Chapter 6 Chemical Bonding Types of Chemical Bonding

Chapter 6 Chemical Bonding
Types of Chemical Bonding l Chemical bond Mutual electrical attraction between nuclei and valence electrons of different atoms that binds the atoms together – Creates more stable compounds – l Ionic – bonds Electrical attraction between large numbers of cations and anions l Covalent – bonds Sharing electron pairs between two atoms
Types of Chemical Bonds l Determine if bond is ionic or covalent by difference in electronegativities – Electronegativity difference 1. 7 or less is covalent l Bonding between diatomic molecule is covalent l Non-polar covalent - e- shared equally (≤ 0. 3) l Polar covalent - unequal attraction for e(0. 3 - 1. 7) – Electronegativity of 1. 7 to 3. 3 is ionic l Polar - uneven distribution of charge
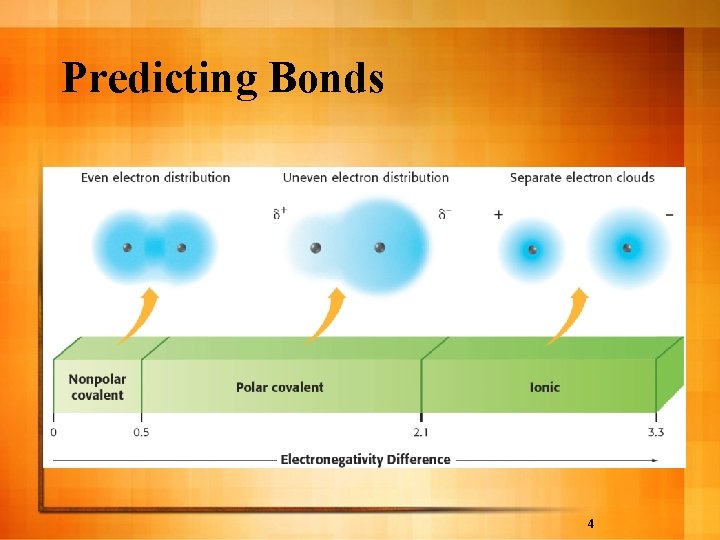
Predicting Bonds 4

Problems l Use electronegativity differences to classify bonding between sulfur, S, and the following elements: hydrogen, H; cesium, Cs; and chlorine, Cl. In each pair, which atom will be more negative?

Problems l Use electronegativity differences to classify bonding between chlorine, Cl, and the following elements: calcium, Ca; oxygen, O; and bromine, Br. Indicate the more negative atom in each pair.
Covalent Bonding l Molecule Neutral group of atoms that are covalently bonded together – H 2 O, sugar, O 2 – l Chemical – formula Shows relative number of atoms in a compound l Diatomic Molecule
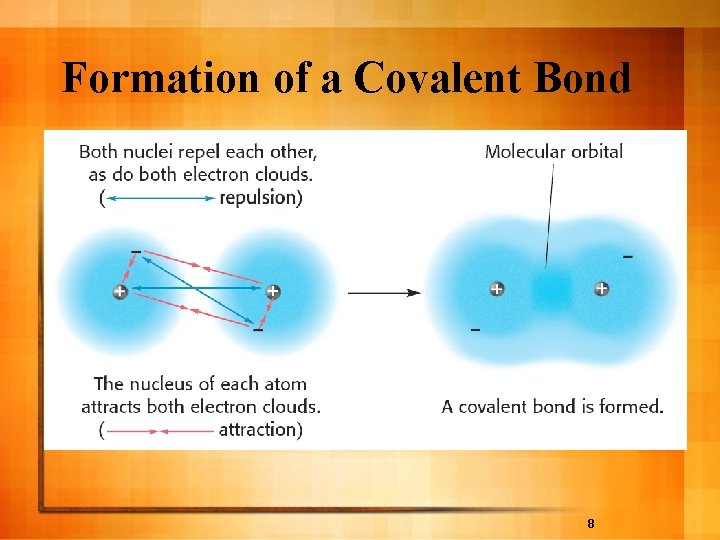
Formation of a Covalent Bond 8
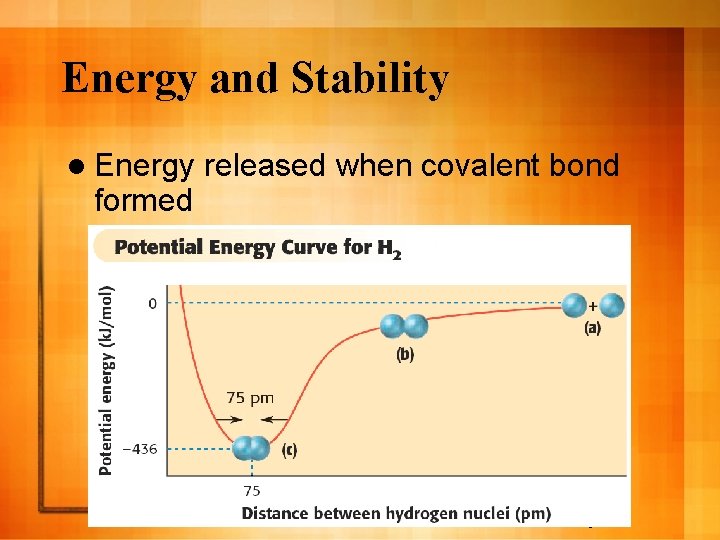
Energy and Stability l Energy formed released when covalent bond 9
Covalent Bonding l Bond – length Distance between two bonded atoms at minimum potential energy l Atoms vibrate back and forth l Depends on atoms that have combined l Bond energy – Energy required to break a chemical bond

Polarity and Bond Strength l The greater the electronegativity difference, the greater the polarity and the stronger the bond 11
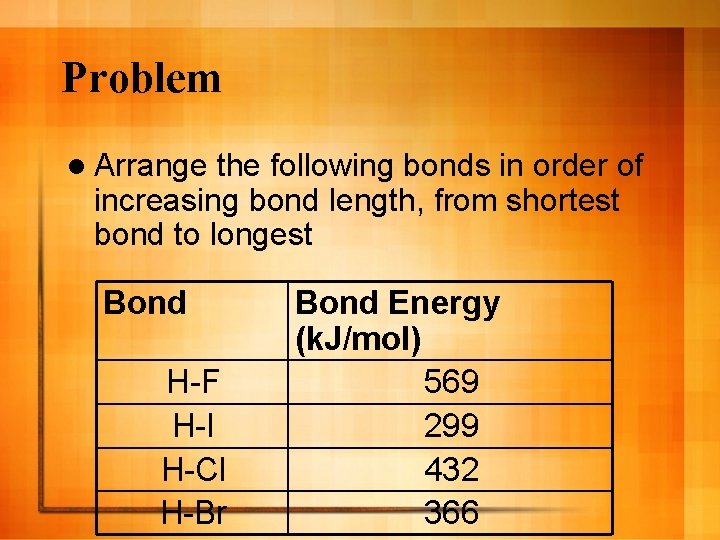
Problem l Arrange the following bonds in order of increasing bond length, from shortest bond to longest Bond H-F H-I H-Cl H-Br Bond Energy (k. J/mol) 569 299 432 366
Octet Rule l Chemical compounds tend to form so that each atom, by gaining, losing, or sharing electrons, has an octet of electrons in its highest occupied energy level l Atoms with 8 electrons in outer shell are stable – Except: l Hydrogen and Helium - stable with 2 electrons l Boron - forms bonds surrounded by 6 electrons l Beryllium - forms bonds surrounded by 4 e-
Electron-Dot Strucutres l Notation in which only valence electrons of element are shown by dots 14
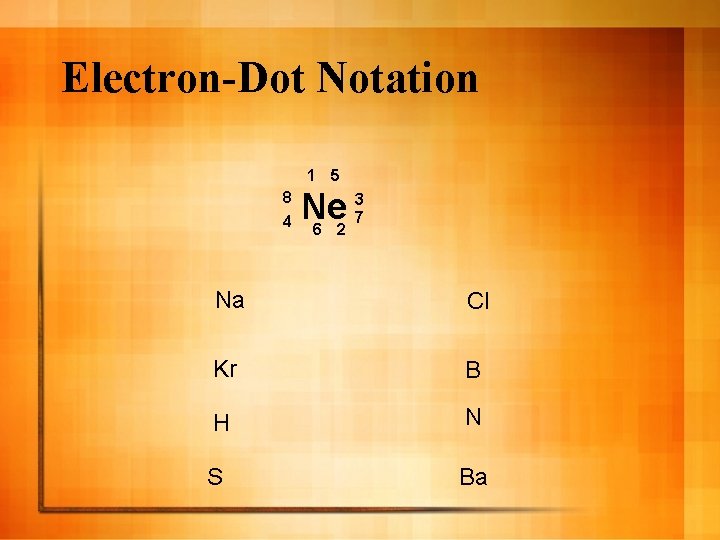
Electron-Dot Notation 1 5 8 4 3 Ne 7 6 2 Na Cl Kr B H N S Ba
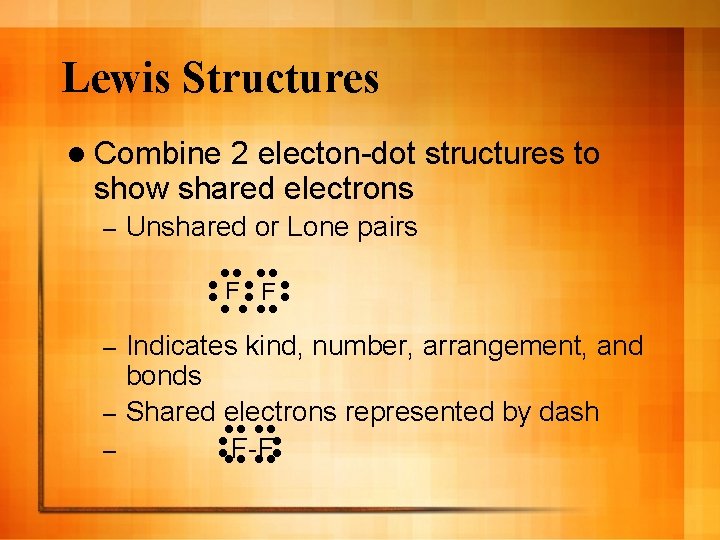
Lewis Structures l Combine 2 electon-dot structures to show shared electrons – Unshared or Lone pairs F F Indicates kind, number, arrangement, and bonds – Shared electrons represented by dash – F-F –
Lewis Structures l Least electronegative atom is central Except hydrogen – Carbon is usually central – l Multiple bond represented Single bond – Double bond – Triple bond –

Lewis Structures 1. Determine the total number of valence electrons in the compound. 2. Arrange the atoms’ symbols to show they are bonded and show valence electrons as dots 3. Compare the number of valence electrons used in the structure to the number available from step 1. 4. Change to a single dash each pair of dots that represents two shared electrons. 5. Be sure that all atoms, with the exception of hydrogen, follow the octet rule.

Problems l Draw the Lewis structure of iodomethane, CH 3 I. l Draw the Lewis structure of ammonia, NH 3 l Draw the Lewis structure for hydrogen sulfide, H 2 S l Draw the Lewis structure for methanal, CH 2 O, which is also known as formaldehyde.
Problems l Draw – – – – the Lewis structure for: Carbon dioxide, CO 2 Hydrogen cyanide, HCN IBr CH 3 Br C 2 HCl Si. Cl 4 F 2 O

Polyatomic Ions l. A charged group of covalently bonded atoms l Combine with other ions to form ionic compounds l Add/subtract appropriate number of electrons l Place brackets around structure l Show the charge of ion outside of brackets
Polyatomic Ions l Draw Lewis structures for: NH 4+ – SO 4– PO 43– CO 32– 22

Ionic Bonding l Composed of cations and anions to make neutral compound – – Na. Cl Cannot be isolated and examined like molecules l Form crystal lattice to stabilize Forces between like-charged ions and opposite-charged ions – Na+ surrounded by 6 Cl– Cl- surrounded by 6 Na+ – l Lattice Energy
Ionic vs. Molecular Compounds l Ionic – – – Stronger bonds Higher melting and boiling points Hard but brittle Electrical conductors when dissolved May separate when dissolved l Molecular Weaker bonds – Lower melting and boiling points –

Metallic Bonding l Chemical bonding the results from attraction between metal atoms and surrounding sea of electrons l Highest energy levels have few electrons l Many vacant orbitals

Metallic Bonding l Metallic – – – Properties High electrical and thermal conductivity Absorb many light frequencies Shiny Malleable Ductile Heat of vaporization →Bond strength

VSEPR Theory l Valence-Shell Electron-Pair Repulsion l Repulsion between sets of valencelevel electrons surrounding an atom causes sets to be oriented as far apart as possible – Electrons of bonded atoms want to be as far away from each other as possible
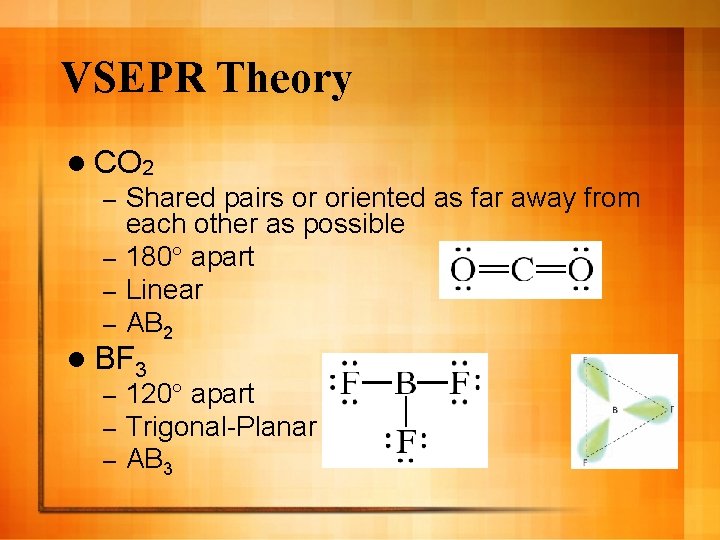
VSEPR Theory l CO 2 Shared pairs or oriented as far away from each other as possible – 180° apart – Linear – AB 2 – l BF 3 – – – 120° apart Trigonal-Planar AB 3
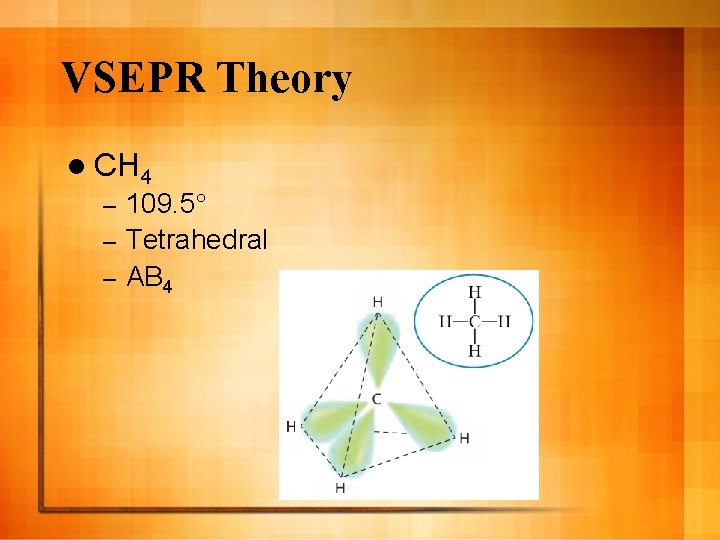
VSEPR Theory l CH 4 109. 5° – Tetrahedral – AB 4 –
VSEPR & Unshared Electrons l Lone pairs occupy space and influence shape of molecule H 2 O →AB 2 E 2 →bent – NH 3 →AB 3 E →trigonal-pyramidal – l Unshared electrons repel electrons more strongly than shared electrons l Double and triple bonds treated like single bonds

Problem l Use VSEPR theory to predict the molecular geometry of: – – – Al. Cl 3 CBr 4 Al. Br 3 SF 6 CH 2 Cl 2

Problem l Use VSEPR theory to predict the molecular geometry of: CO 2 – Cl. O 3– SF 2 – PCl 3 –

Hybridization l Mixing of two or more atomic orbitals of similar energies on the same atom to produce new orbitals of equal energies l Example →CH 4 – 2 s and 2 p orbitals hybridize to form 4 identical orbitals called sp 3 orbitals l Group – 15 and 16 Nitrogen - sp 3
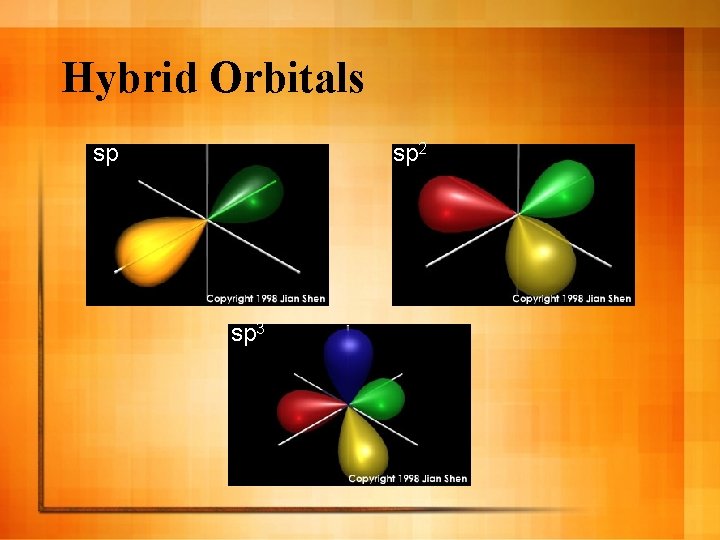
Hybrid Orbitals sp sp 2 sp 3

Intermolecular Forces l Forces of attraction between molecules l Boiling point is used to measure force of attraction between particles – – Weaker than bonds Boiling points of ionic compounds higher than covalent molecules l Dipole-Dipole Forces l Hydrogen Bonding l London Dispersion Forces

Dipole-Dipole Forces l Strongest molecules l Dipole force is between polar Equal but opposite charges that are separated by short distance – Direction is from positive pole to negative – Represented by arrow pointing toward negative pole with a crossed tail –

Dipole-Dipole Forces l Forces of attraction between polar molecules are short-range forces l Dipole-Dipole forces cause higher boiling points

Hydrogen Bonding l Many bonds with hydrogen are highly polar because of large electronegativity difference l Hydrogen therefore has positive charge in many compounds l Hydrogen atoms bonded to highly electronegative atom is attracted to an unshared pair of electrons – Example →H 2 O l Represented by dotted lines
Hydrogen Bonding
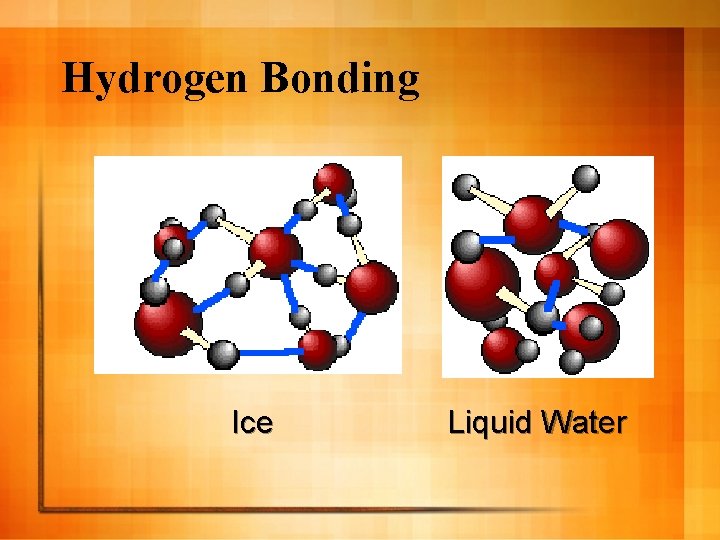
Hydrogen Bonding Ice Liquid Water

London Dispersion Force l Electrons are in continuous motion l Slight uneven electron distribution l Temporary dipole l Intermolecular attractions resulting from constant motion of electrons and creation of instantaneous dipole l Only intermolecular force acting on noble gases l Strength increases with number of e-

Chapter Review l Pg. – 209 3, 6 acfg, 9, 16, 19 abcfg, 21 bde, 22, 24 c, 29 ab, 33, 45 bc, 46 bdf, 47 cd, 48 acdf, 49 a
- Slides: 42