Chapter 6 Bonding Types of Bonding l l

Chapter 6 Bonding
Types of Bonding l l l ________: a mutual ______________ between the nuclei and valence electrons of different atoms that ____ the atoms together. ______: chemical bonding that results from the _______ between cations and anions. Electonegativity difference of _______: sharing of electron pairs between atoms. 6 -2
Types of Bonding l The degree to which bonding between atoms is ionic or covalent can be estimated by calculating the difference in the electronegativity of the bonding atoms. 6 -3
Covalent Bonding l ___________: a covalent bond in which the bonding electrons are _________ by the bonded atoms, resulting in a ________ of electrical charge. Electronegativity difference is _____. 6 -4
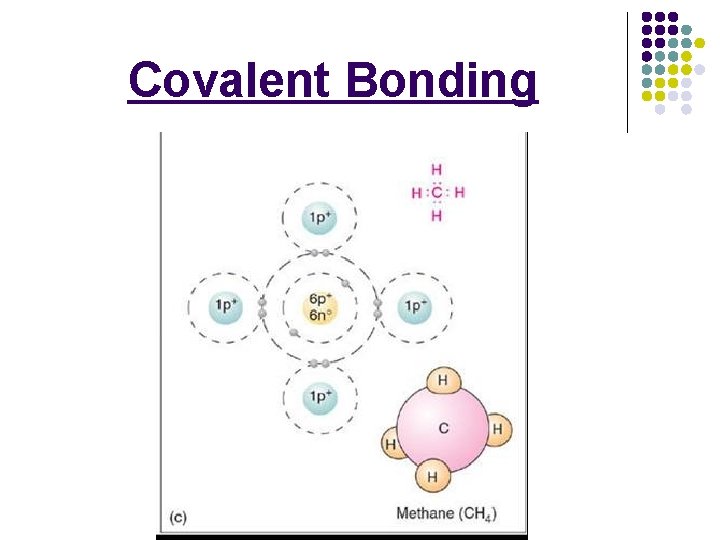
Covalent Bonding
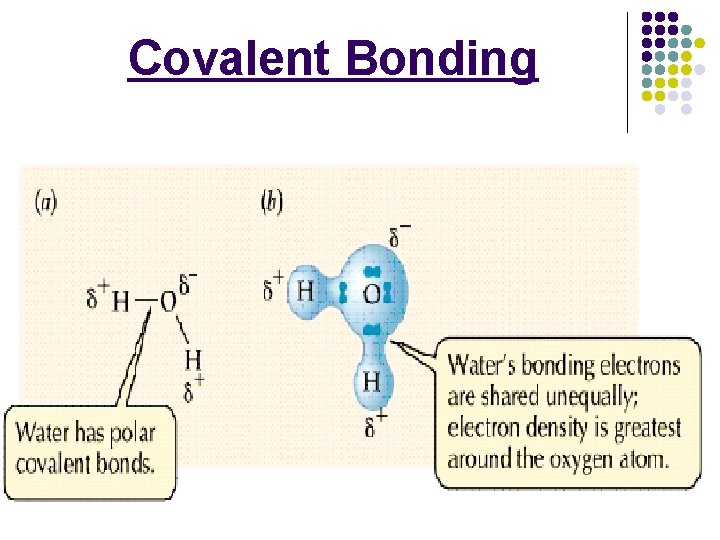
Covalent Bonding l ________: a covalent bond in which the bonded atoms have an ________ for the shared electrons. Polar bonds result in a _______ charge around the _______________ atom (δ-) and a _________ charge around the _____electronegative atom (δ+). Electronegativity difference is between ______. 6 -6
Covalent Bonding
Practice 1) What type of bond would form between the following atoms? a) O-H__________ b) C-H__________ c) Cl-Cl_________
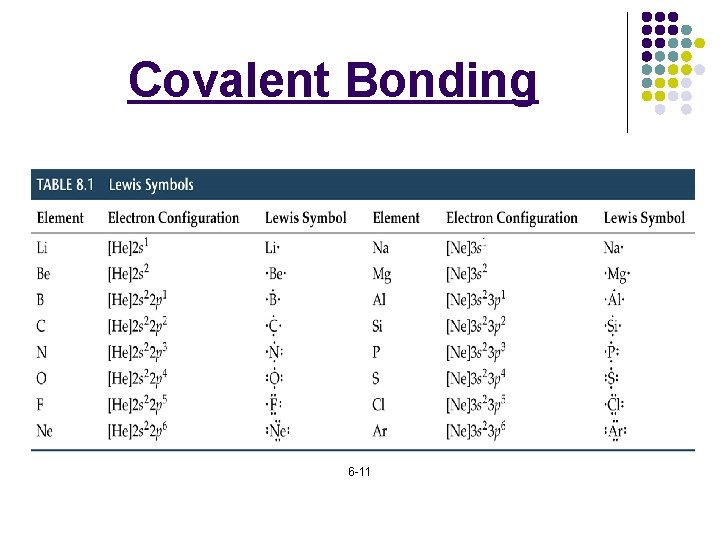
Covalent Bonding l l _____: a neutral group of atoms that are held together by _________: a chemical compound whose simplest units are ________: indicates the relative numbers of atoms of each kind in a chemical compound using ________ and ________: shows the type and numbers of atoms combined in a single molecule of a molecular compound. 6 -9
Covalent Bonding l l l _______: the energy required to _____ a chemical bond and form ______ isolated atoms _____: the average _______ between two bonded atoms. ________: only _____ e- of an atom of a particular element are shown, indicated by ______ placed around the _____. 6 -10
Covalent Bonding 6 -11
Covalent Bonding l l ________: formulas in which atomic symbols represent _______, dot-pairs or dashes between two atomic symbols represent ______ in _______ and dots adjacent to only one atomic symbol represent ______electrons. _______: indicates the kind, number, arrangement and bonds but not the ________ of the atoms in a molecule. 6 -12
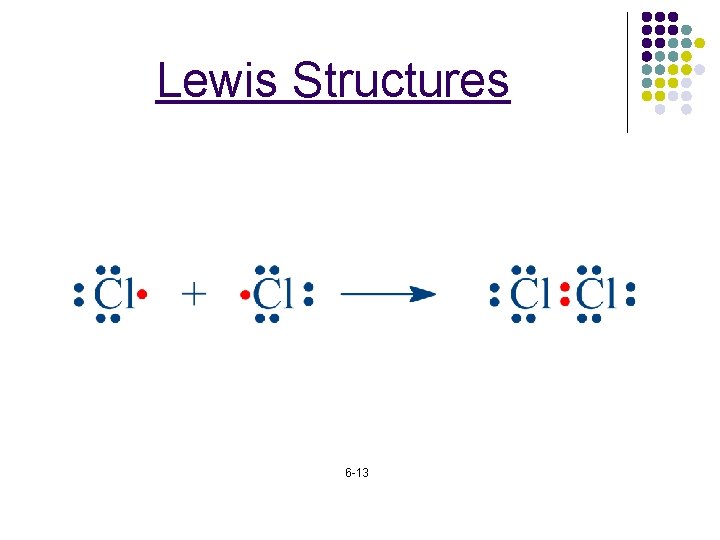
Lewis Structures 6 -13
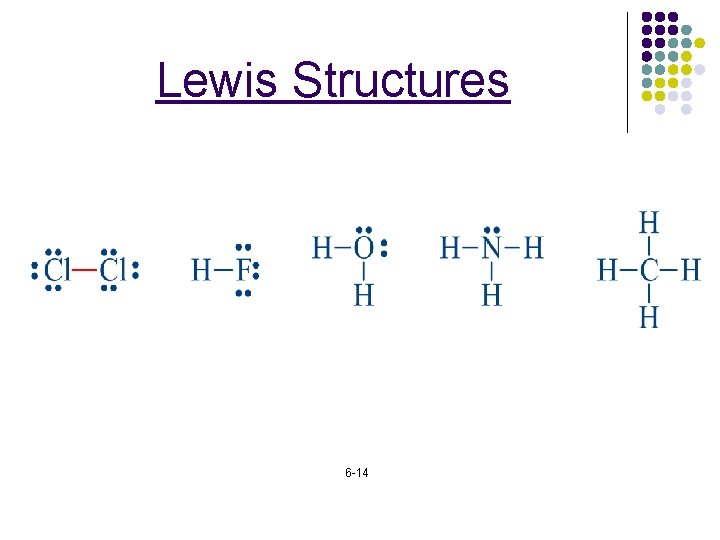
Lewis Structures 6 -14
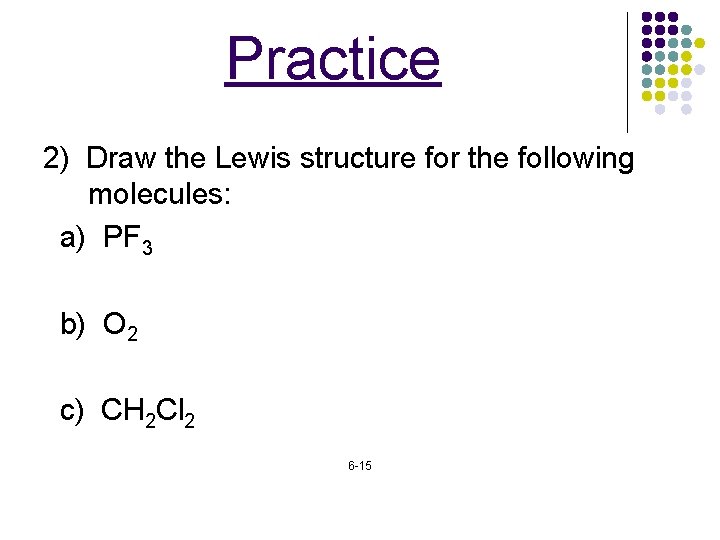
Practice 2) Draw the Lewis structure for the following molecules: a) PF 3 b) O 2 c) CH 2 Cl 2 6 -15
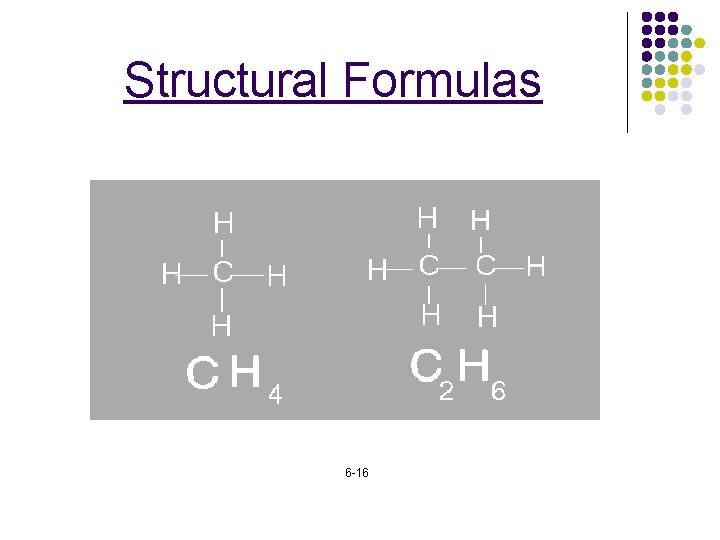
Structural Formulas 6 -16
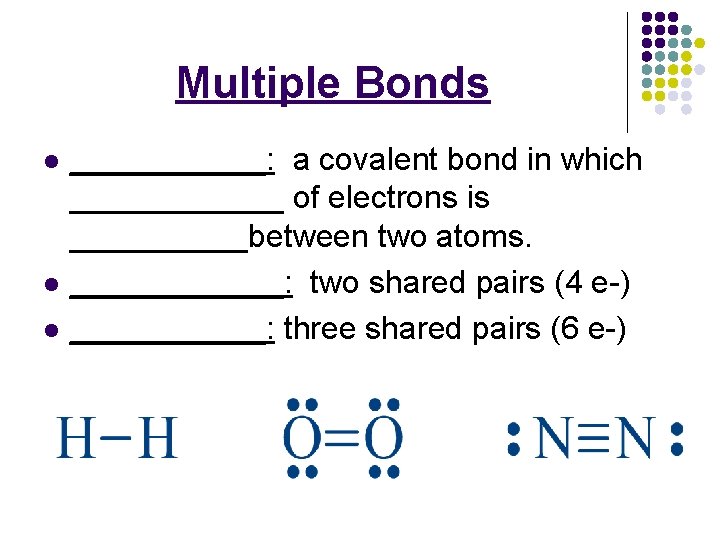
Multiple Bonds l l l ______: a covalent bond in which ______ of electrons is _____between two atoms. ______: two shared pairs (4 e-) ______: three shared pairs (6 e-)
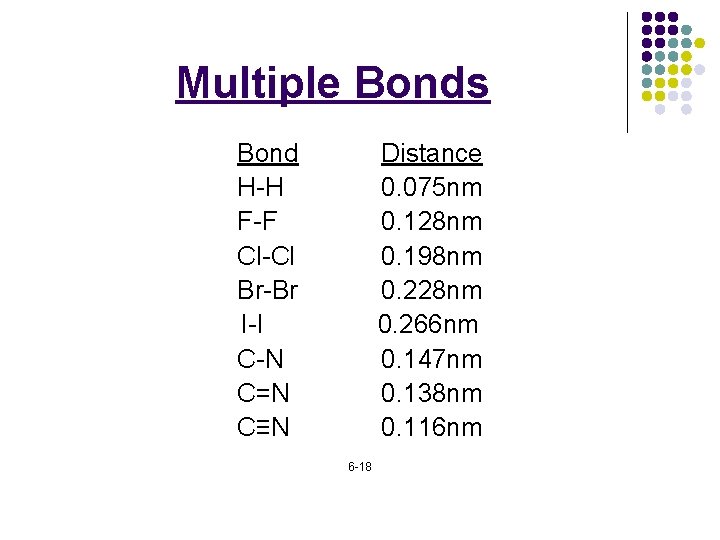
Multiple Bonds Bond H-H F-F Cl-Cl Br-Br I-I C-N C=N C≡N Distance 0. 075 nm 0. 128 nm 0. 198 nm 0. 228 nm 0. 266 nm 0. 147 nm 0. 138 nm 0. 116 nm 6 -18
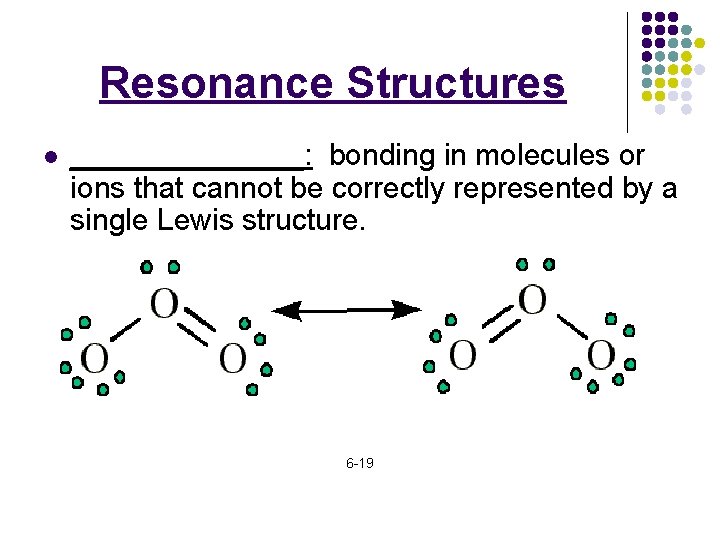
Resonance Structures l _______: bonding in molecules or ions that cannot be correctly represented by a single Lewis structure. 6 -19
Ionic Bonding l l ________: composed of positive and negative ions that are combined so that the _____ of positive and negative charges _______: the ____ collection of atoms from which an _______ compounds _____ can be established. 6 -20
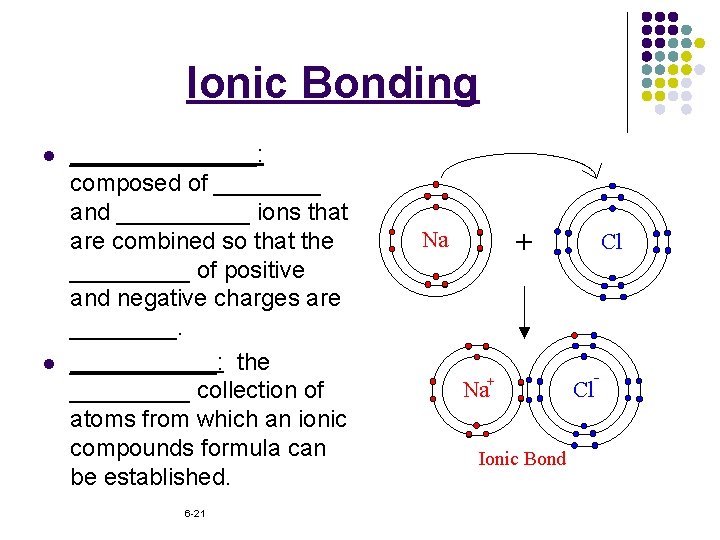
Ionic Bonding l l _______: composed of ____ and _____ ions that are combined so that the _____ of positive and negative charges are ______: the _____ collection of atoms from which an ionic compounds formula can be established. 6 -21
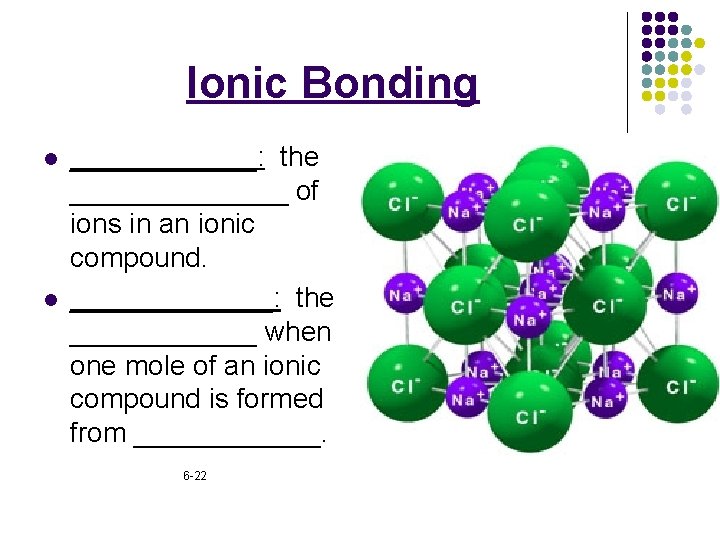
Ionic Bonding l l ______: the _______ of ions in an ionic compound. _______: the ______ when one mole of an ionic compound is formed from ______. 6 -22
Bonding Comparison l l ______ compounds tend to have ______, ______ and are not as _______ as ionic compounds. Many are _____ at room temp. _____ compounds tend to have _______, ____ and are ______. Ionic solutions are ________ of electricity. 6 -23
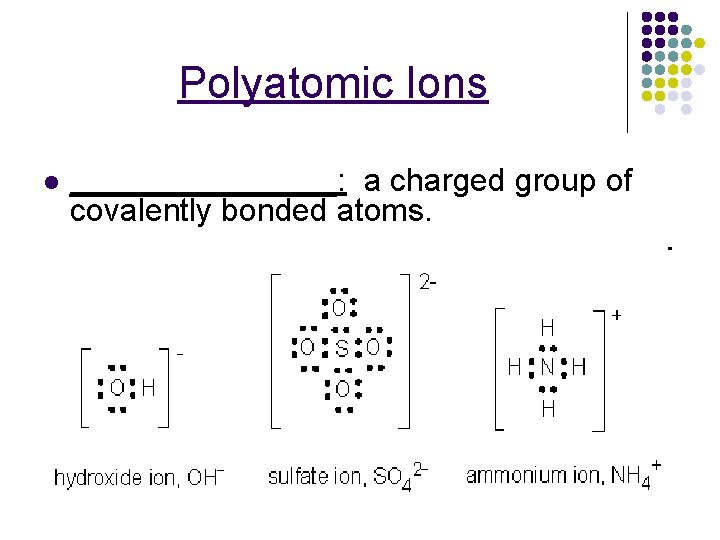
Polyatomic Ions l ________: a charged group of covalently bonded atoms.
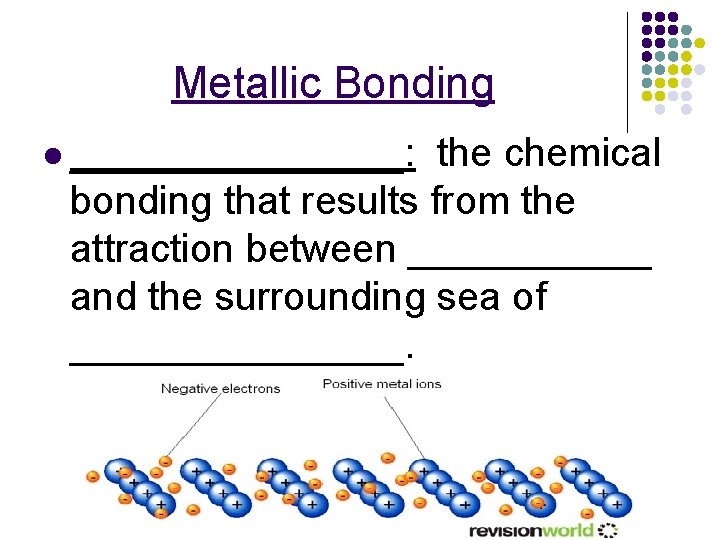
Metallic Bonding l ________: the chemical bonding that results from the attraction between ______ and the surrounding sea of ________.
Molecular Geometry l l ________: whether a molecule is polar or not is determined by the ___________ and the _____ of the molecule. _____: ________________ states that repulsion between sets of valence-level electrons surrounding an atom causes these sets to be oriented _______ possible. 6 -26
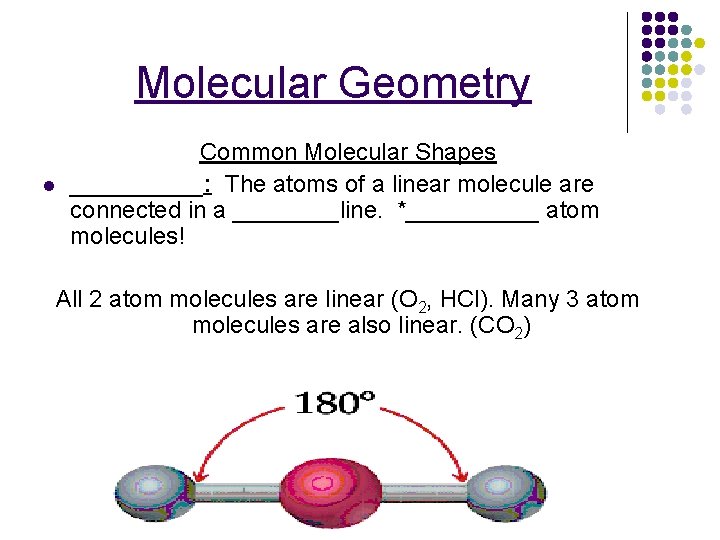
Molecular Geometry l Common Molecular Shapes _____: The atoms of a linear molecule are connected in a ____line. *_____ atom molecules! All 2 atom molecules are linear (O 2, HCl). Many 3 atom molecules are also linear. (CO 2) 6 -26
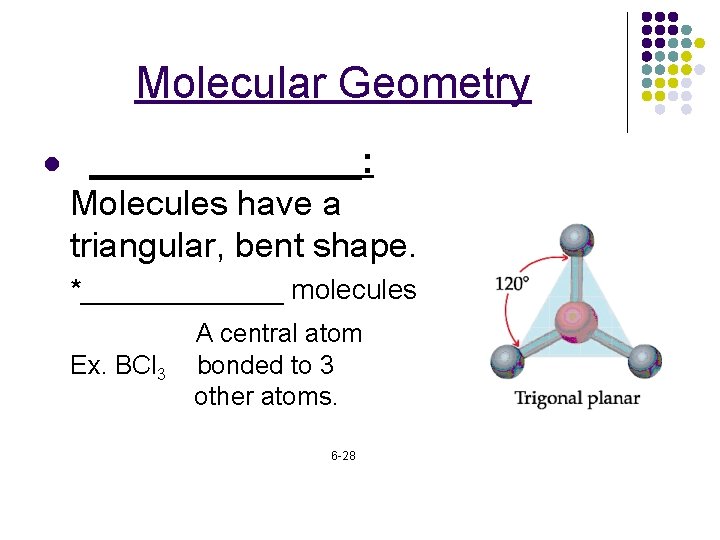
Molecular Geometry l _______: Molecules have a triangular, bent shape. *_______ molecules Ex. BCl 3 A central atom bonded to 3 other atoms. 6 -28
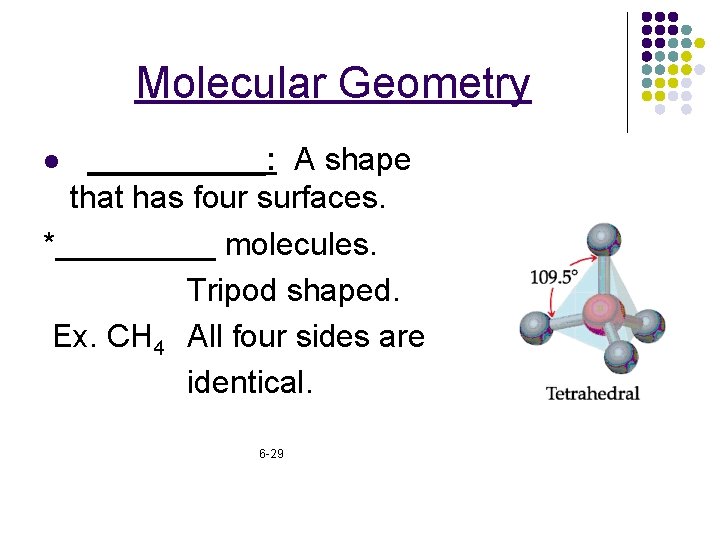
Molecular Geometry _____: A shape that has four surfaces. *_____ molecules. Tripod shaped. Ex. CH 4 All four sides are identical. l 6 -29
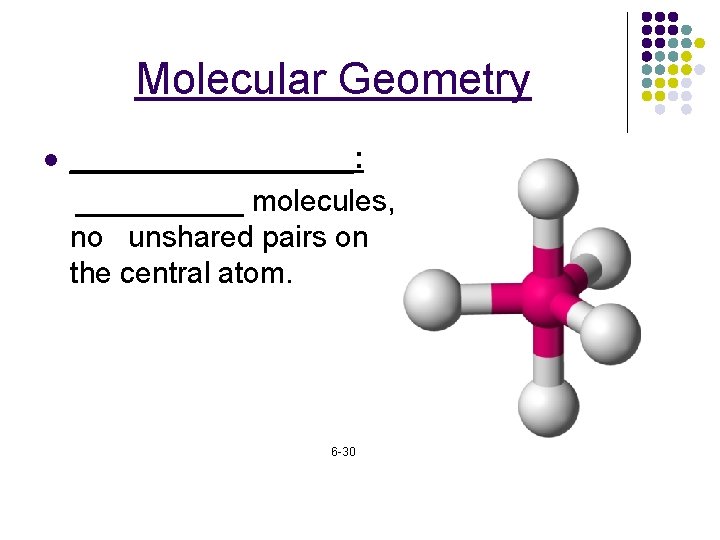
Molecular Geometry l _________: _____ molecules, no unshared pairs on the central atom. 6 -30
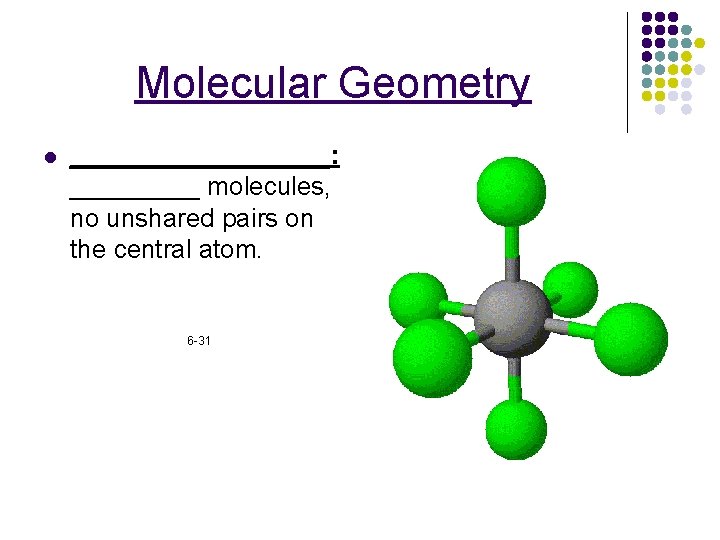
Molecular Geometry l _________: _____ molecules, no unshared pairs on the central atom. 6 -31
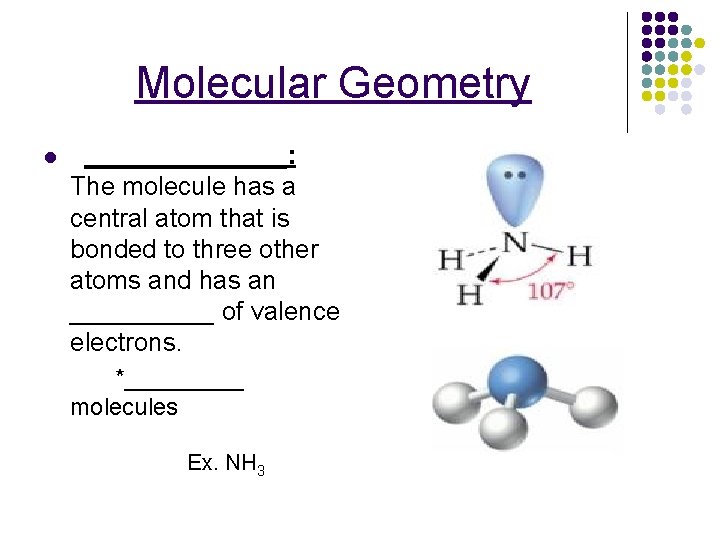
Molecular Geometry l _______: The molecule has a central atom that is bonded to three other atoms and has an _____ of valence electrons. *_____ molecules Ex. NH 3

Molecular Geometry l _______: ______ result in a slightly smaller bond angle, due to an even greater repulsion force. *______ molecules Ex. H 2 O

Practice 3) What shape would the following molecules have and would they be polar or nonpolar: a) CH 4: b) SF 6: c) NH 3: 6 -34

Hybrid Orbitals l ____________: the mixing of _______ atomic orbitals of similar energies on the same atom to produce new hybrid atomic orbitals of ________. 6 -35
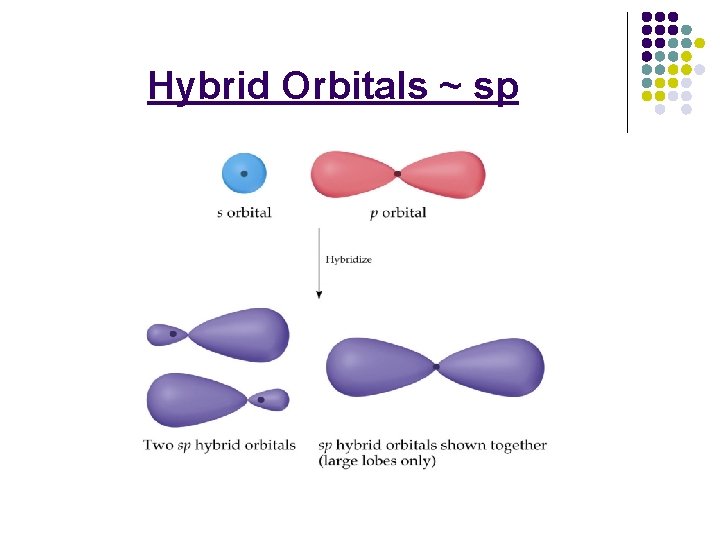
Hybrid Orbitals ~ sp
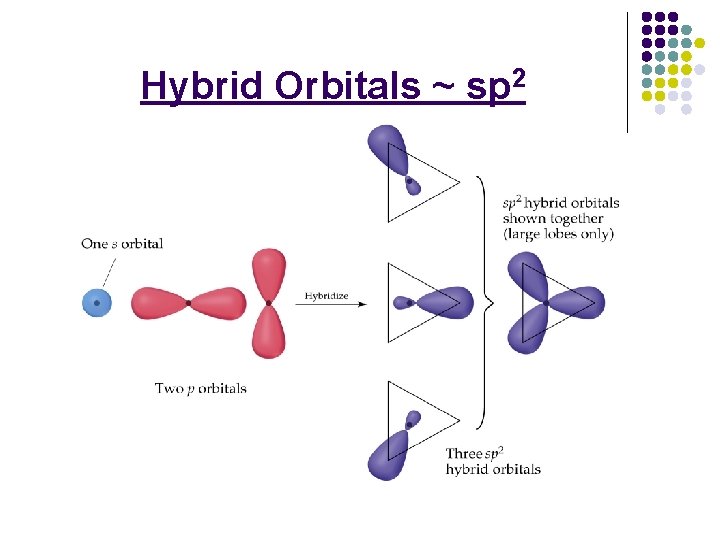
Hybrid Orbitals ~ sp 2
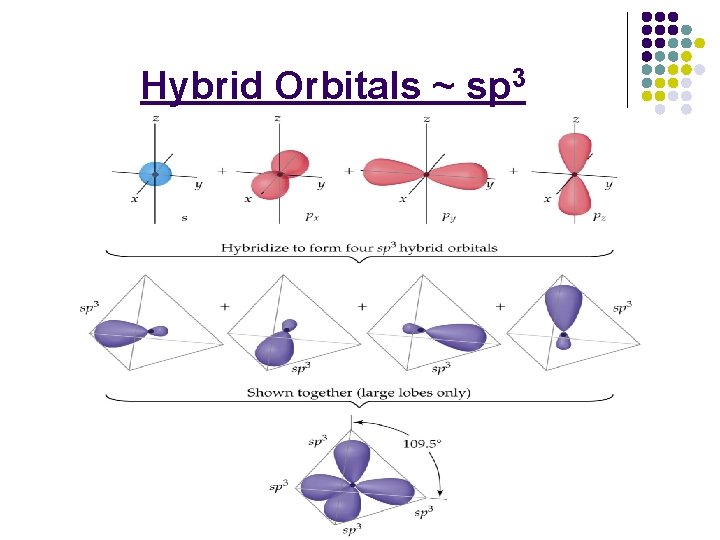
Hybrid Orbitals ~ sp 3
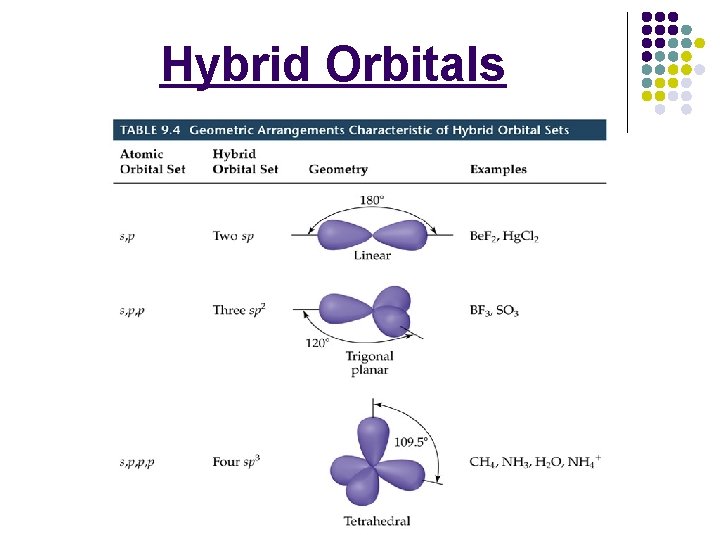
Hybrid Orbitals
Intermolecular Forces l l ____________: forces of _______ between molecules. The _________ are between polar molecules. _________: is created by ______ but ______ charges that are separated by a short distance. 6 -40
Intermolecular Forces l l _________: an intermolecular force in which a hydrogen atom that is bonded to a _________ atom is attracted to an _________ of electrons of an _______ atom in a nearby molecule. __________: the intermolecular attractions resulting from the ______ of electrons and the creation of _____________. 6 -41

Ch. 6 The End!
- Slides: 42