Chapter 6 Chemical Bonds Terms Molecule a neutral

Chapter 6 Chemical Bonds
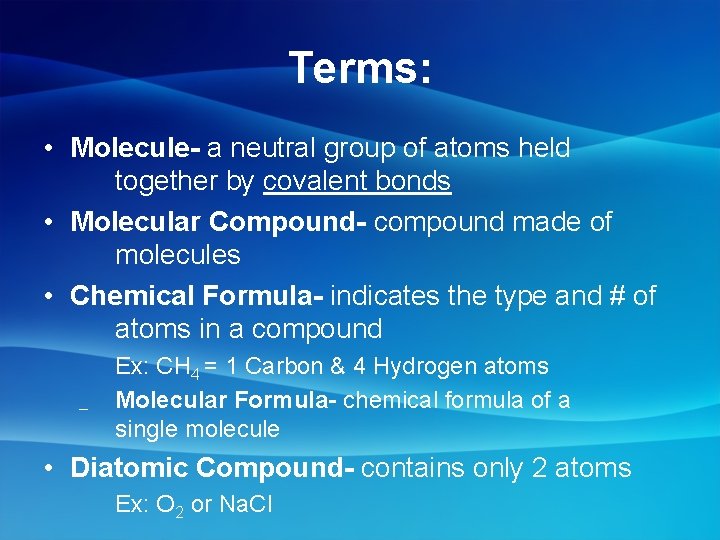
Terms: • Molecule- a neutral group of atoms held together by covalent bonds • Molecular Compound- compound made of molecules • Chemical Formula- indicates the type and # of atoms in a compound – Ex: CH 4 = 1 Carbon & 4 Hydrogen atoms Molecular Formula- chemical formula of a single molecule • Diatomic Compound- contains only 2 atoms Ex: O 2 or Na. Cl
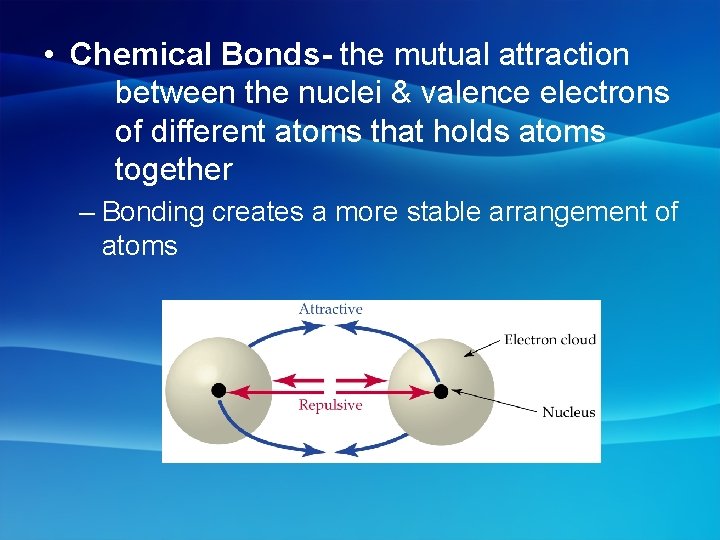
• Chemical Bonds- the mutual attraction between the nuclei & valence electrons of different atoms that holds atoms together – Bonding creates a more stable arrangement of atoms

Ionic Bonds- result from the electrical attraction between cations (+) and anions (-) *Electronegativity of Ionic Bonds: ionic bonds form when the electronegativity difference between the atoms is 1. 8 or more Ex: Na + Cl Na. Cl Na = 0. 9 Difference = 2. 1 = ionic bond Cl = 3. 0
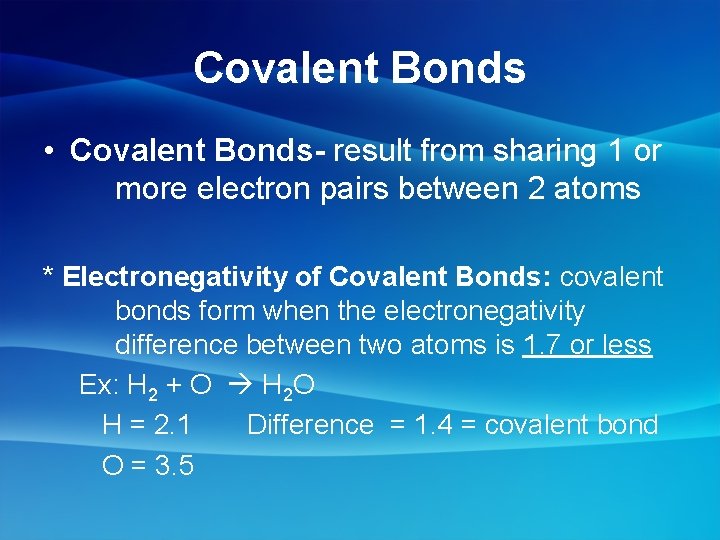
Covalent Bonds • Covalent Bonds- result from sharing 1 or more electron pairs between 2 atoms * Electronegativity of Covalent Bonds: covalent bonds form when the electronegativity difference between two atoms is 1. 7 or less Ex: H 2 + O H 2 O H = 2. 1 Difference = 1. 4 = covalent bond O = 3. 5
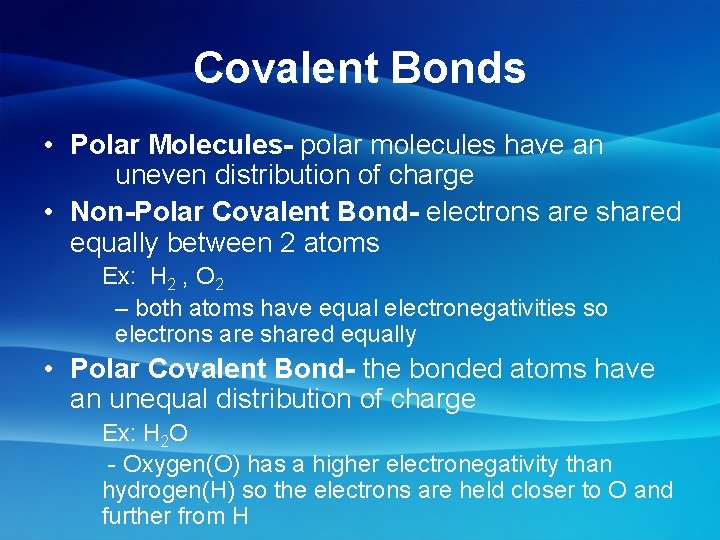
Covalent Bonds • Polar Molecules- polar molecules have an uneven distribution of charge • Non-Polar Covalent Bond- electrons are shared equally between 2 atoms Ex: H 2 , O 2 – both atoms have equal electronegativities so electrons are shared equally • Polar Covalent Bond- the bonded atoms have an unequal distribution of charge Ex: H 2 O - Oxygen(O) has a higher electronegativity than hydrogen(H) so the electrons are held closer to O and further from H
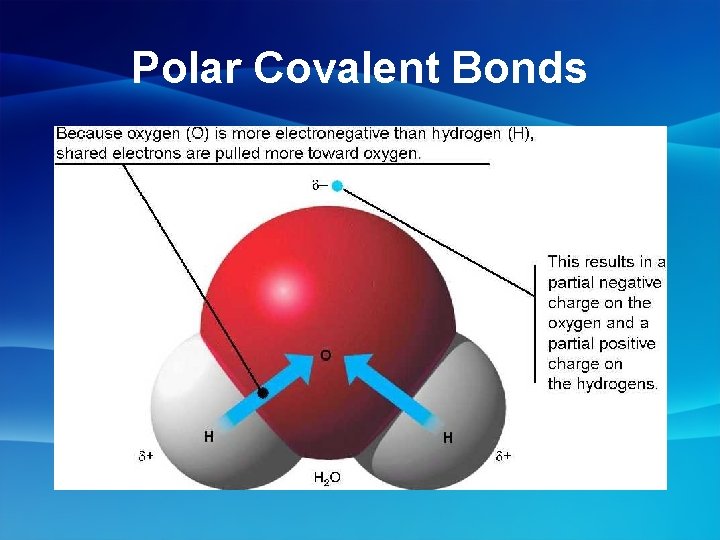
Polar Covalent Bonds

Bond Properties • Bond Length- distance between the nuclei of two bonded atoms • Bond Energy- The energy required to break a chemical bond Single bond = long, low energy Double bond = med length, med energy Triple bond = short, high energy * Increase bond length, Decrease bond strength
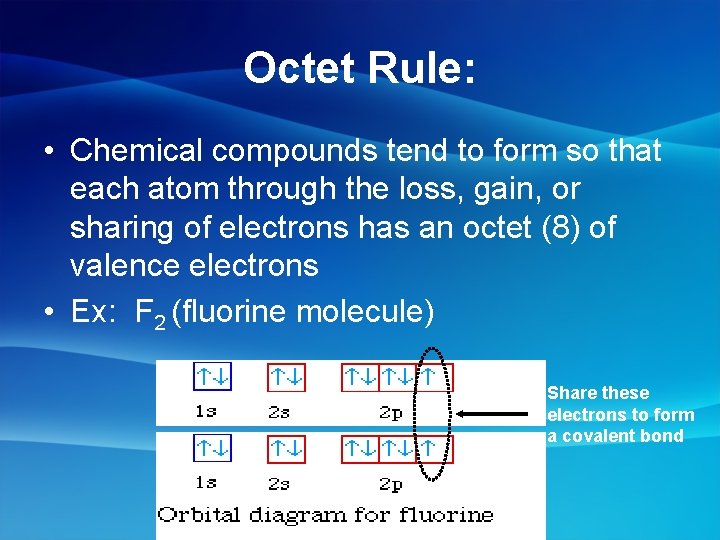
Octet Rule: • Chemical compounds tend to form so that each atom through the loss, gain, or sharing of electrons has an octet (8) of valence electrons • Ex: F 2 (fluorine molecule) Share these electrons to form a covalent bond
Exceptions to the Octet Rule: • Some elements are stable with less than 8 valence electrons – H and He are full with 2 electrons because they only have a 1 s orbital – Boron is usually surrounded with 6 electrons • BF 3 – Expanded Valence: some elements such as sulfur (S) and phosphorus (P) may exist with more than 8 valence electrons (involve d orbital)
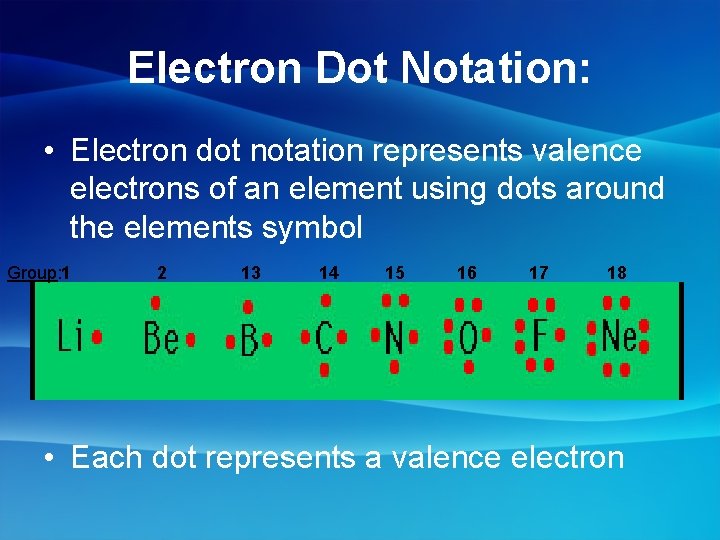
Electron Dot Notation: • Electron dot notation represents valence electrons of an element using dots around the elements symbol Group: 1 2 13 14 15 16 17 18 • Each dot represents a valence electron
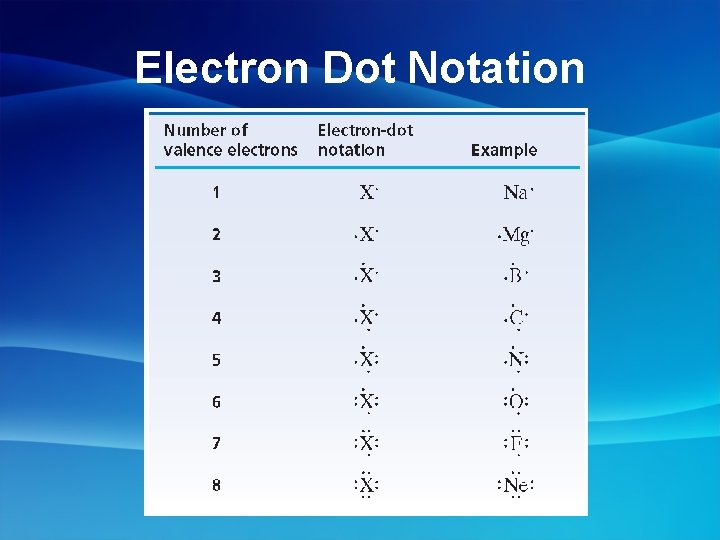
Electron Dot Notation
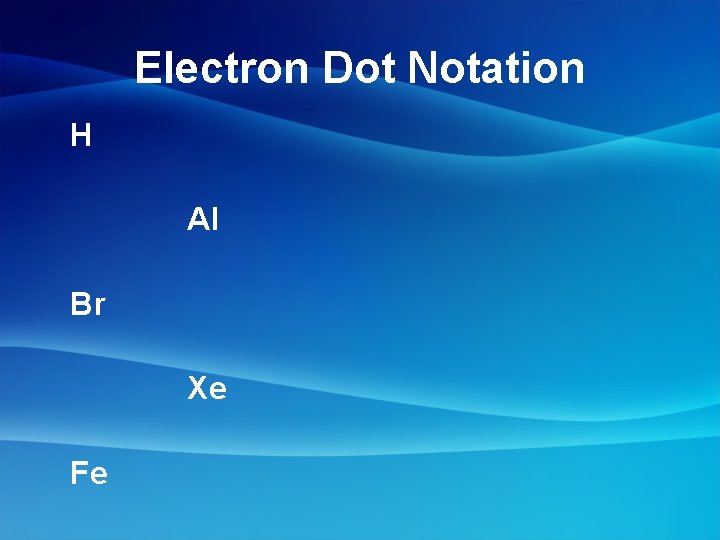
Electron Dot Notation H Al Br Xe Fe
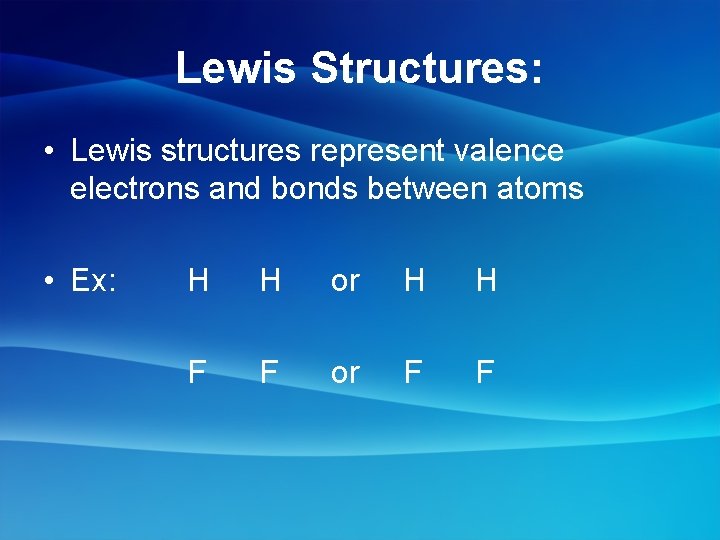
Lewis Structures: • Lewis structures represent valence electrons and bonds between atoms • Ex: H H or H H F F or F F
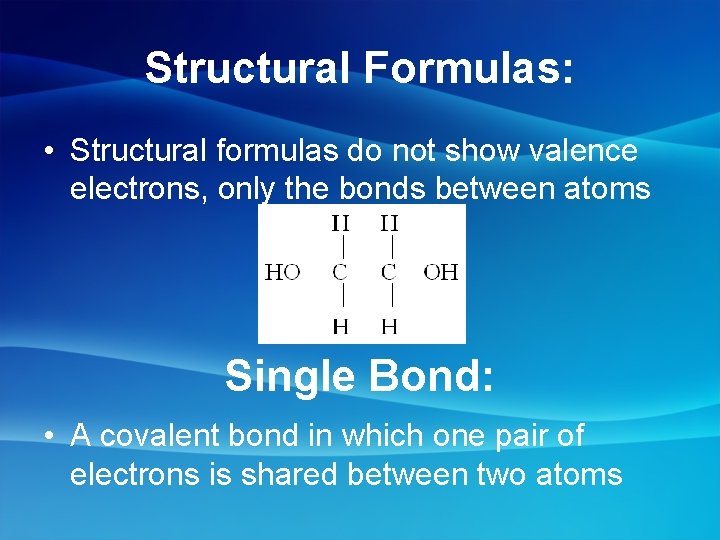
Structural Formulas: • Structural formulas do not show valence electrons, only the bonds between atoms Single Bond: • A covalent bond in which one pair of electrons is shared between two atoms
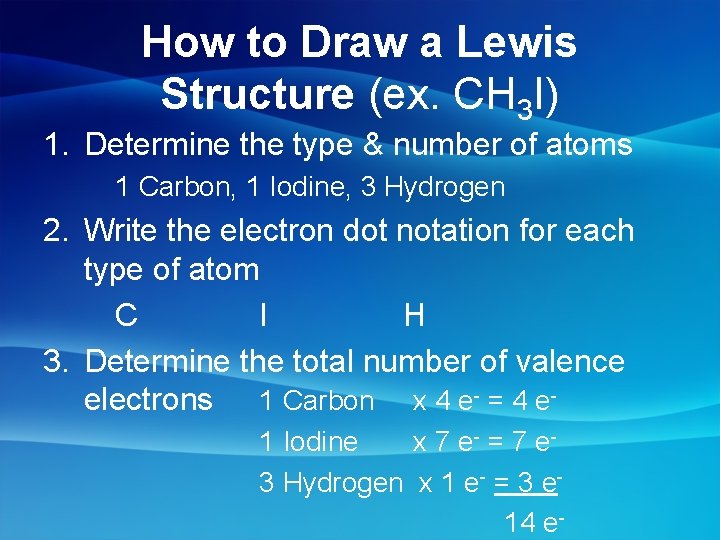
How to Draw a Lewis Structure (ex. CH 3 I) 1. Determine the type & number of atoms 1 Carbon, 1 Iodine, 3 Hydrogen 2. Write the electron dot notation for each type of atom C I H 3. Determine the total number of valence electrons 1 Carbon x 4 e- = 4 e 1 Iodine x 7 e - = 7 e 3 Hydrogen x 1 e- = 3 e 14 e-
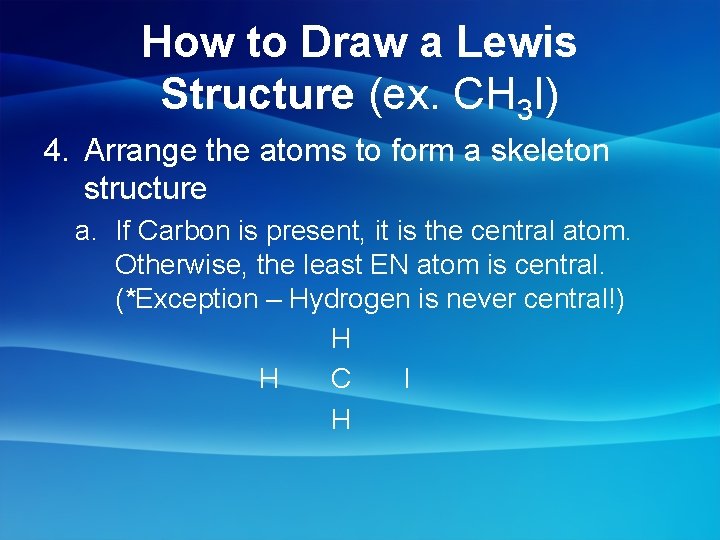
How to Draw a Lewis Structure (ex. CH 3 I) 4. Arrange the atoms to form a skeleton structure a. If Carbon is present, it is the central atom. Otherwise, the least EN atom is central. (*Exception – Hydrogen is never central!) H H C I H
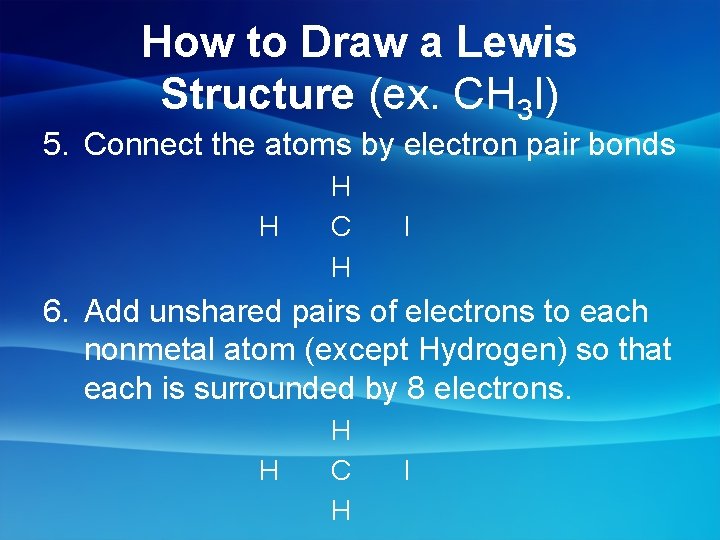
How to Draw a Lewis Structure (ex. CH 3 I) 5. Connect the atoms by electron pair bonds H H C H I 6. Add unshared pairs of electrons to each nonmetal atom (except Hydrogen) so that each is surrounded by 8 electrons. H H C H I

How to Draw a Lewis Structure (ex. CH 3 I) 7. Count the electrons in the structure to be sure the number of valence electrons used = the number of available electrons (from step 3) a. Be sure the central atom and other atoms (except H) have an octet!

How to Draw a Lewis Structure - Example NH 3
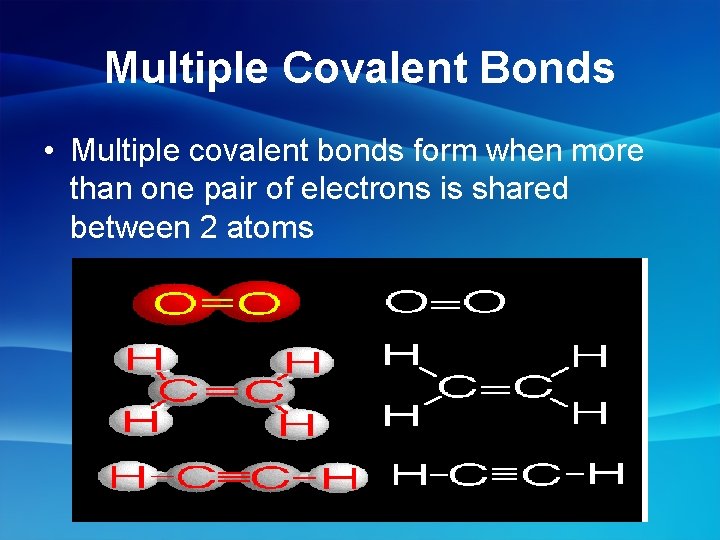
Multiple Covalent Bonds • Multiple covalent bonds form when more than one pair of electrons is shared between 2 atoms
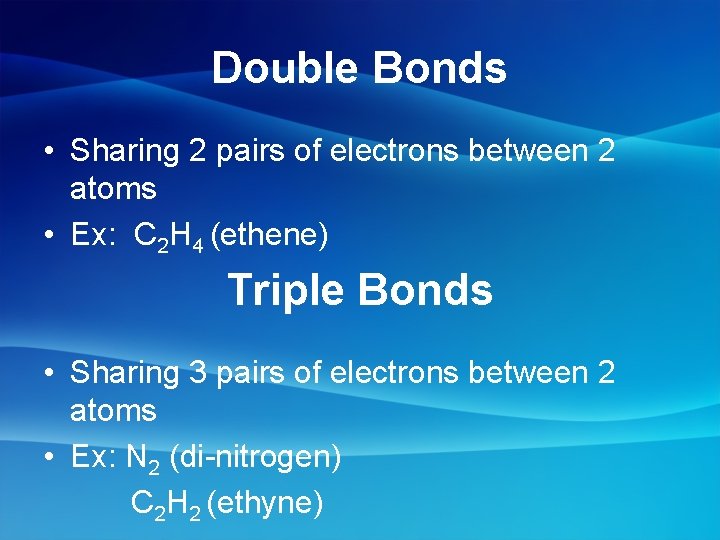
Double Bonds • Sharing 2 pairs of electrons between 2 atoms • Ex: C 2 H 4 (ethene) Triple Bonds • Sharing 3 pairs of electrons between 2 atoms • Ex: N 2 (di-nitrogen) C 2 H 2 (ethyne)
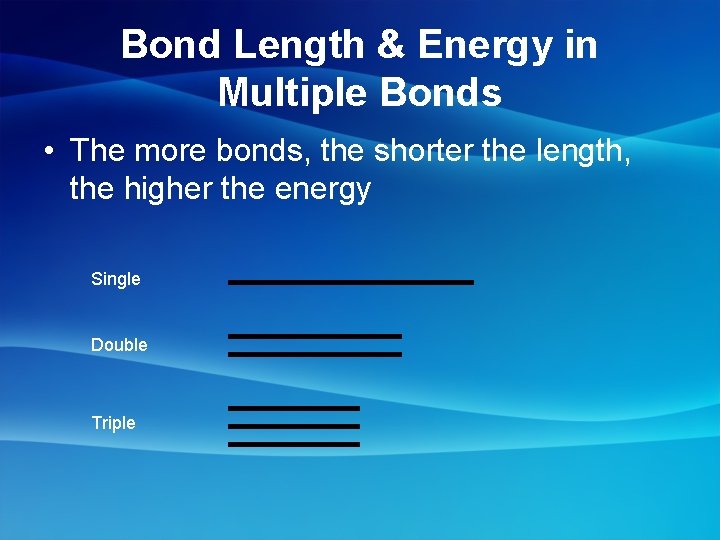
Bond Length & Energy in Multiple Bonds • The more bonds, the shorter the length, the higher the energy Single Double Triple

How to Draw a Lewis Structure for Multiple Bonds – • Steps 1 -7 are the same. • If you get to step 7 and there are too many valence electrons… 8. Subtract lone pairs and move them to become multiple bonds and fill the shells.

How to Draw a Lewis Structure for Multiple Bonds – Example: CH 2 O

Resonance Structures(Hybrids) • Resonance structures have 2 or more acceptable Lewis Structures • Ex: O 3 (ozone)

Ionic Bonding & Ionic Compounds • Ionic Compound- a compound composed of cations (+) and anions (-) with an overall neutral charge • Ex: Na. Cl (sodium chloride) Ca. F 2 (calcium fluoride)
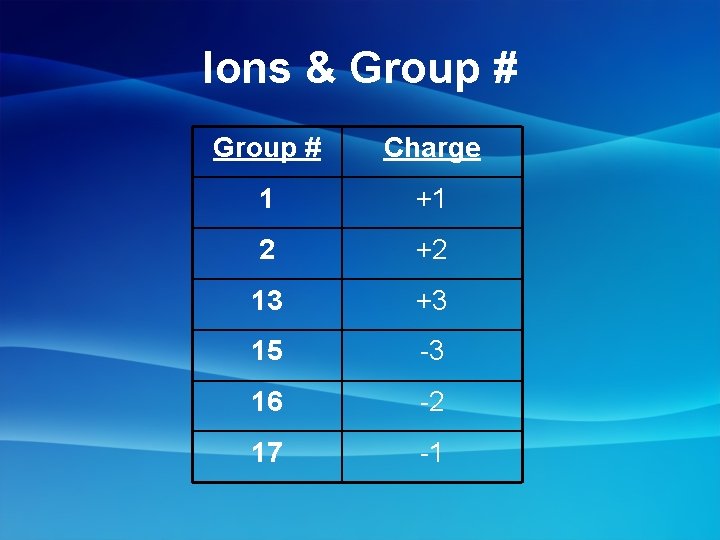
Ions & Group # Charge 1 +1 2 +2 13 +3 15 -3 16 -2 17 -1

Formula Unit • A formula unit is the simplest form of an ionic compound • Written with the lowest possible whole # ratio between the ions • Ex: Mg 3 Br 6 Mg. Br 2
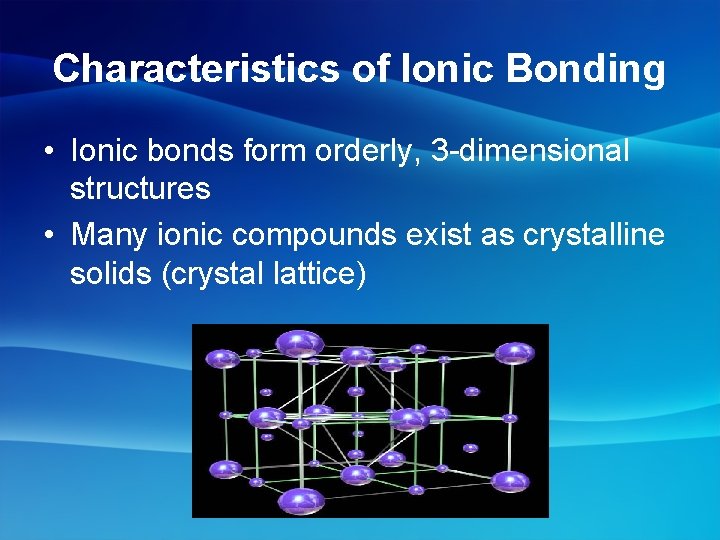
Characteristics of Ionic Bonding • Ionic bonds form orderly, 3 -dimensional structures • Many ionic compounds exist as crystalline solids (crystal lattice)
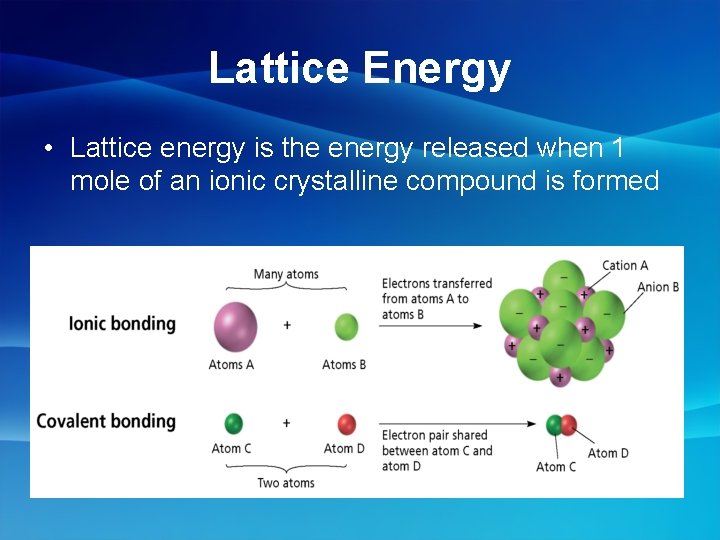
Lattice Energy • Lattice energy is the energy released when 1 mole of an ionic crystalline compound is formed
Molecular Forces vs. Atomic Forces • Ionic and Covalent Bonds are very strong (forces between atoms) • Molecular Bonds are weak between molecules) (forces • Solubility- ionic compounds are soluble in water (dissolve in water)

Polyatomic Ions • Polyatomic Ions are charged groups of atoms held together by covalent bonds • These molecules have an overall positive(+) or negative(-) charge • Ex: NH 4+ (ammonium)

Metallic Bonding • Metallic bonding results from the attraction metal atoms and the surrounding “sea of electrons” • Sea of Electrons- mobile electrons roaming freely within a metal – Electrons can pass from metal atom to metal through overlapping orbitals

Properties of Metals • Malleability- ability of a metal to bend without breaking • Ductility- ability to be drawn into a fine wire • Luster- shiny

Metallic Bond Strength • Metallic Bond Strength = heat of vaporization • Amount of heat energy required to vaporize a metal (solid gas) • Metallic bonds are weaker than ionic & covalent bonds
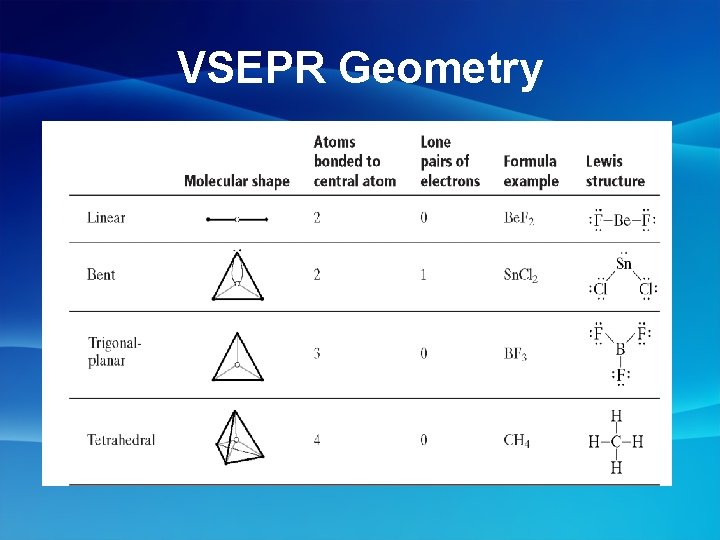
Molecular Geometry • The 3 D shapes of molecules are determined by the atoms and electrons within the molecule VSEPR Theory- Valence Shell Electron Pair Repulsion Theory - the VSEPR theory states that atoms will space themselves as far apart from one another when bonds are formed
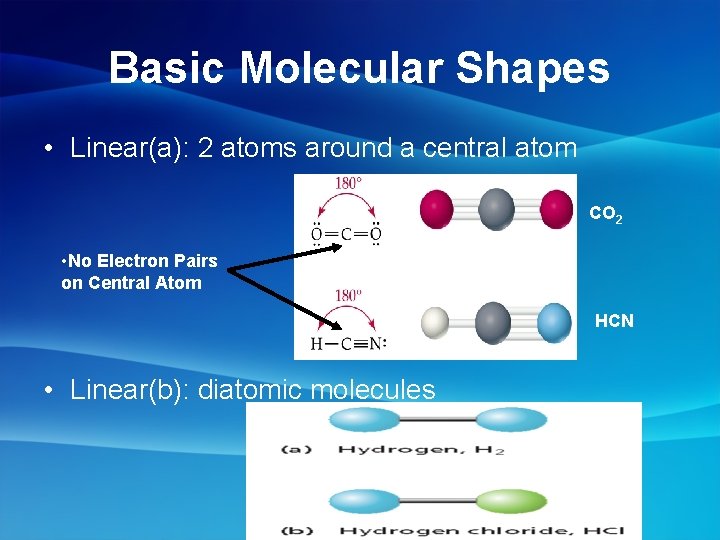
Basic Molecular Shapes • Linear(a): 2 atoms around a central atom CO 2 • No Electron Pairs on Central Atom HCN • Linear(b): diatomic molecules
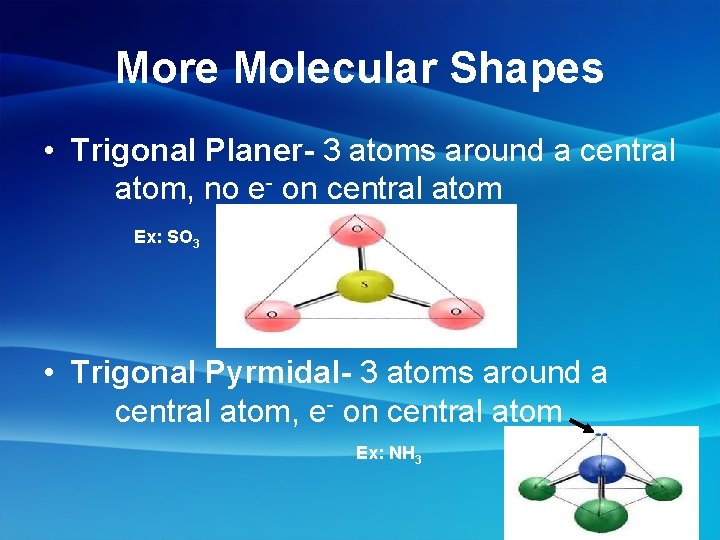
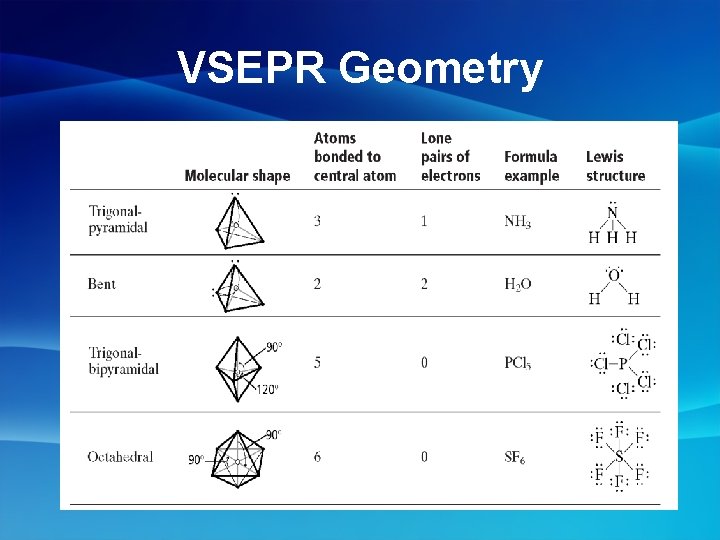
More Molecular Shapes • Trigonal Planer- 3 atoms around a central atom, no e- on central atom Ex: SO 3 • Trigonal Pyrmidal- 3 atoms around a central atom, e- on central atom Ex: NH 3
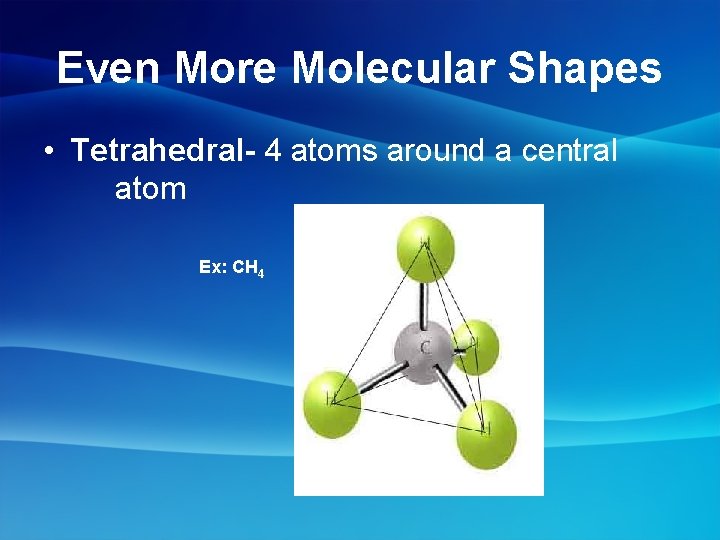
Even More Molecular Shapes • Tetrahedral- 4 atoms around a central atom Ex: CH 4
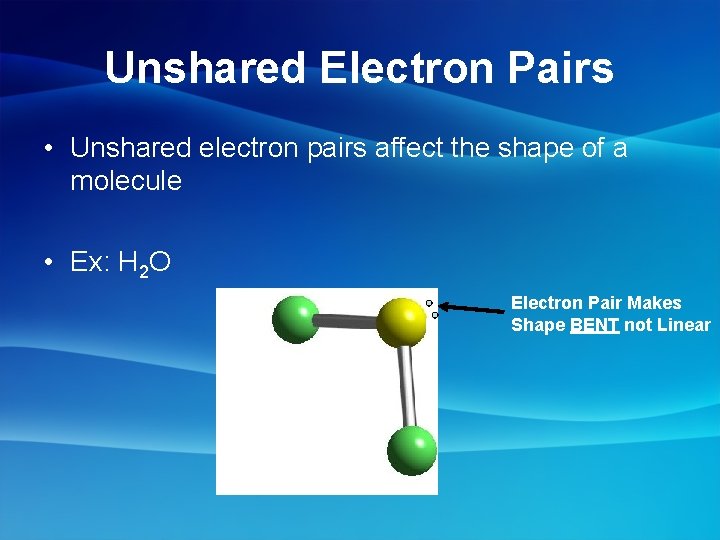
Unshared Electron Pairs • Unshared electron pairs affect the shape of a molecule • Ex: H 2 O Electron Pair Makes Shape BENT not Linear
VSEPR Geometry
VSEPR Geometry
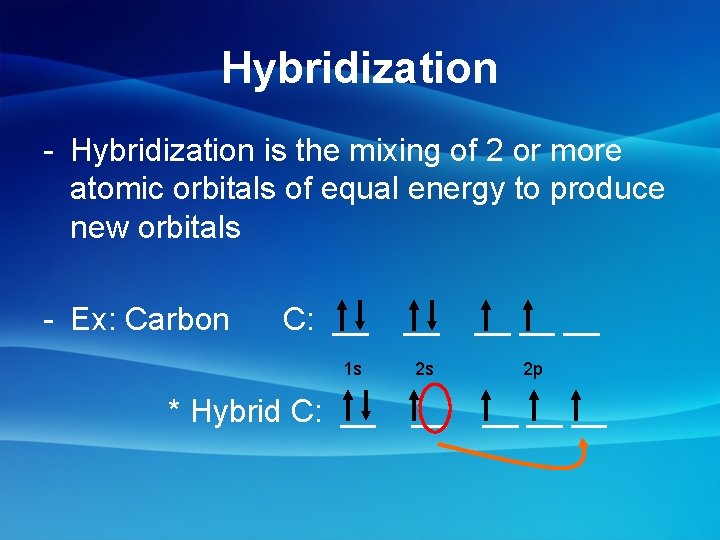
Hybridization - Hybridization is the mixing of 2 or more atomic orbitals of equal energy to produce new orbitals - Ex: Carbon C: __ 1 s * Hybrid C: __ __ __ 2 s 2 p __ __
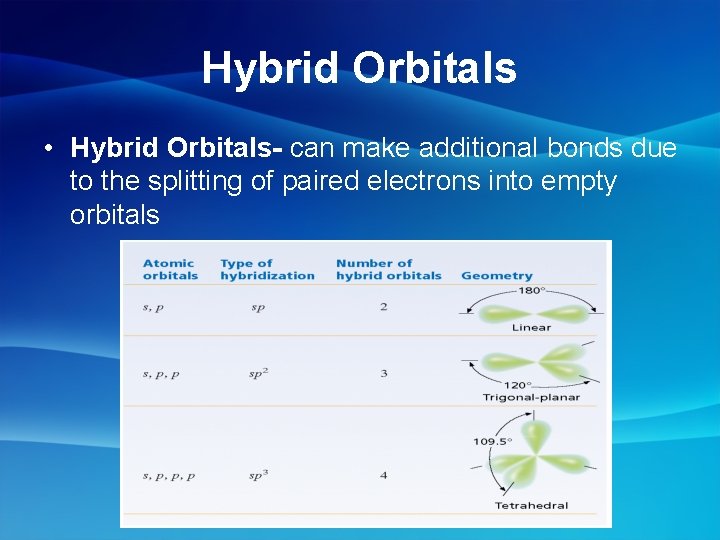
Hybrid Orbitals • Hybrid Orbitals- can make additional bonds due to the splitting of paired electrons into empty orbitals
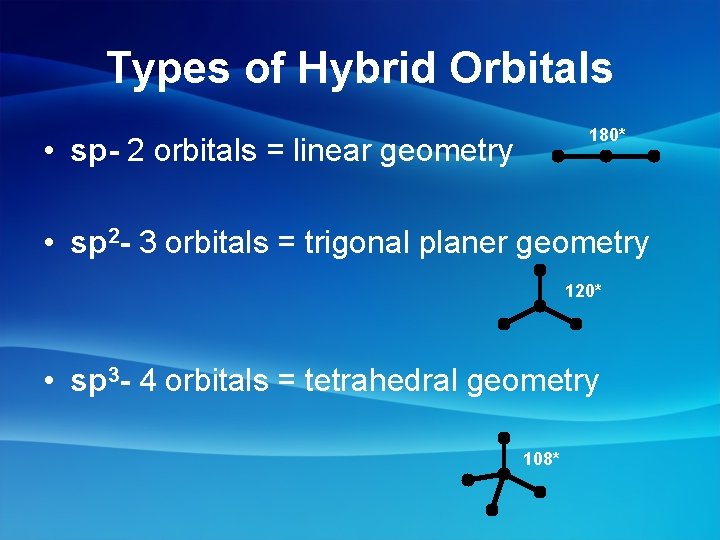
Types of Hybrid Orbitals 180* • sp- 2 orbitals = linear geometry • sp 2 - 3 orbitals = trigonal planer geometry 120* • sp 3 - 4 orbitals = tetrahedral geometry 108*
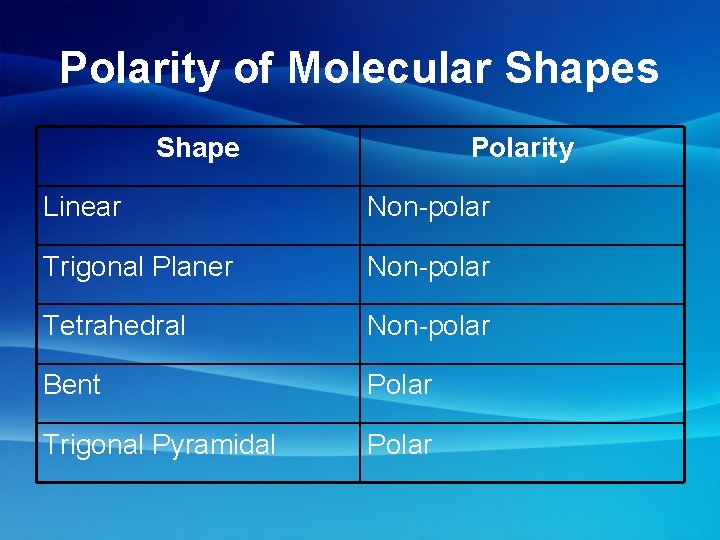
Polarity of Molecular Shapes Shape Polarity Linear Non-polar Trigonal Planer Non-polar Tetrahedral Non-polar Bent Polar Trigonal Pyramidal Polar
Intermolecular Forces • Intermolecular Forces are forces holding separate molecules together – Intermolecular forces are weaker than ionic and covalent bonds • Dipole-Dipole: force of attraction between 2 polar molecules • Ex: HCl (hydrochloric acid)

Intermolecular Forces • Hydrogen Bonding: is a special type of dipole-dipole force between water molecules • London Dispersion Forces: attraction between 2 non-polar molecules

END OF CHAPTER 6 NOTES!!!
- Slides: 51