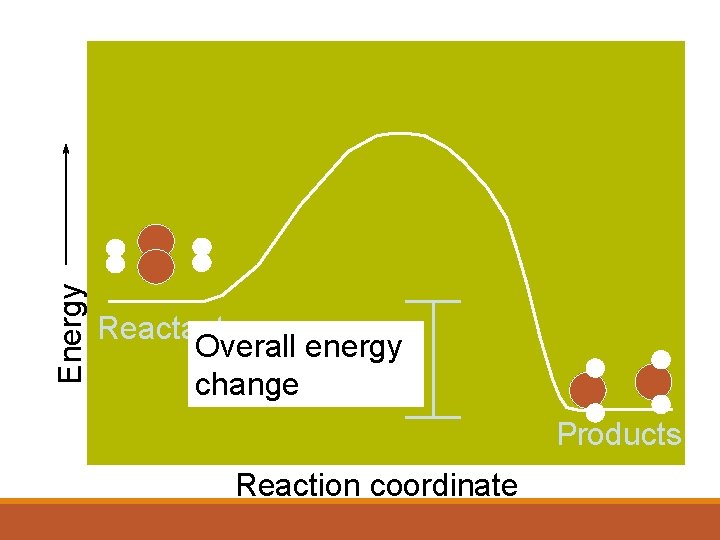
Energy Reactants Overall energy change Products Reaction coordinate

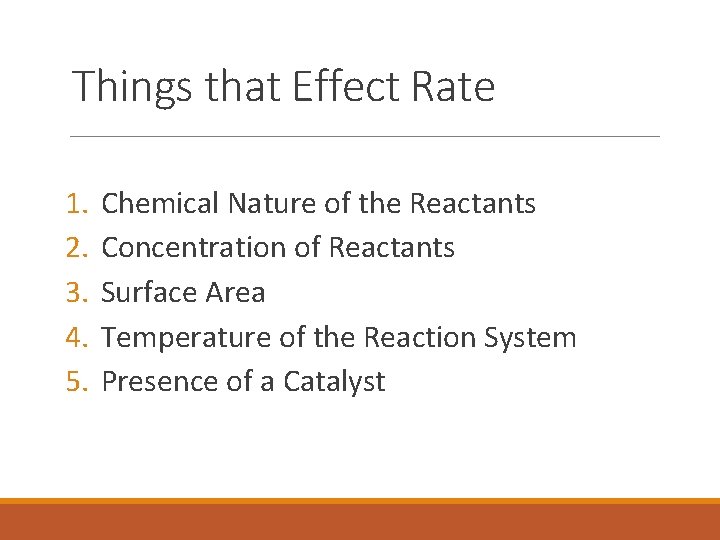
Things that Effect Rate 1. 2. 3. 4. 5. Chemical Nature of the Reactants Concentration of Reactants Surface Area Temperature of the Reaction System Presence of a Catalyst

1. Chemical Nature of the Reactants

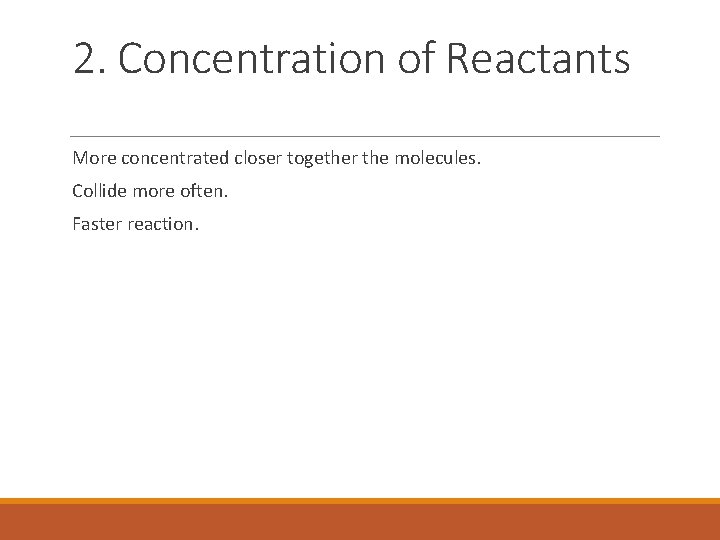
2. Concentration of Reactants More concentrated closer together the molecules. Collide more often. Faster reaction.

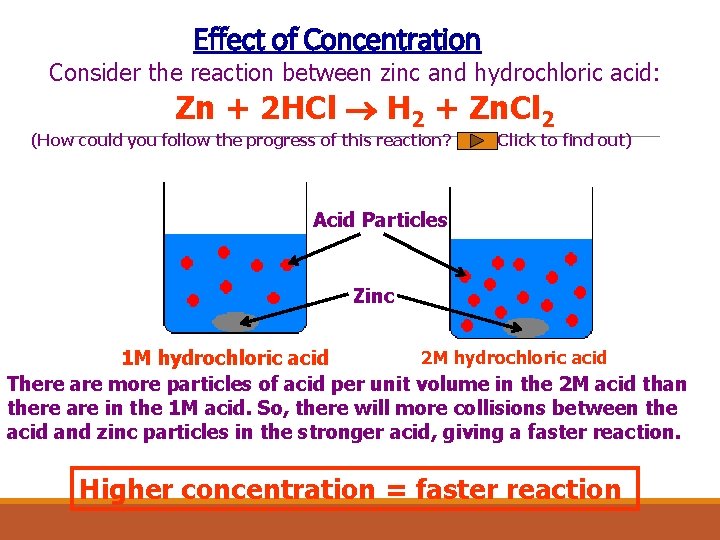
Effect of Concentration Consider the reaction between zinc and hydrochloric acid: Zn + 2 HCl H 2 + Zn. Cl 2 (How could you follow the progress of this reaction? Click to find out) Acid Particles Zinc 2 M hydrochloric acid 1 M hydrochloric acid There are more particles of acid per unit volume in the 2 M acid than there are in the 1 M acid. So, there will more collisions between the acid and zinc particles in the stronger acid, giving a faster reaction. Higher concentration = faster reaction

3. Surface Area Particle size Molecules can only collide at the surface. Smaller particles bigger surface area. Smaller particles faster reaction. Smallest possible is molecules or ions. Dissolving speeds up reactions. Getting two solids to react with each other is slow.

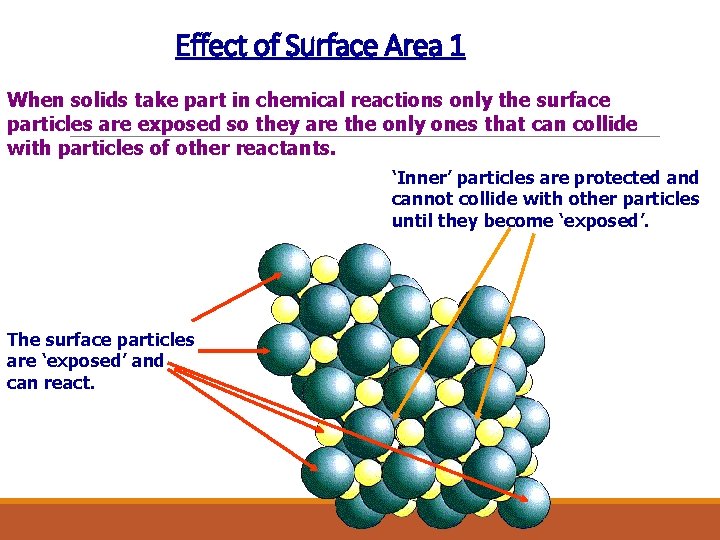
Effect of Surface Area 1 When solids take part in chemical reactions only the surface particles are exposed so they are the only ones that can collide with particles of other reactants. ‘Inner’ particles are protected and cannot collide with other particles until they become ‘exposed’. The surface particles are ‘exposed’ and can react.

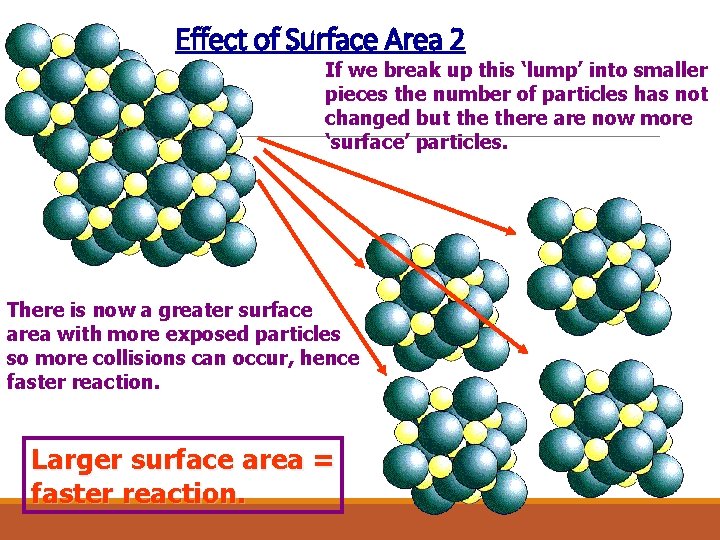
Effect of Surface Area 2 If we break up this ‘lump’ into smaller pieces the number of particles has not changed but there are now more ‘surface’ particles. There is now a greater surface area with more exposed particles so more collisions can occur, hence faster reaction. Larger surface area = faster reaction.

4. Temperature of the Reaction System Higher temperature faster particles. More and harder collisions. Faster Reactions.

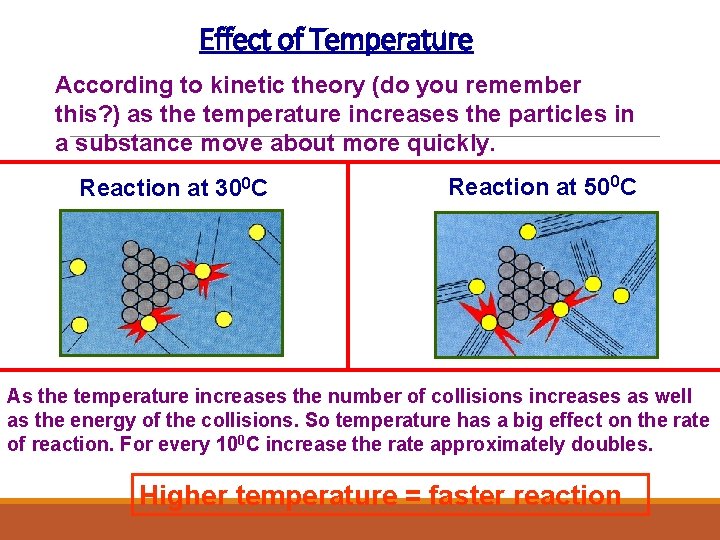
Effect of Temperature According to kinetic theory (do you remember this? ) as the temperature increases the particles in a substance move about more quickly. Reaction at 300 C Reaction at 500 C As the temperature increases the number of collisions increases as well as the energy of the collisions. So temperature has a big effect on the rate of reaction. For every 100 C increase the rate approximately doubles. Higher temperature = faster reaction

5. Presence of a Catalysts- substances that speed up a reaction without being used up. Speeds up reaction by giving the reaction a new path. The new path has a lower activation energy. More molecules have this energy. The reaction goes faster. Inhibitor- a substance that blocks a catalyst.

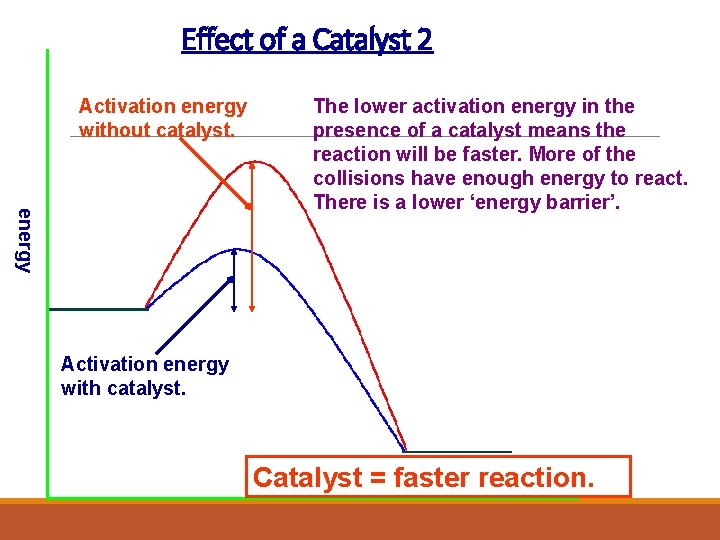
Effect of a Catalyst 2 Activation energy without catalyst. energy The lower activation energy in the presence of a catalyst means the reaction will be faster. More of the collisions have enough energy to react. There is a lower ‘energy barrier’. Activation energy with catalyst. Catalyst = faster reaction.

Energy Reactants Products Reaction coordinate

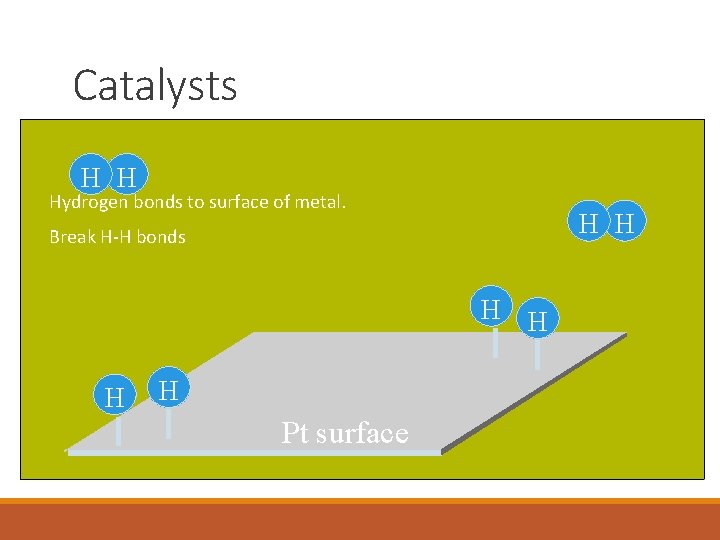
Catalysts H H Hydrogen bonds to surface of metal. H H Break H-H bonds H H Pt surface

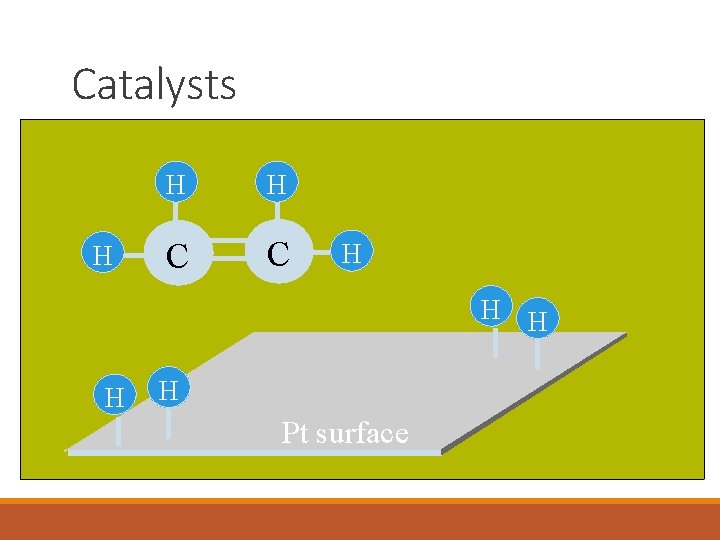
Catalysts H H H C C H H H Pt surface

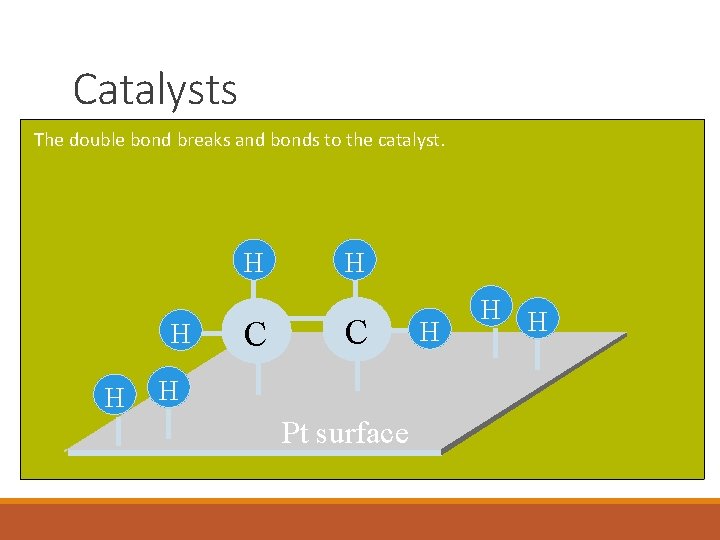
Catalysts The double bond breaks and bonds to the catalyst. H H H C H Pt surface H H H

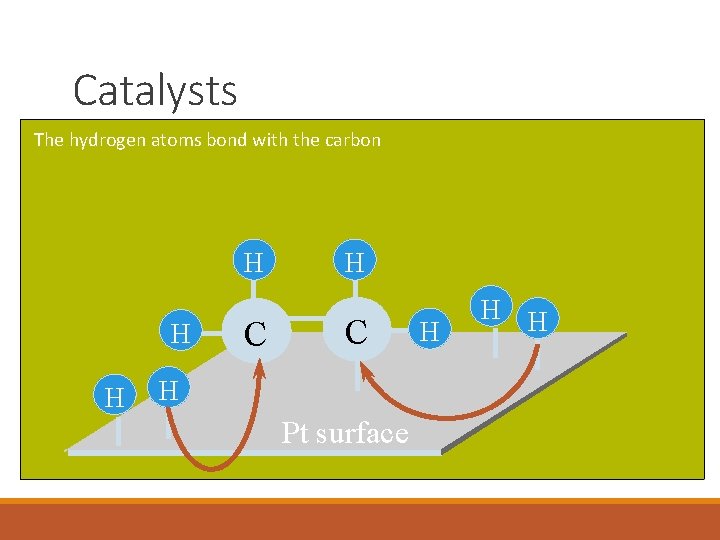
Catalysts The hydrogen atoms bond with the carbon H H H C H Pt surface H H H

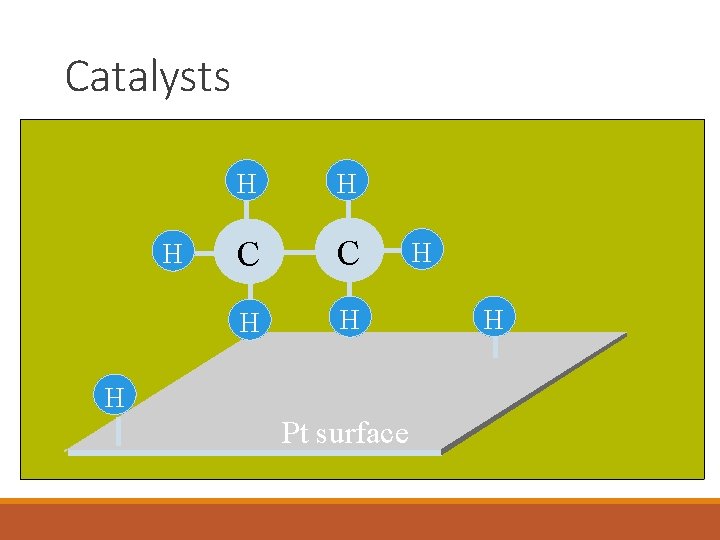
Catalysts H H H C C H H H Pt surface H H

Summary 1. Increasing the surface area gives a faster reaction because more particles are ‘exposed’ to the other reactant. 2. Increasing the concentration increases the rate of reaction because there are more collisions between the reactant particles. 3. Increasing the temperature increases the rate of reaction because the particles move quickly and so collide more often and with greater energy. 4. A catalyst increases the rate of a reaction because it reduces the activation energy so more of the collisions have enough energy to react.