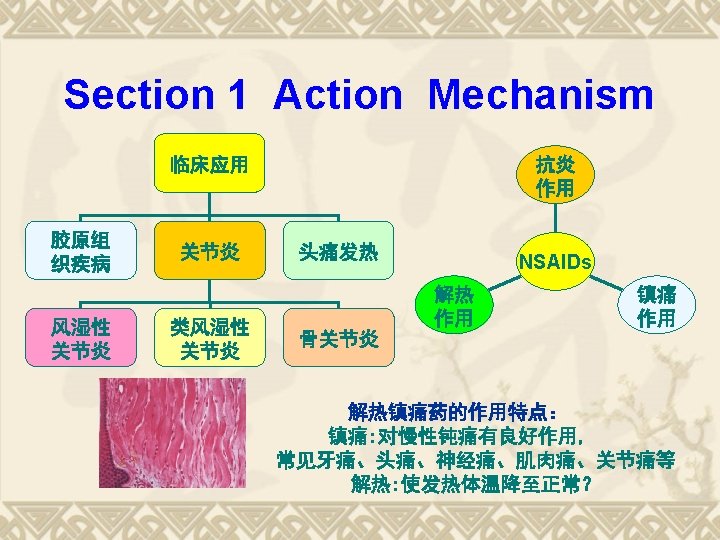
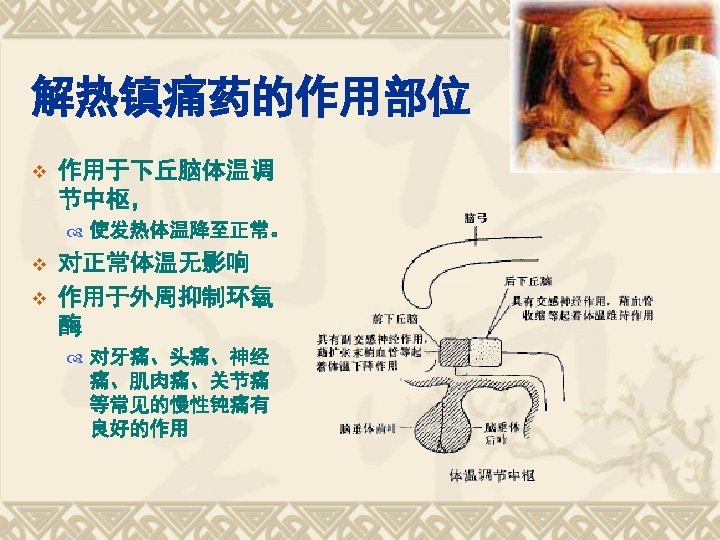
Chapter 6 Antipyretic Analgesics and NSAIDs October 2729

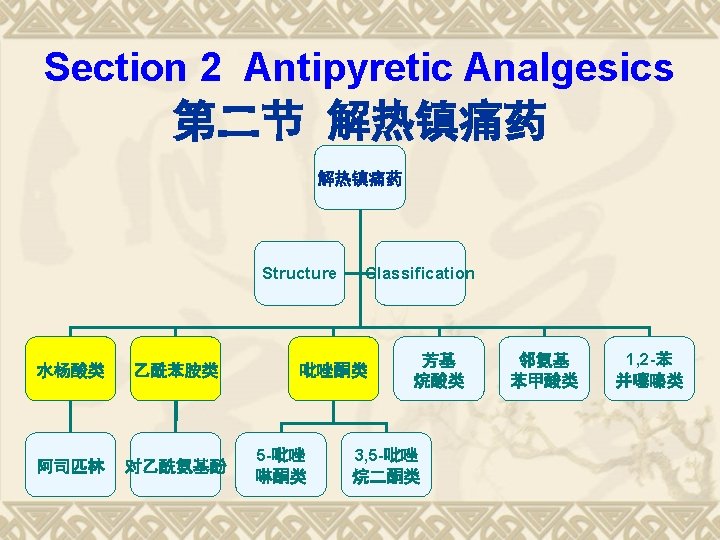
第六章 解热镇痛药和非甾体抗炎药 Chapter 6 Antipyretic Analgesics and NSAIDs October, 27~29, 2014


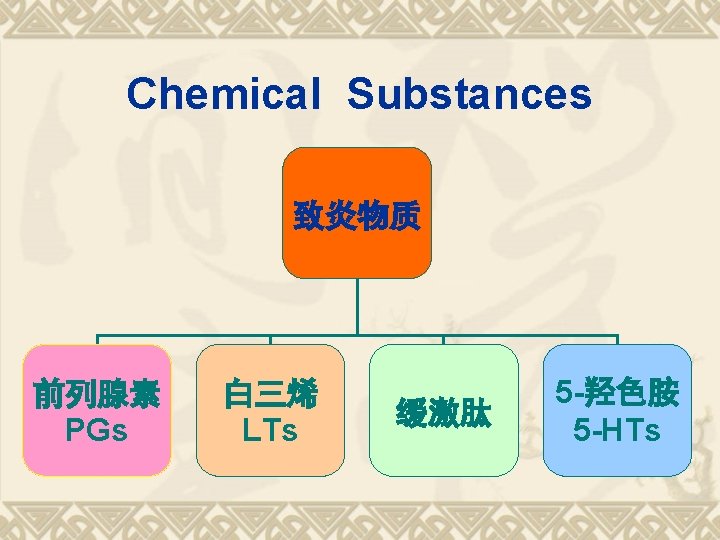
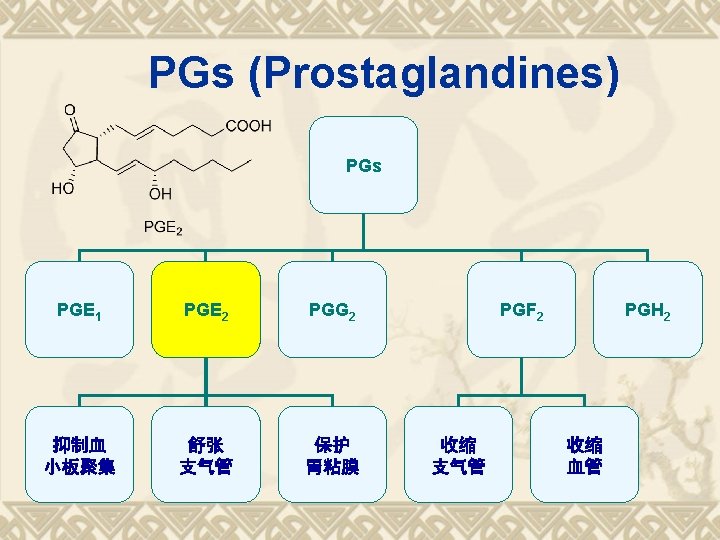
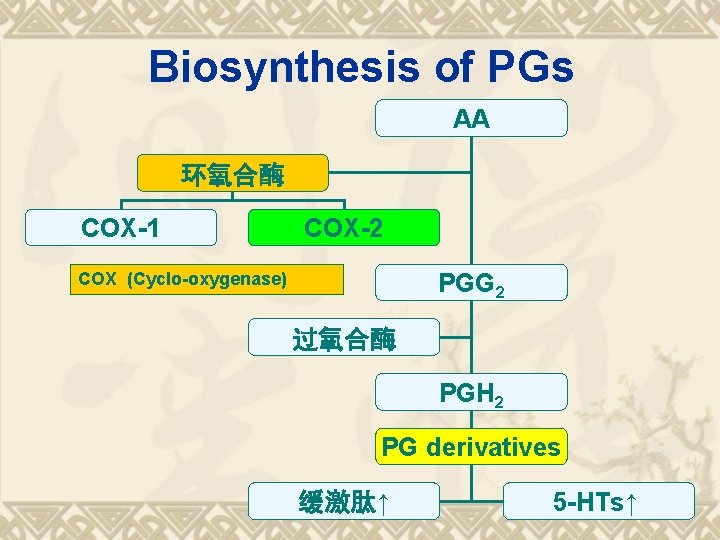
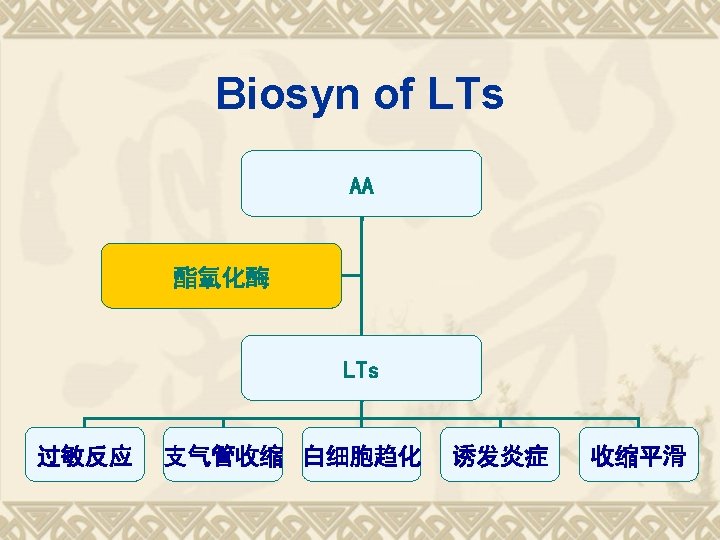
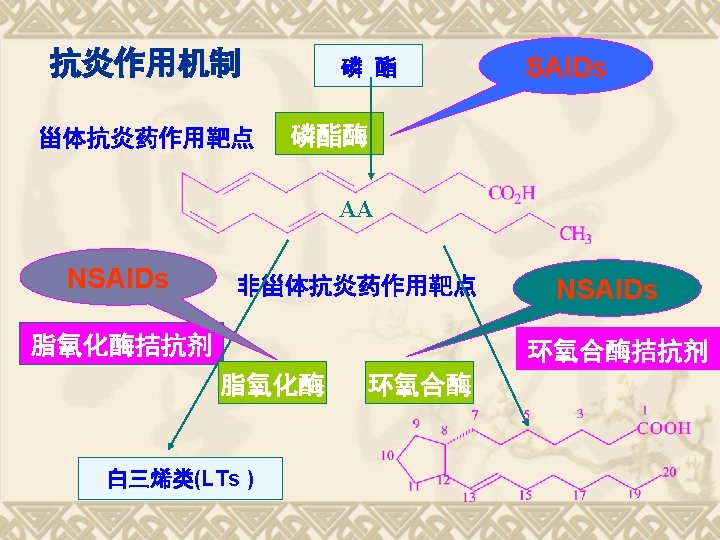
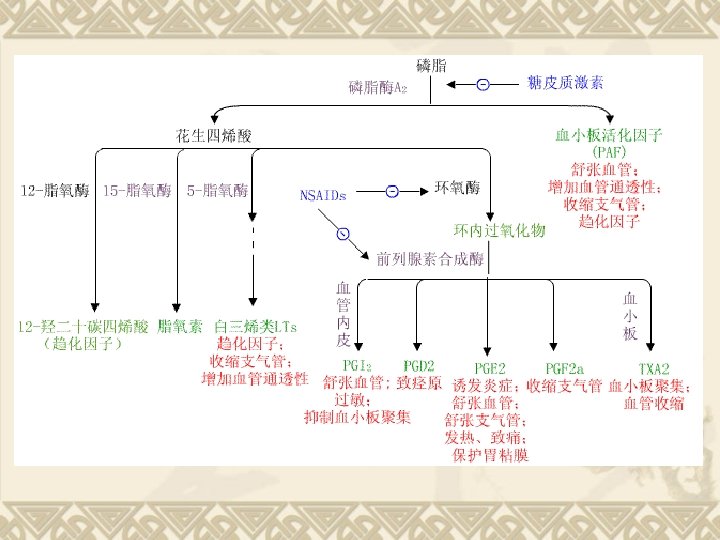
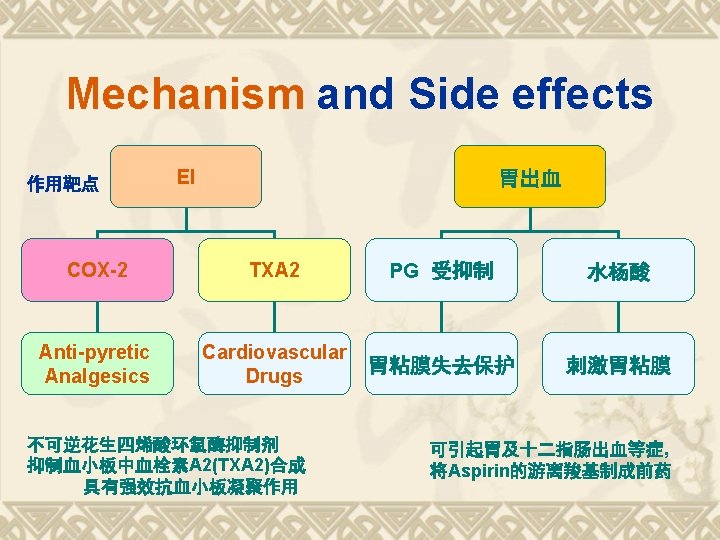
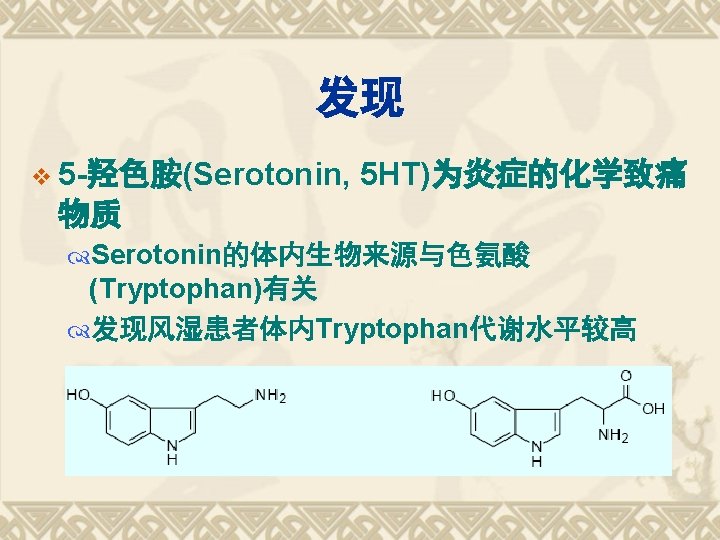
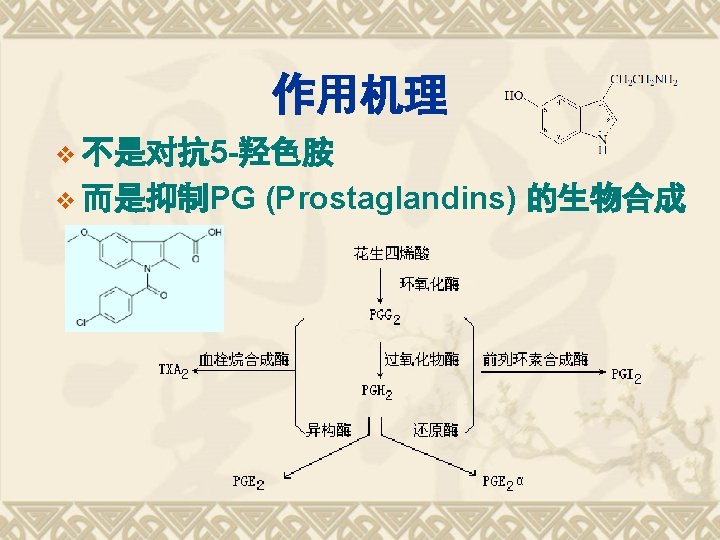
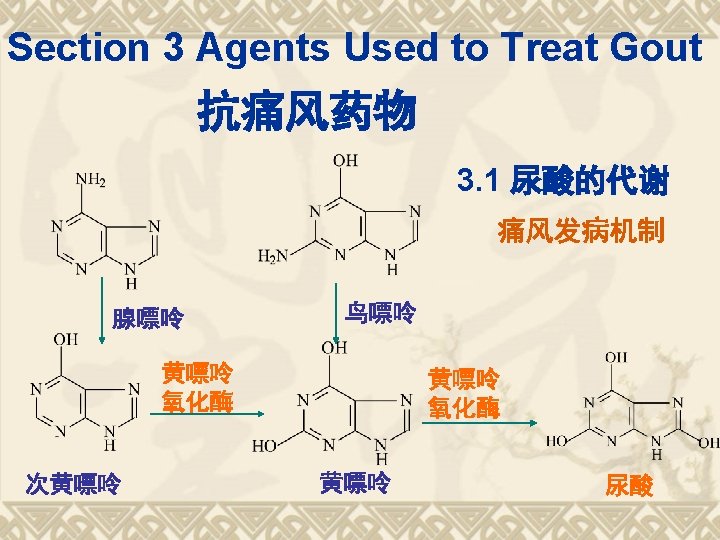
Biosynthesis of PGs AA 环氧合酶 COX-1 COX-2 PGG 2 COX (Cyclo-oxygenase) 过氧合酶 PGH 2 PG derivatives 缓激肽↑ 5 -HTs↑
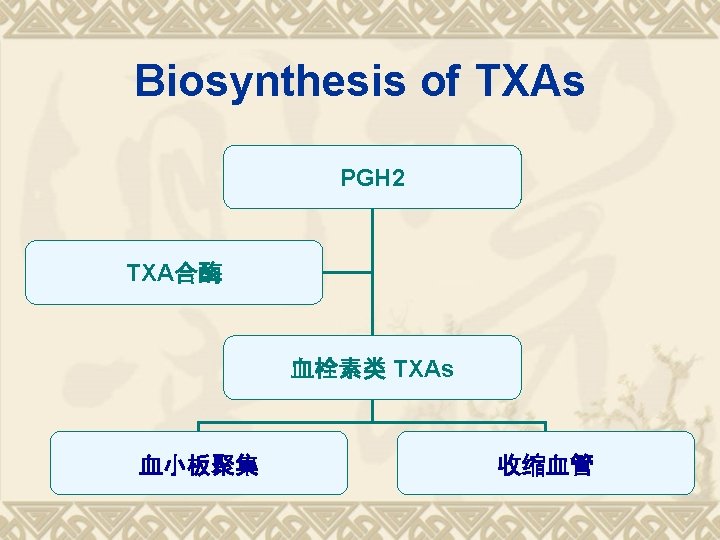
Biosynthesis of TXAs PGH 2 TXA合酶 血栓素类 TXAs 血小板聚集 收缩血管

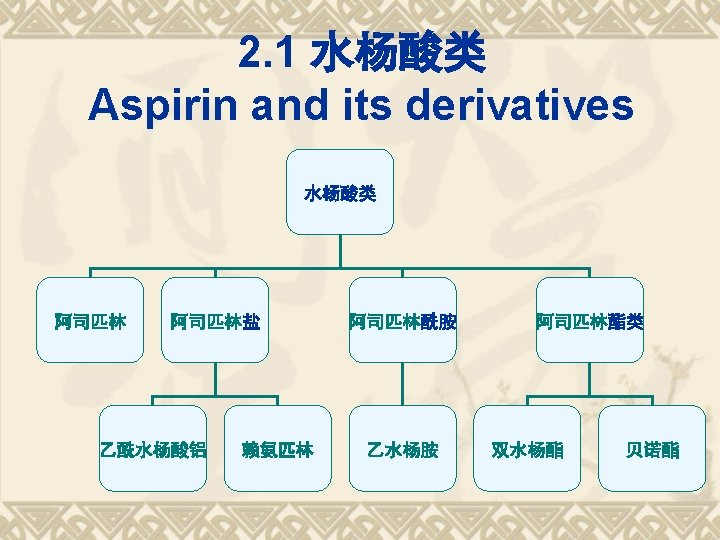


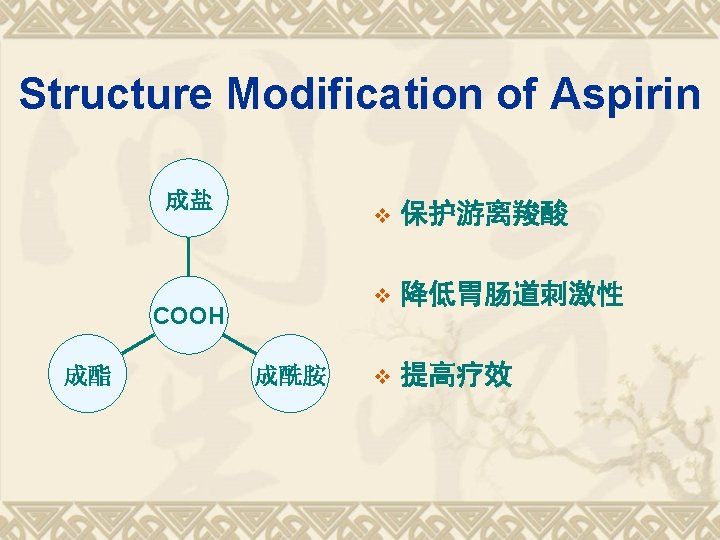
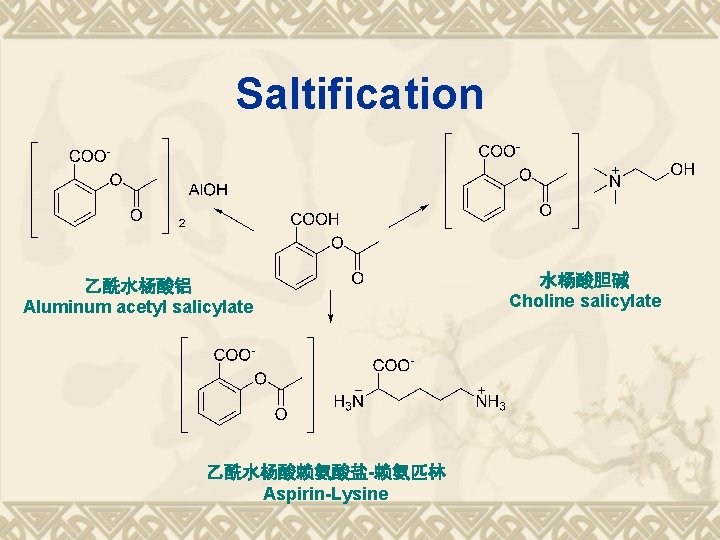
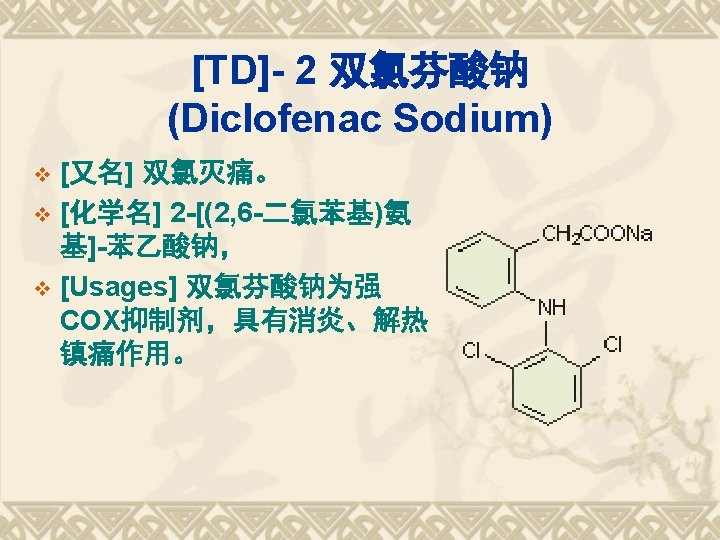
Saltification 乙酰水杨酸铝 Aluminum acetyl salicylate 乙酰水杨酸赖氨酸盐-赖氨匹林 Aspirin-Lysine 水杨酸胆碱 Choline salicylate

Esterification 双水杨酯Salsalate 贝诺酯Benorilate
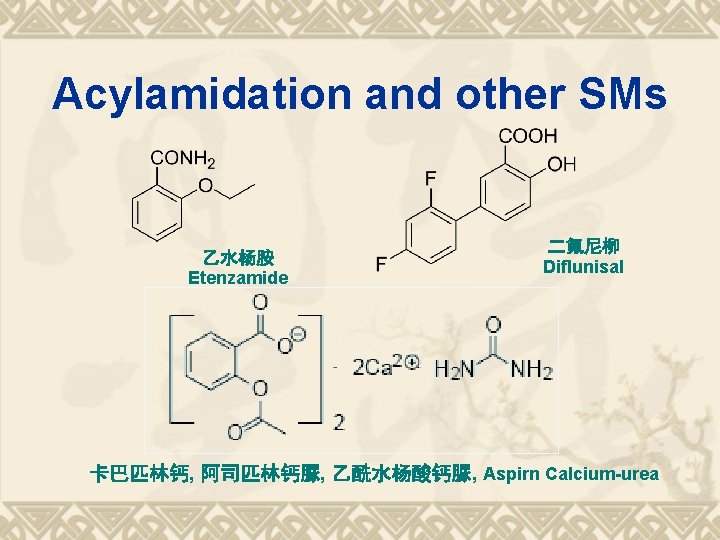
Acylamidation and other SMs 乙水杨胺 Etenzamide 二氟尼柳 Diflunisal 卡巴匹林钙, 阿司匹林钙脲, 乙酰水杨酸钙脲, Aspirn Calcium-urea
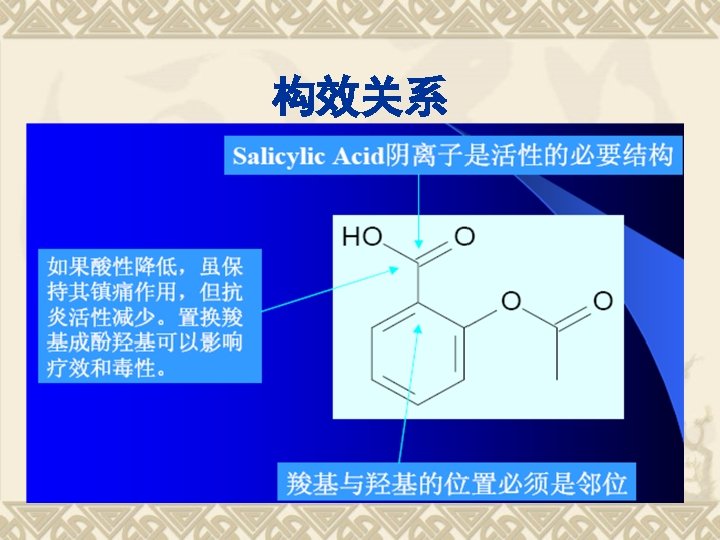
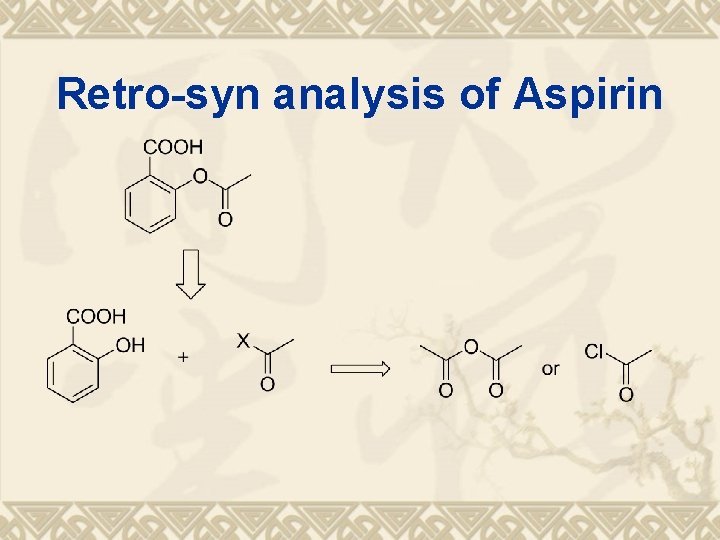
Retro-syn analysis of Aspirin
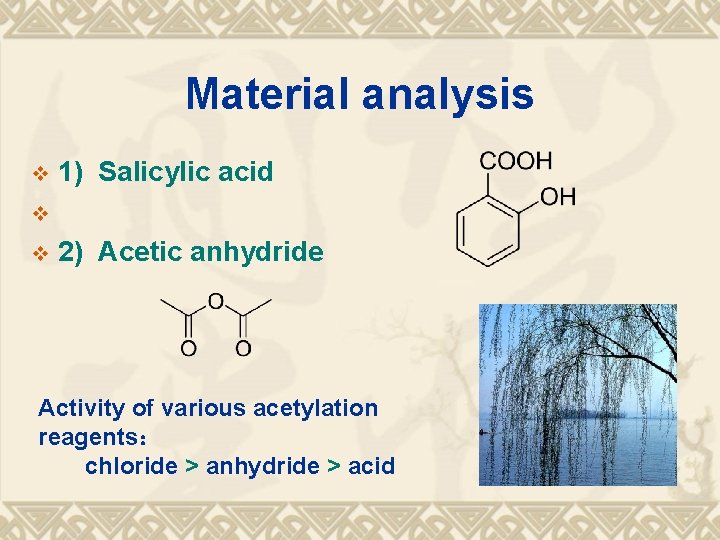
Material analysis v 1) Salicylic acid v v 2) Acetic anhydride Activity of various acetylation reagents: chloride > anhydride > acid
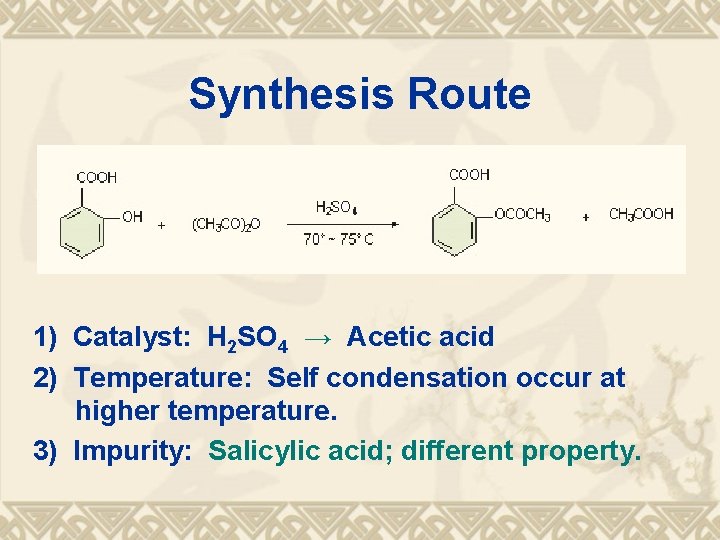
Synthesis Route 1) Catalyst: H 2 SO 4 → Acetic acid 2) Temperature: Self condensation occur at higher temperature. 3) Impurity: Salicylic acid; different property.

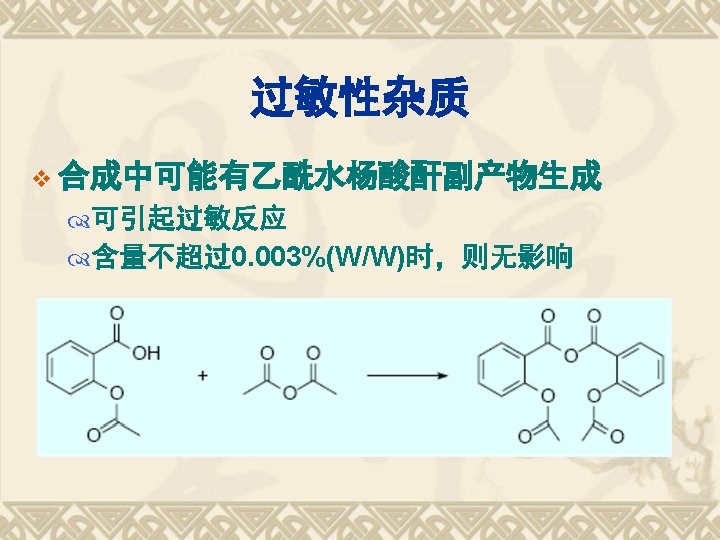
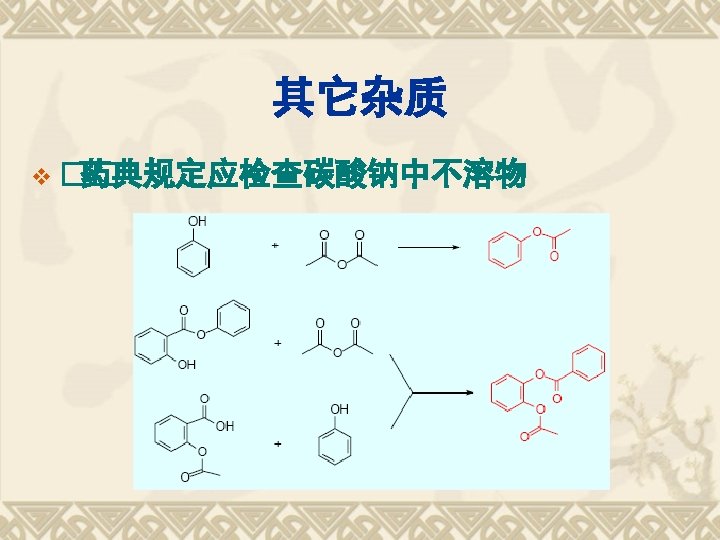
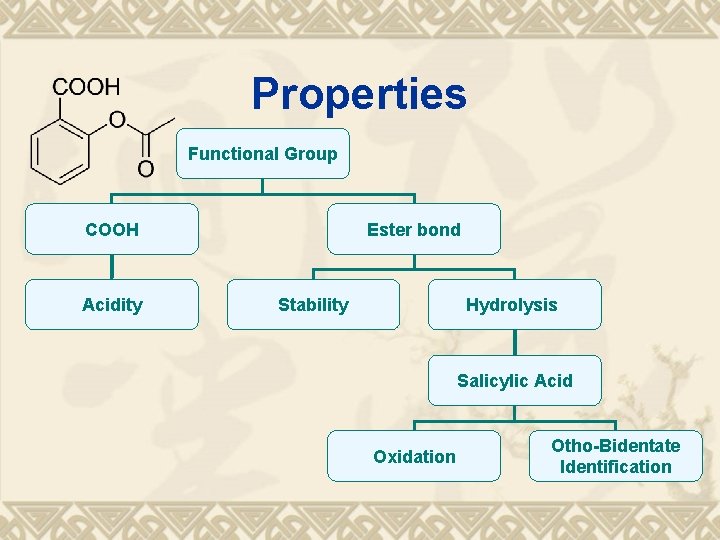
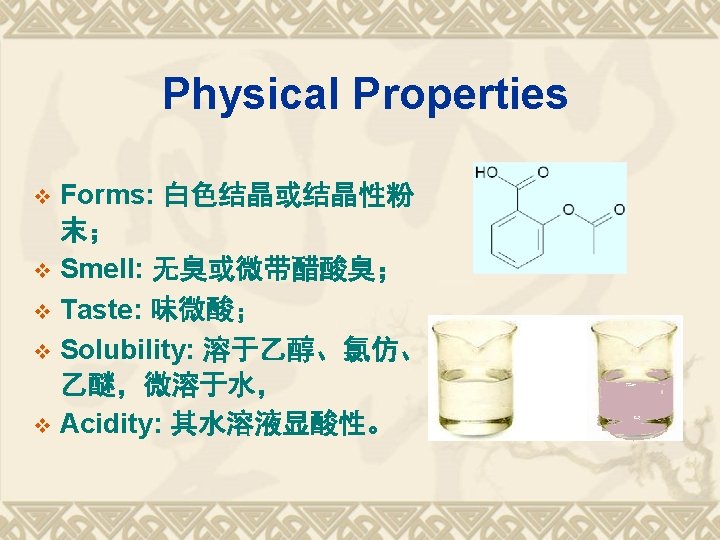
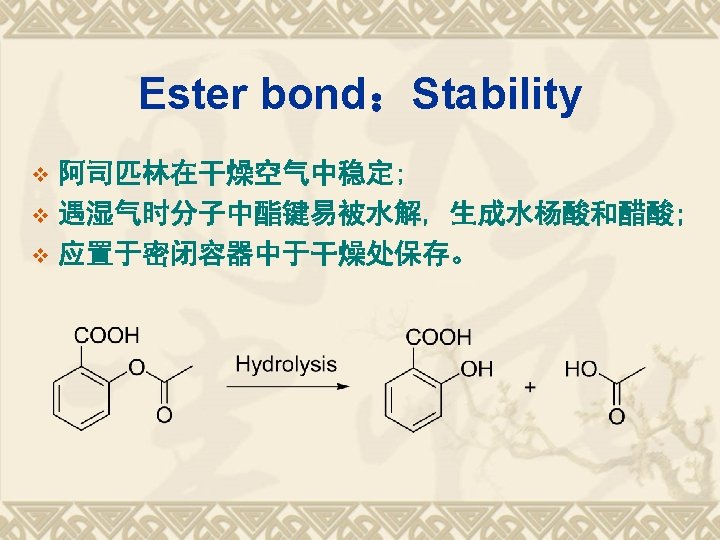
Properties Functional Group COOH Acidity Ester bond Stability Hydrolysis Salicylic Acid Oxidation Otho-Bidentate Identification

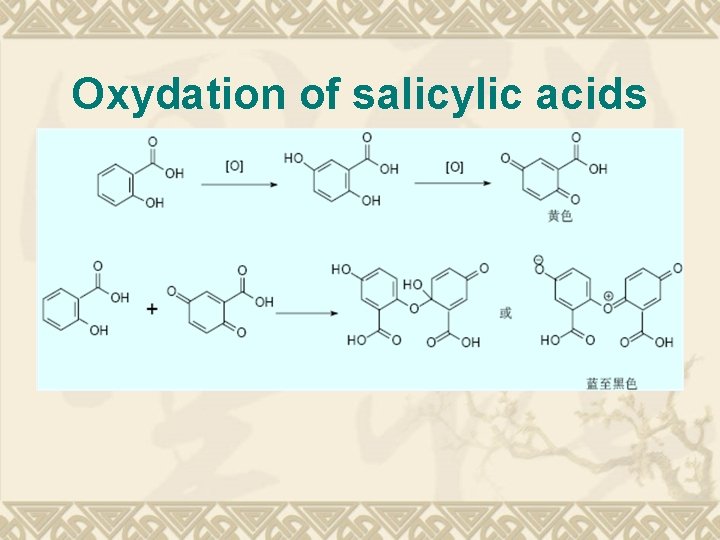
Oxydation of salicylic acids
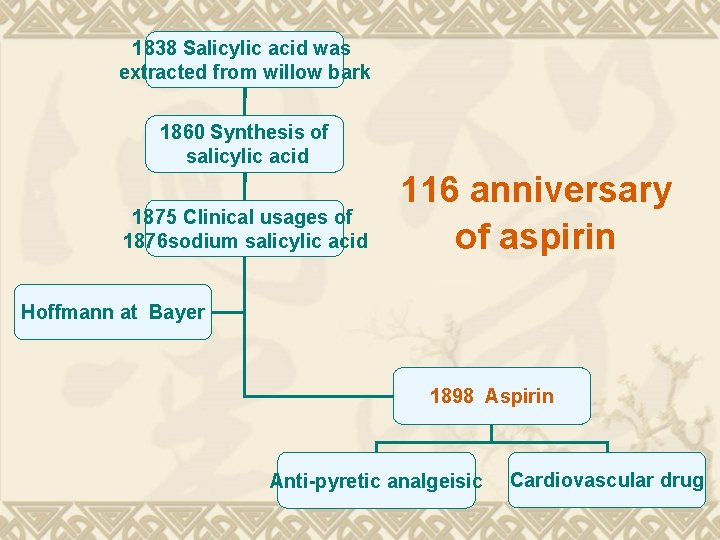
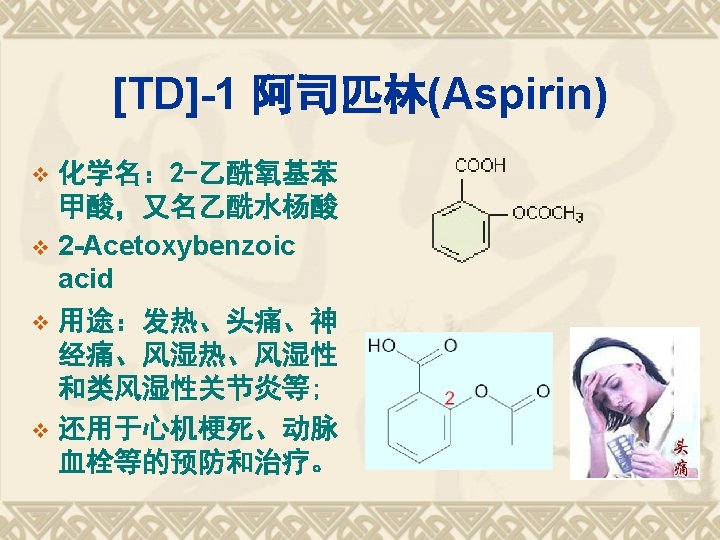
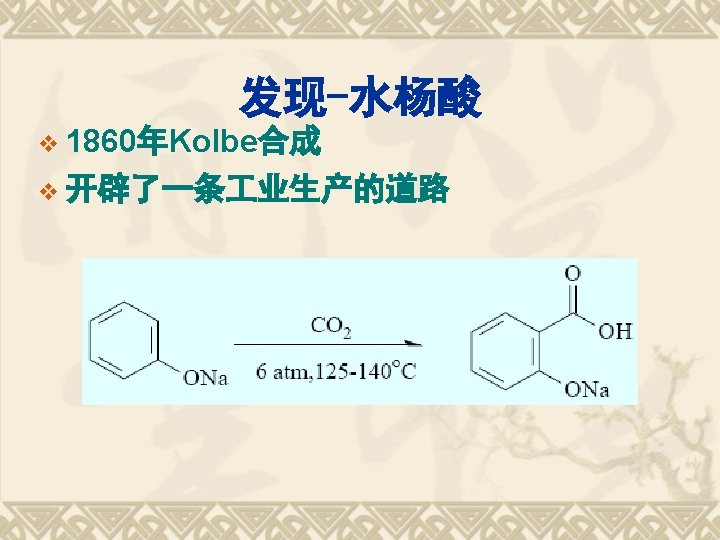
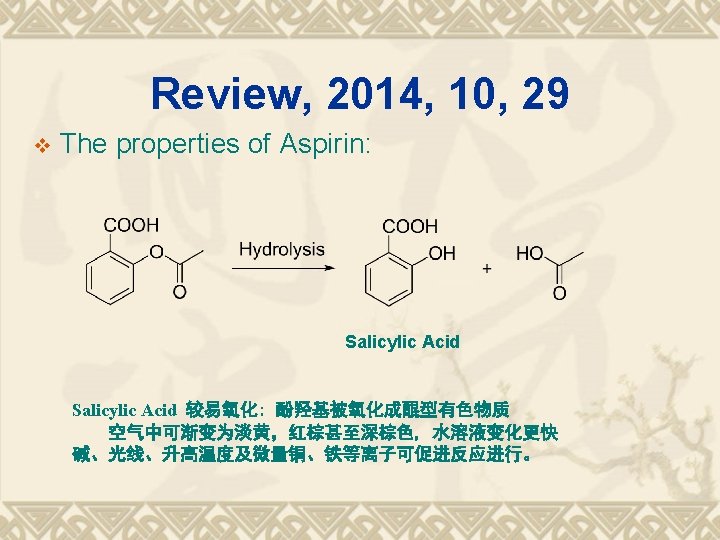
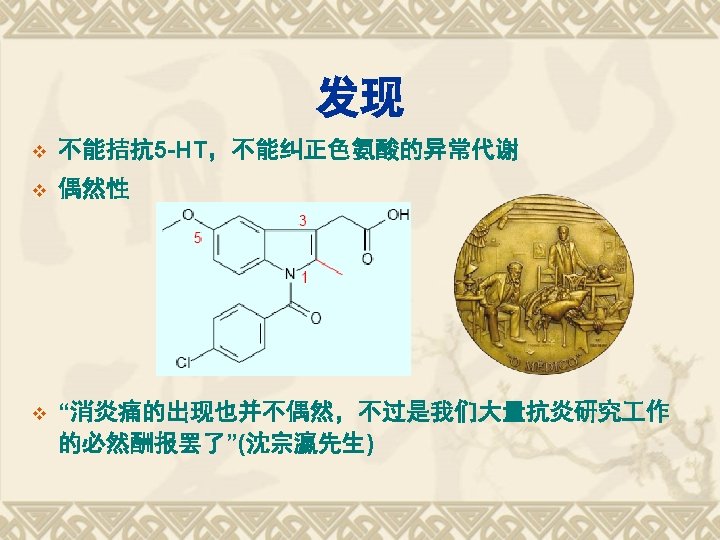
1838 Salicylic acid was extracted from willow bark 1860 Synthesis of salicylic acid 1875 Clinical usages of 1876 sodium salicylic acid 116 anniversary of aspirin Hoffmann at Bayer 1898 Aspirin Anti-pyretic analgeisic Cardiovascular drug
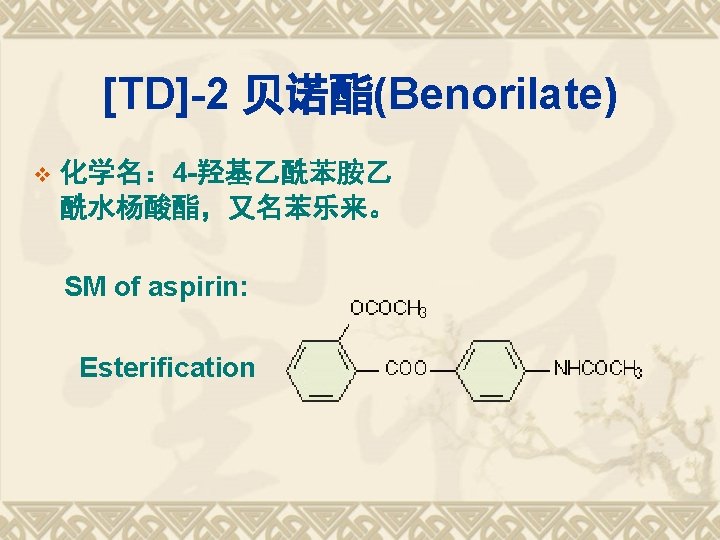
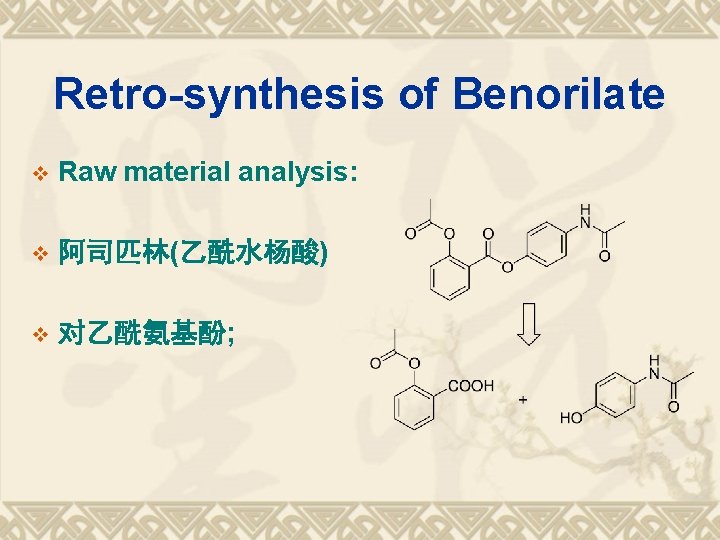
Retro-synthesis of Benorilate v Raw material analysis: v 阿司匹林(乙酰水杨酸) v 对乙酰氨基酚;
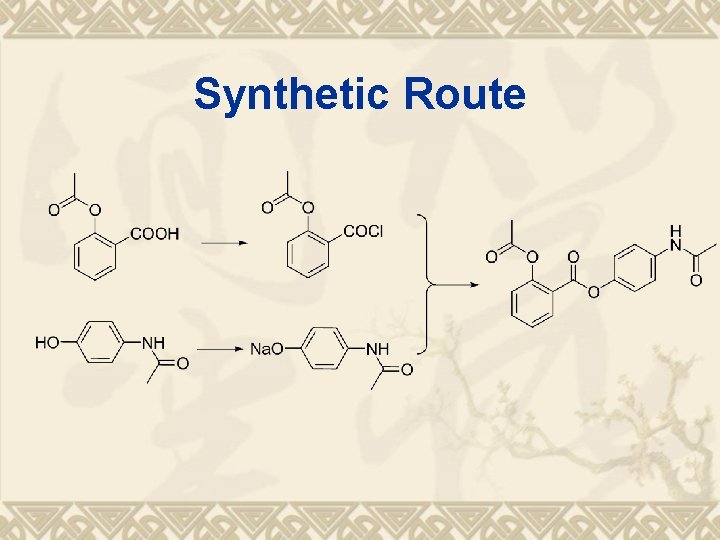
Synthetic Route

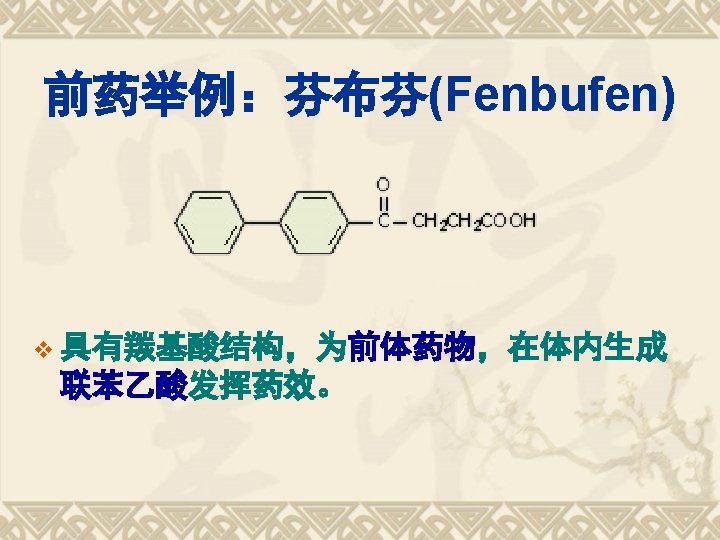
前药原理 Pro-drug Principle v v v A prodrug is a pharmacological substance that is administered in an inactive (or less than fully active) form, and is subsequently converted to an active pharmacological agent (drug) through normal metabolic processes (bioactivation). A prodrug serves as a type of 'precursor' to the intended drug. Prodrugs can be used to improve how the intended drug is absorbed, distributed, metabolized and excreted (ADME). Prodrugs are often designed to improve oral bioavailability in cases where the intended drug is poorly absorbed through the gastrointestinal tract. A prodrug may also be used to improve how selectively the intended drug interacts with cells or processes that are not its intended target. This reduces the adverse or unintended effects of the intended drug, especially important in treatments like chemotherapy, which can have severe unintended and undesirable side effects. 阿司匹林及扑热息痛均具有解热镇痛作用; 阿司匹林为酸性药物, 对胃粘 膜有刺激作用; 为减小其副作用, 提高药物吸收度, 使二者成酯得苯乐来; 苯 乐来为前药Pro-drug, 在体内被水解为乙酰水杨酸及对乙酰氨基酚.
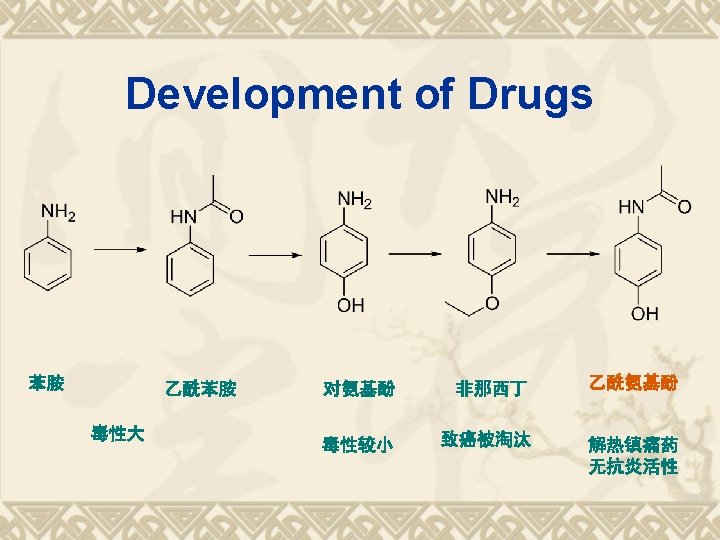
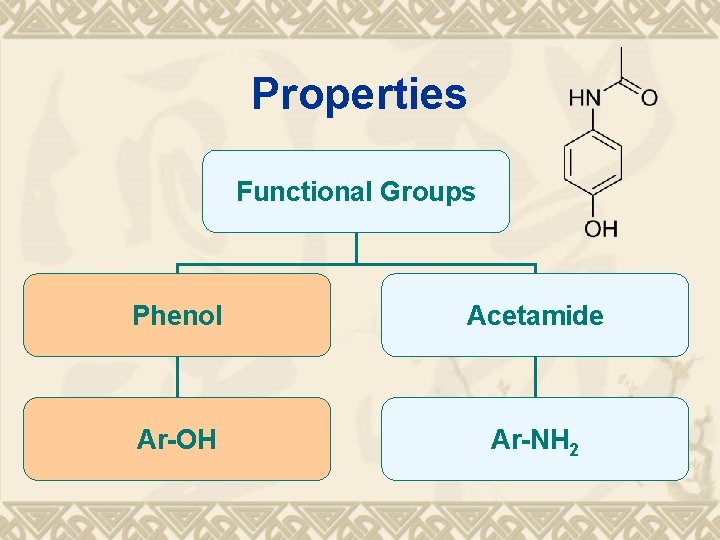
Properties Functional Groups Phenol Acetamide Ar-OH Ar-NH 2

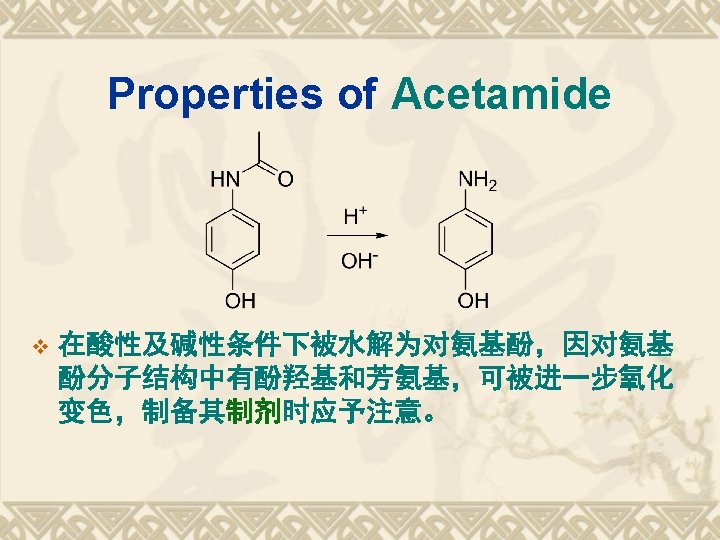
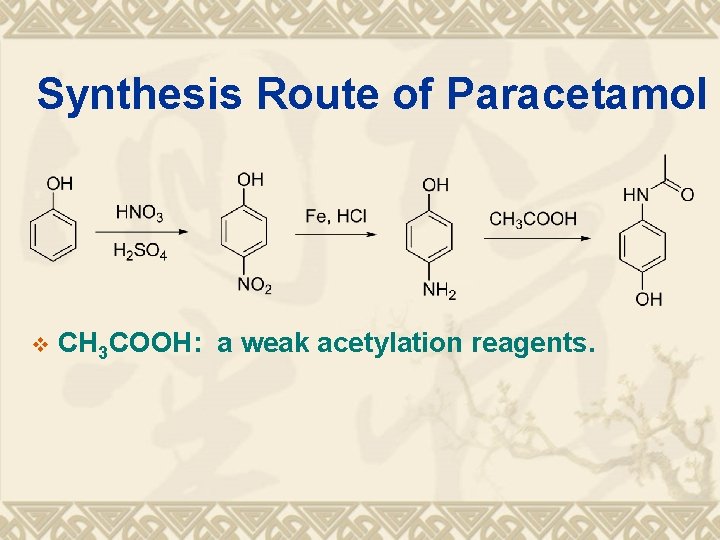
Synthesis Route of Paracetamol v CH 3 COOH: a weak acetylation reagents.
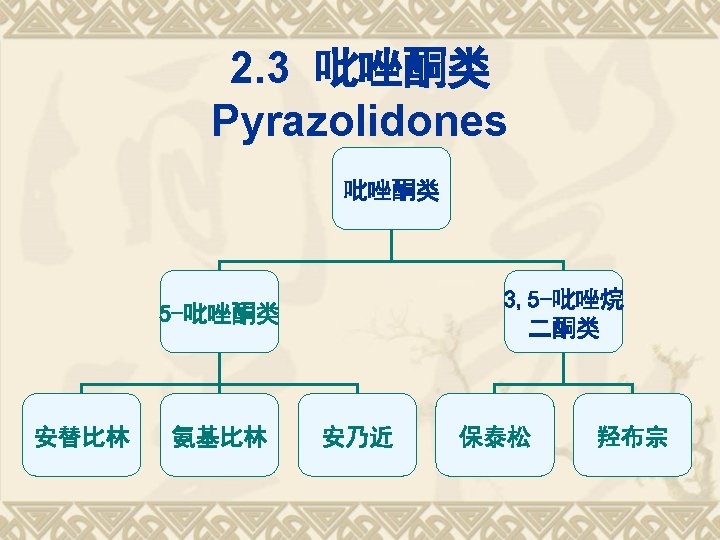
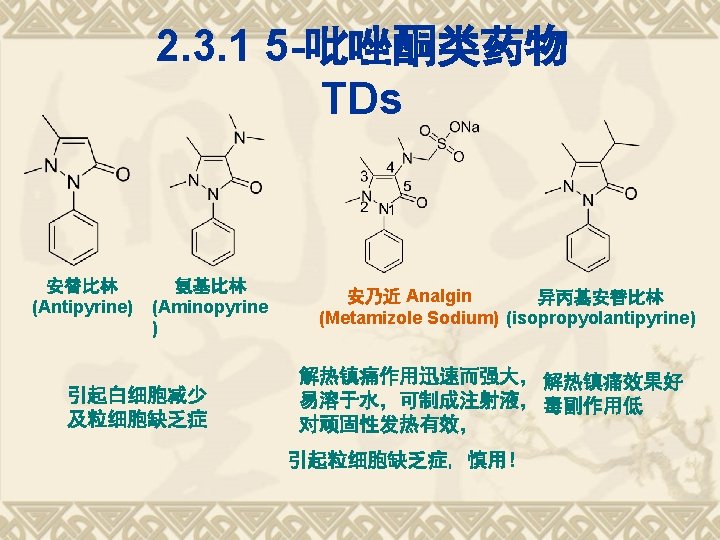
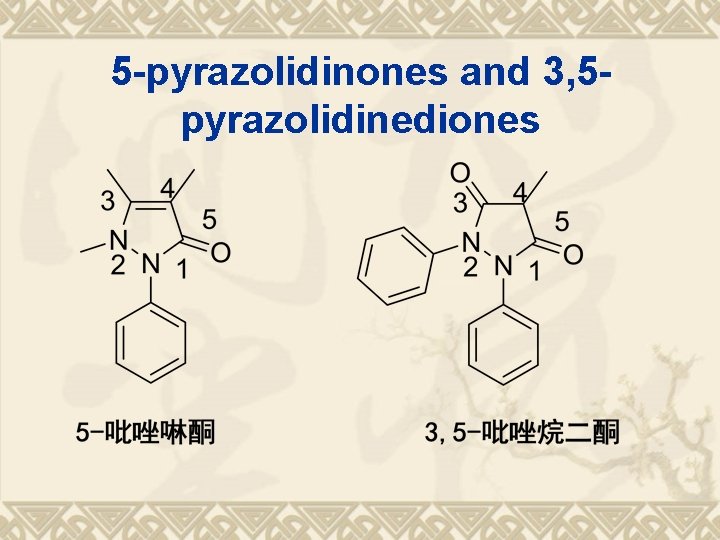
5 -pyrazolidinones and 3, 5 pyrazolidinediones
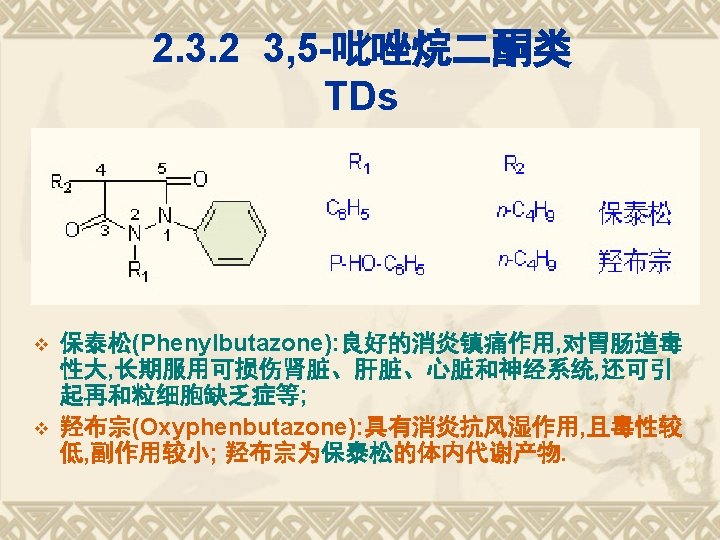

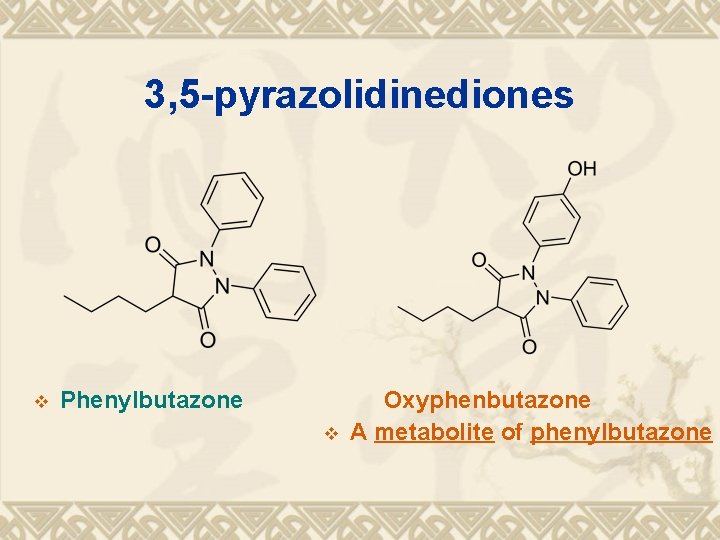
3, 5 -pyrazolidinediones v Phenylbutazone v Oxyphenbutazone A metabolite of phenylbutazone
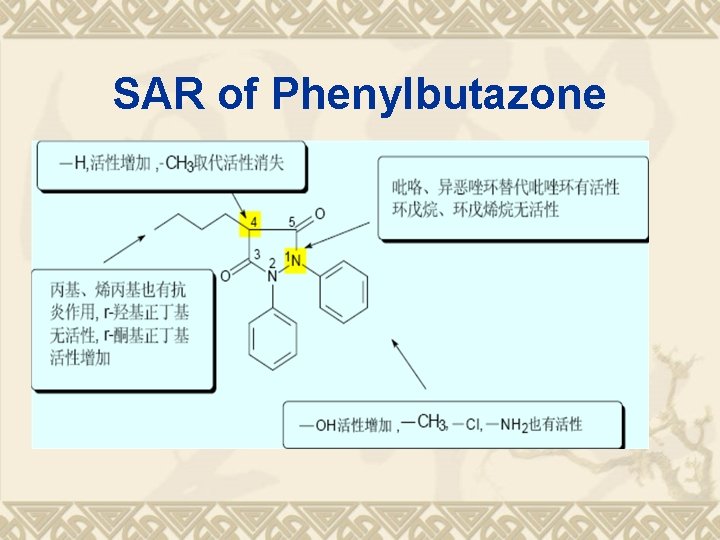
SAR of Phenylbutazone
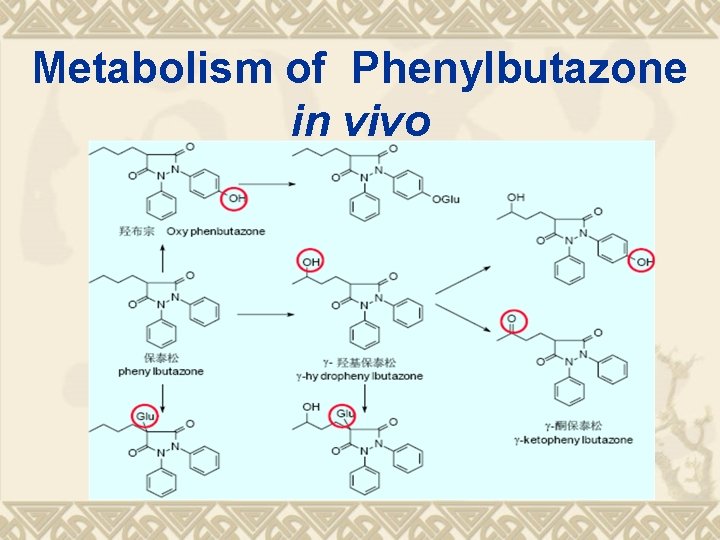
Metabolism of Phenylbutazone in vivo
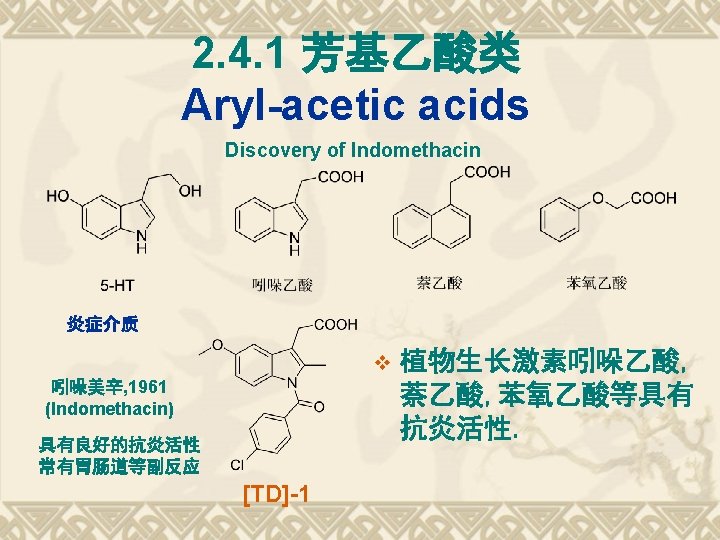
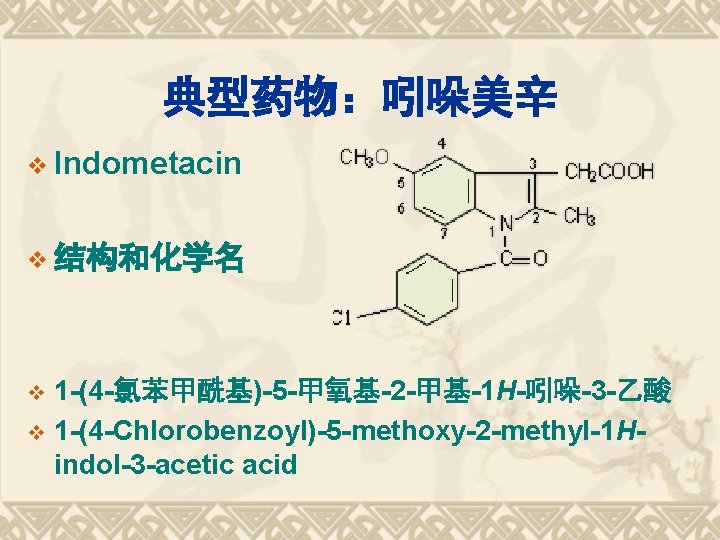
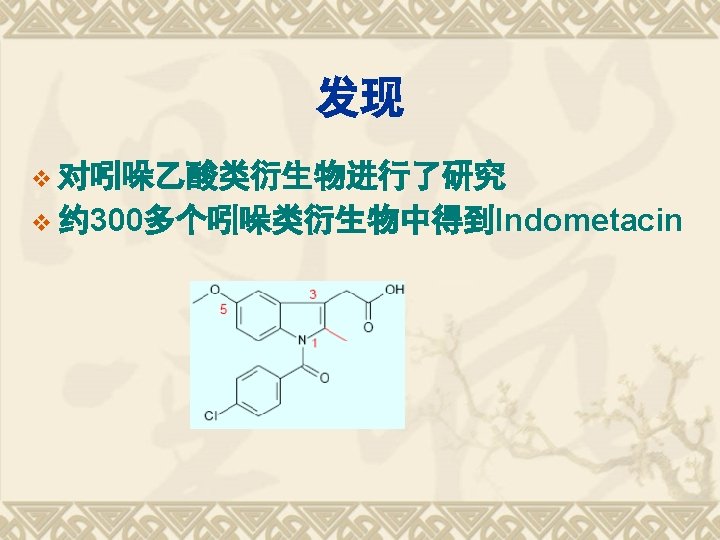
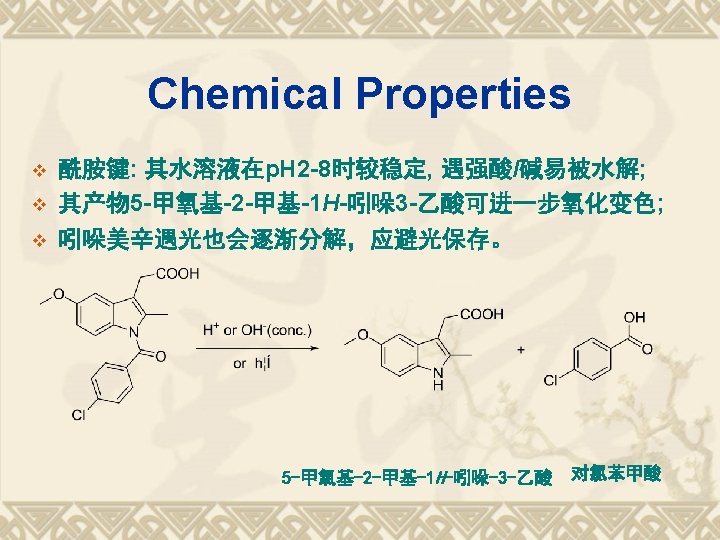
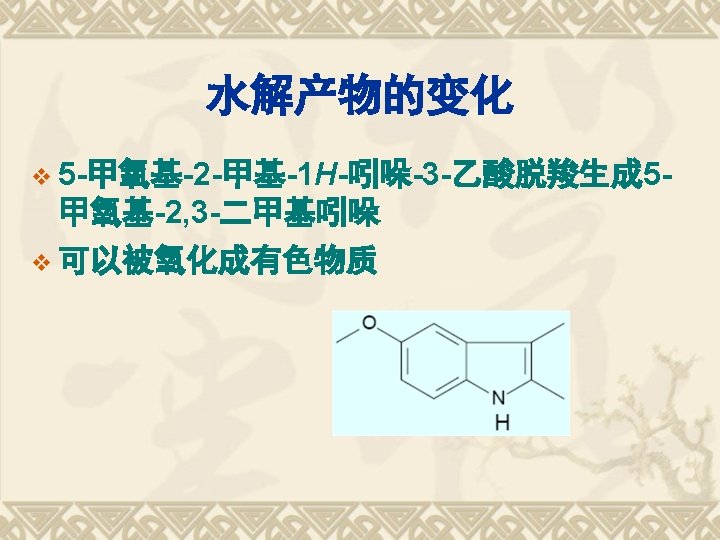
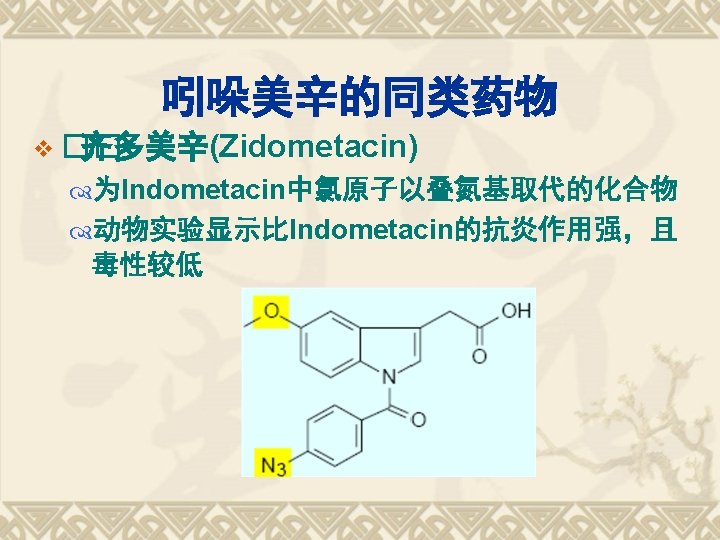
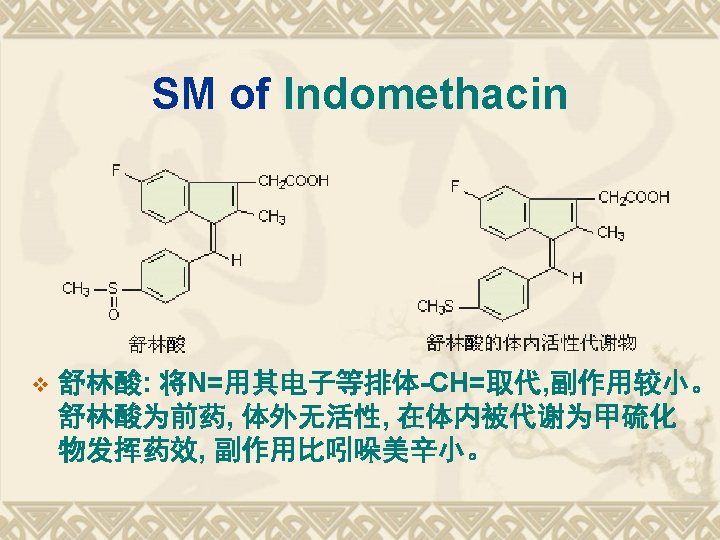
典型药物:吲哚美辛 v Indometacin v 结构和化学名 1 -(4 -氯苯甲酰基)-5 -甲氧基-2 -甲基-1 H-吲哚-3 -乙酸 v 1 -(4 -Chlorobenzoyl)-5 -methoxy-2 -methyl-1 Hindol-3 -acetic acid v
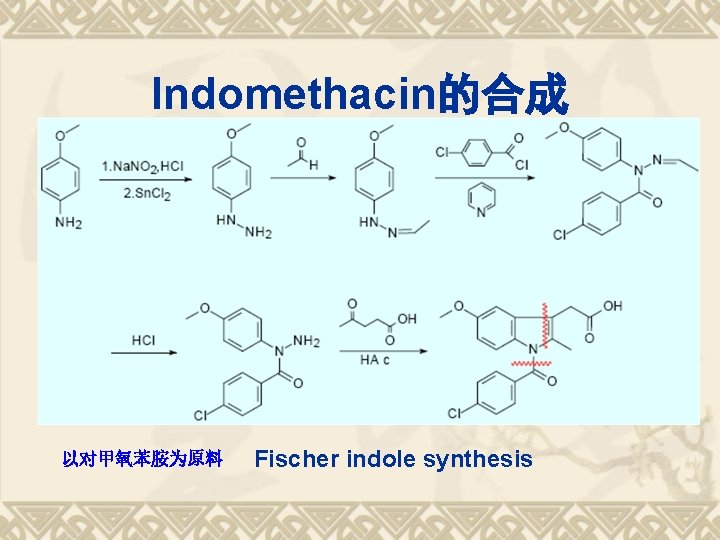
Indomethacin的合成 以对甲氧苯胺为原料 Fischer indole synthesis
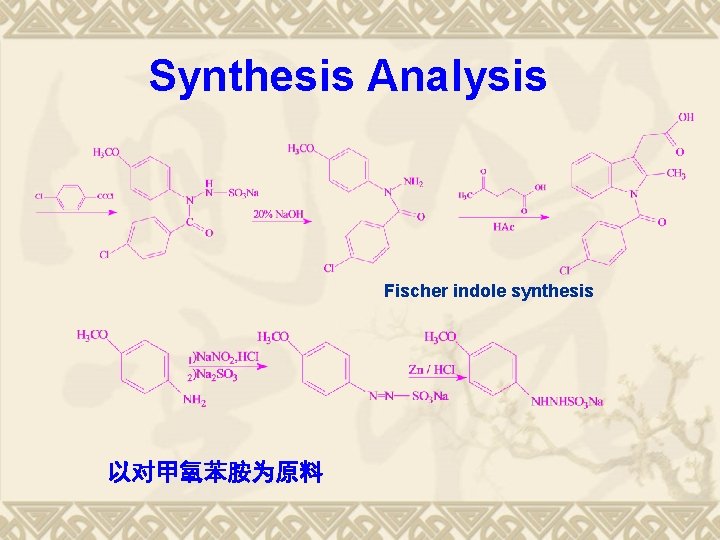
Synthesis Analysis Fischer indole synthesis 以对甲氧苯胺为原料
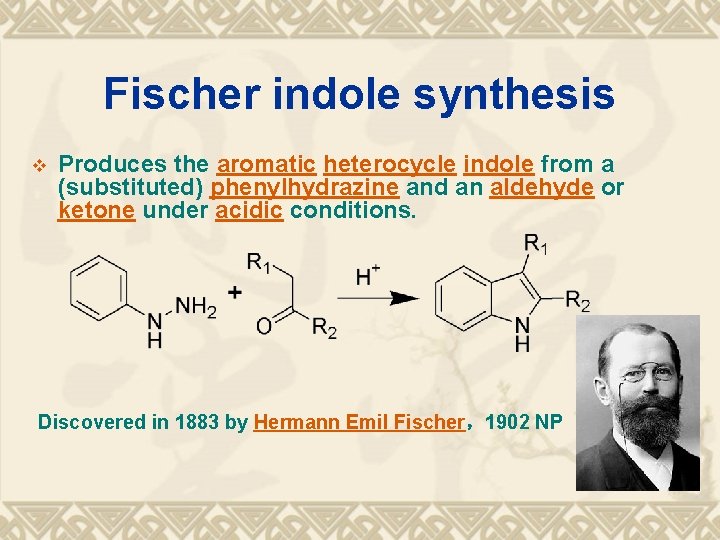
Fischer indole synthesis v Produces the aromatic heterocycle indole from a (substituted) phenylhydrazine and an aldehyde or ketone under acidic conditions. Discovered in 1883 by Hermann Emil Fischer,1902 NP
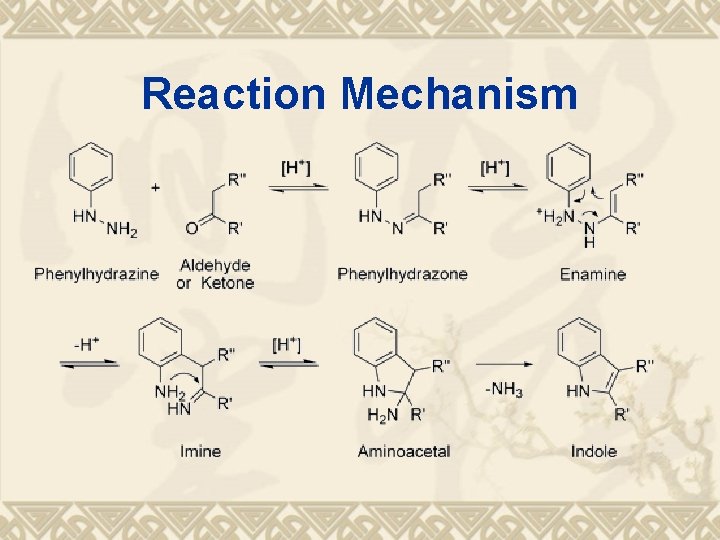
Reaction Mechanism
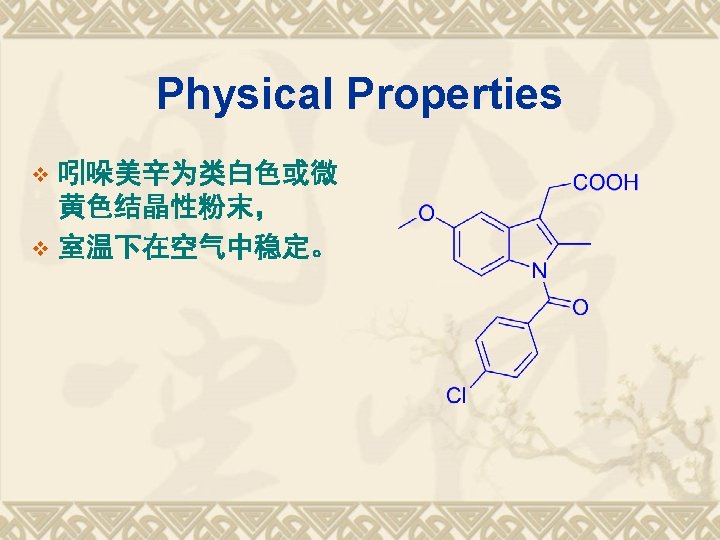
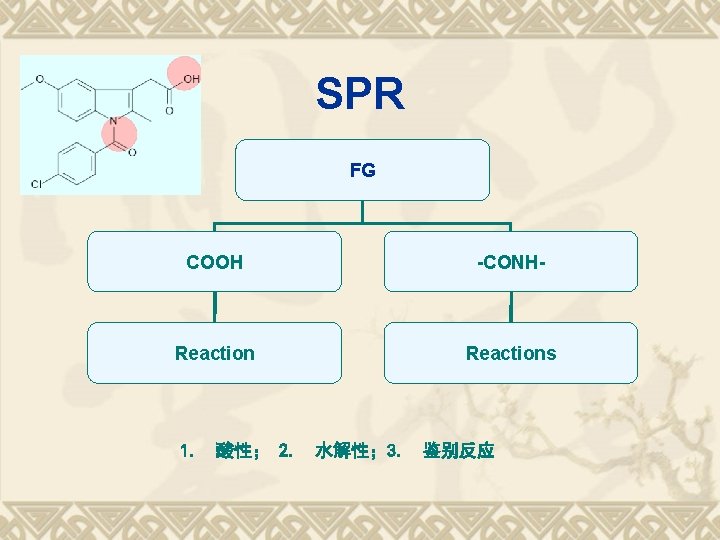
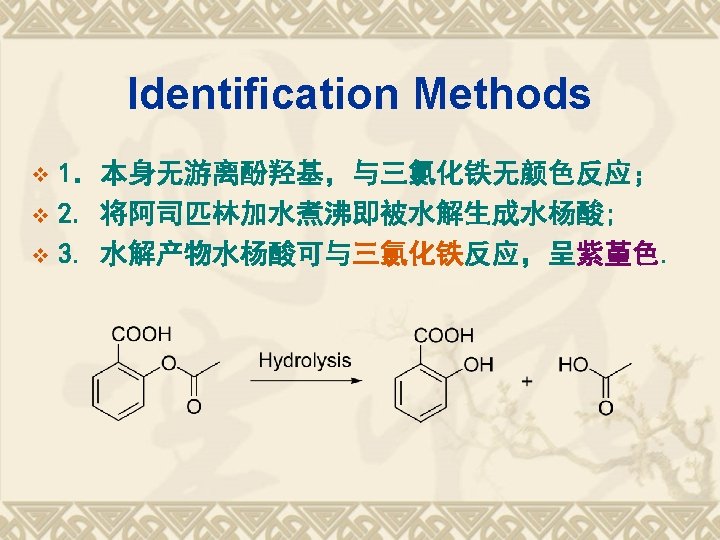
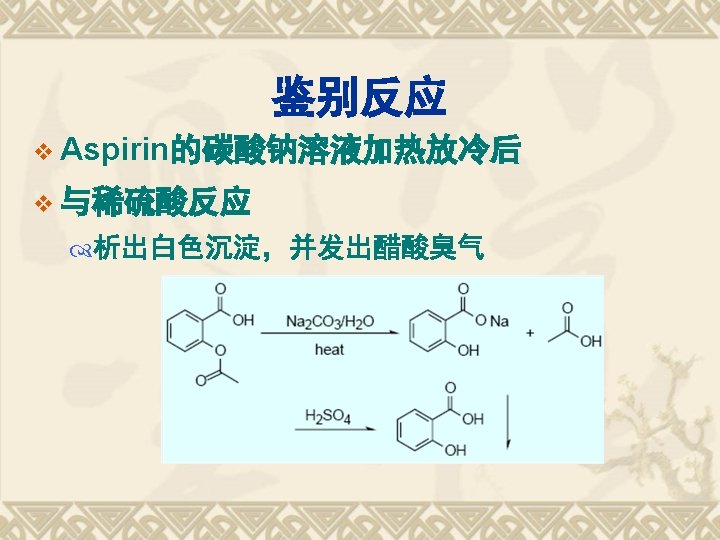
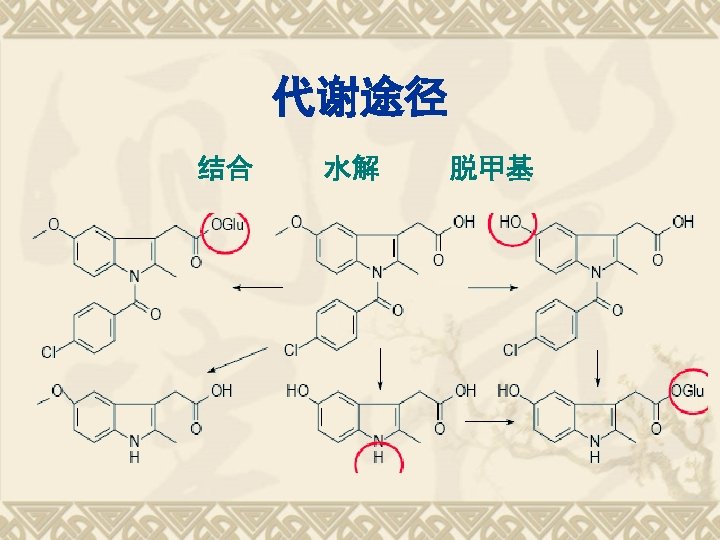
SPR FG COOH -CONH- Reactions 1. 酸性; 2. 水解性; 3. 鉴别反应
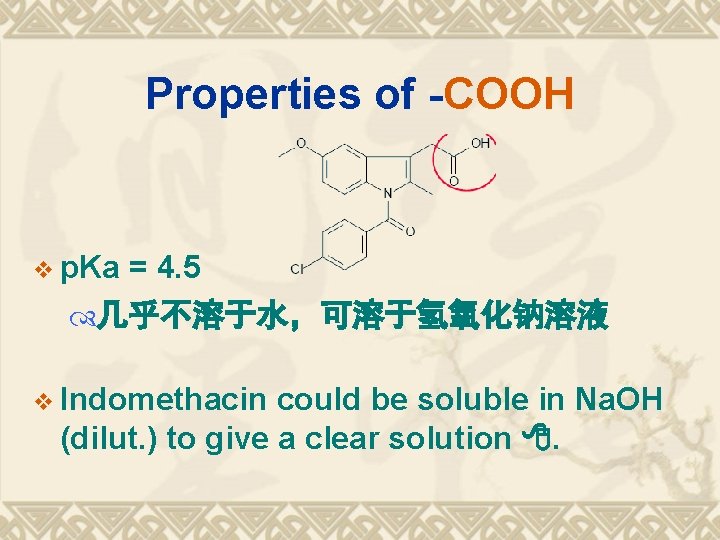
Properties of -COOH v p. Ka = 4. 5 几乎不溶于水,可溶于氢氧化钠溶液 v Indomethacin could be soluble in Na. OH (dilut. ) to give a clear solution .


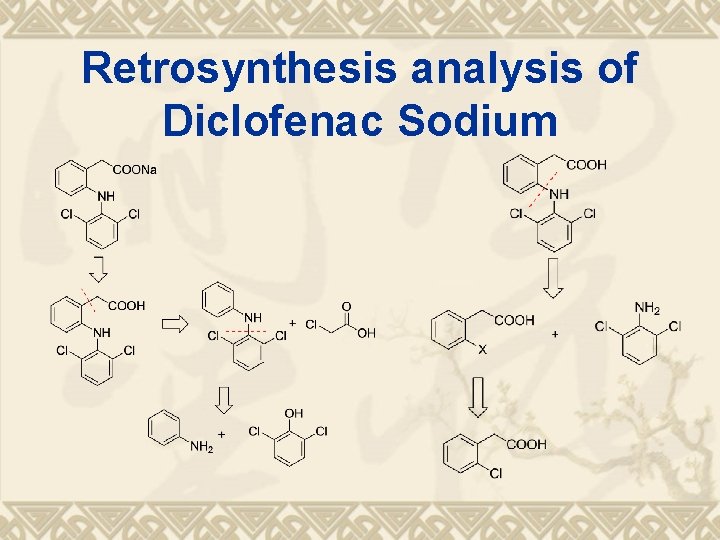
Retrosynthesis analysis of Diclofenac Sodium
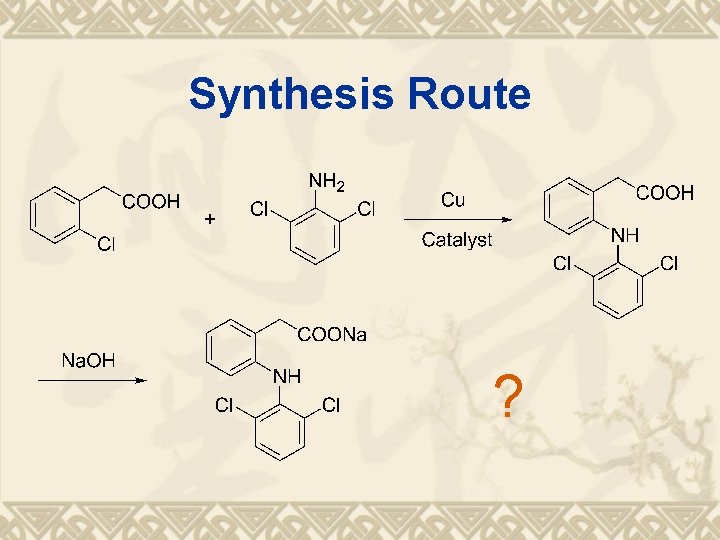
Synthesis Route ?
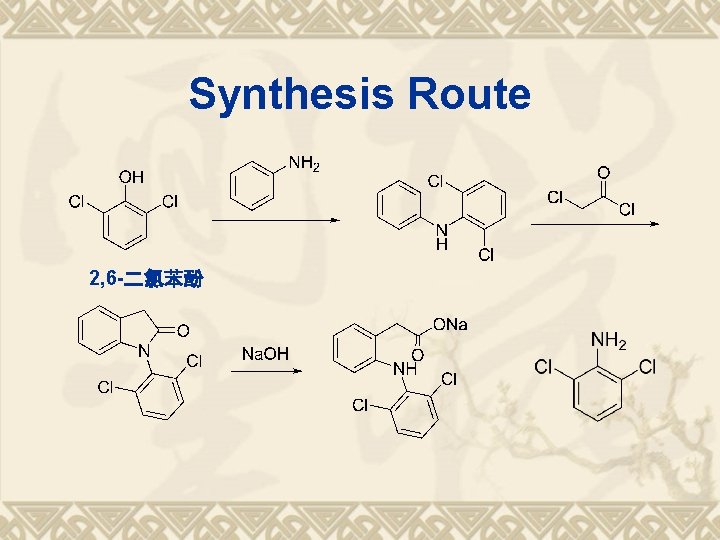
Synthesis Route 2, 6 -二氯苯酚

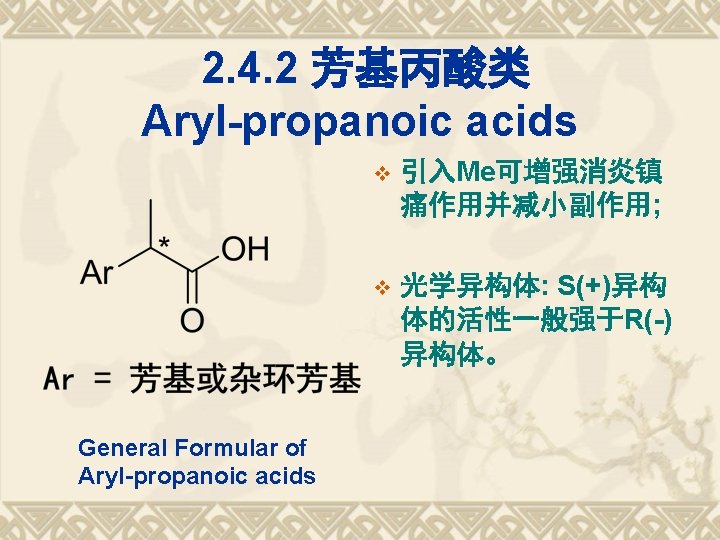
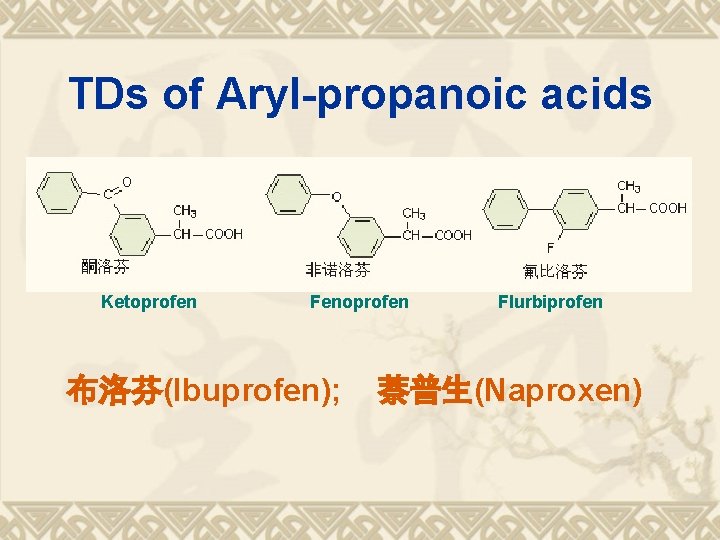
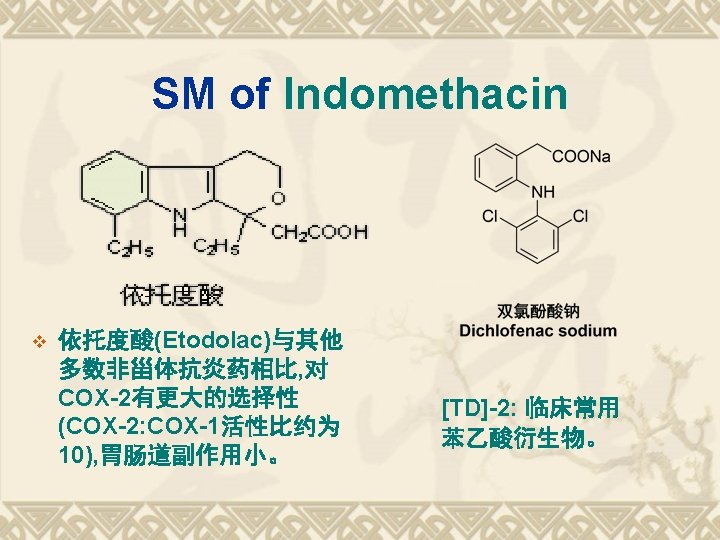
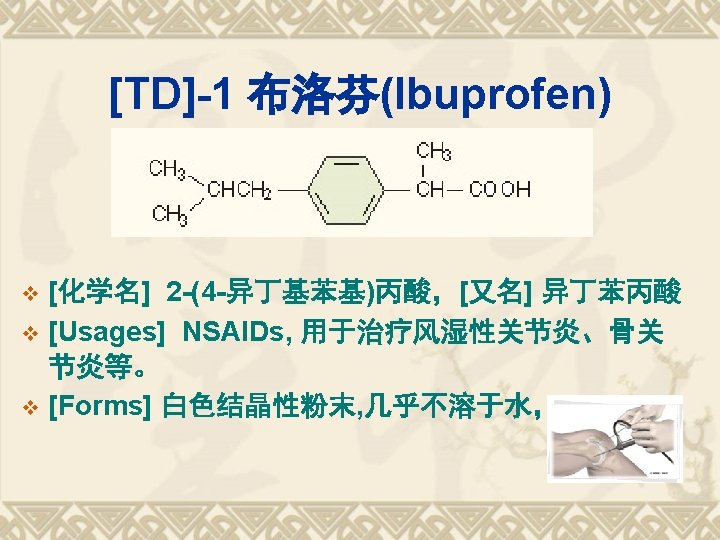
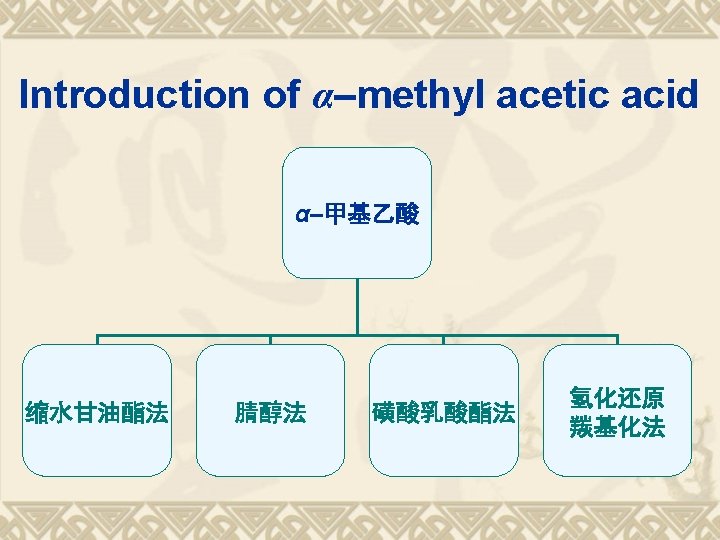
TDs of Aryl-propanoic acids Ketoprofen Fenoprofen 布洛芬(Ibuprofen); Flurbiprofen 萘普生(Naproxen)
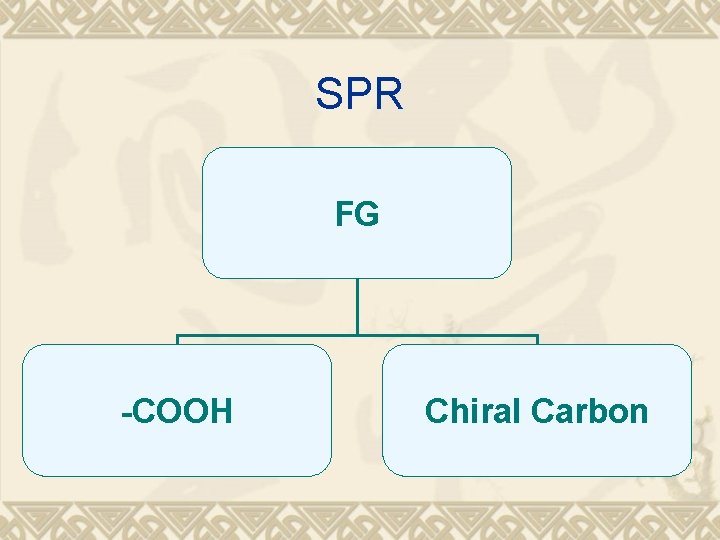
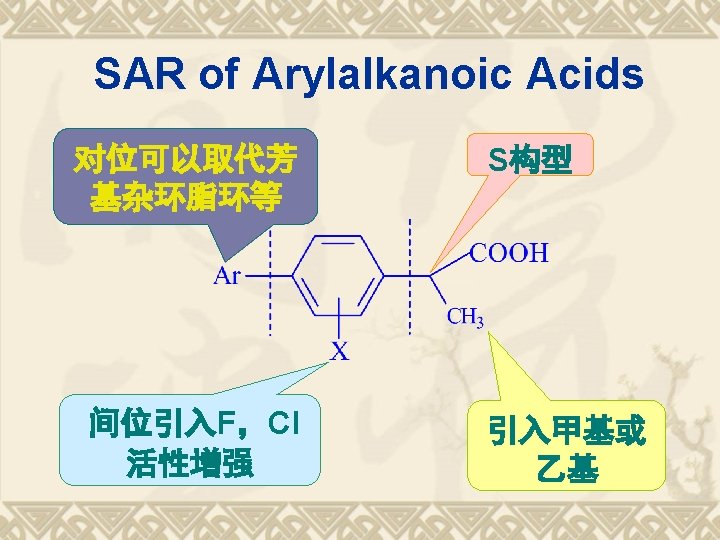
SPR FG -COOH Chiral Carbon


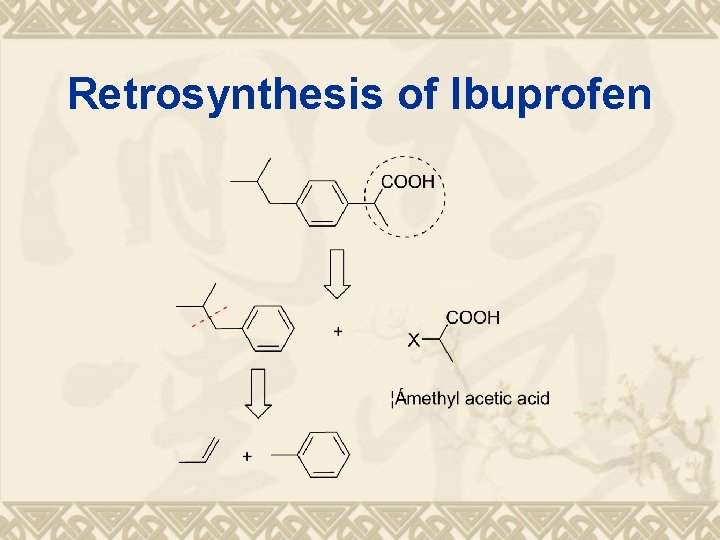
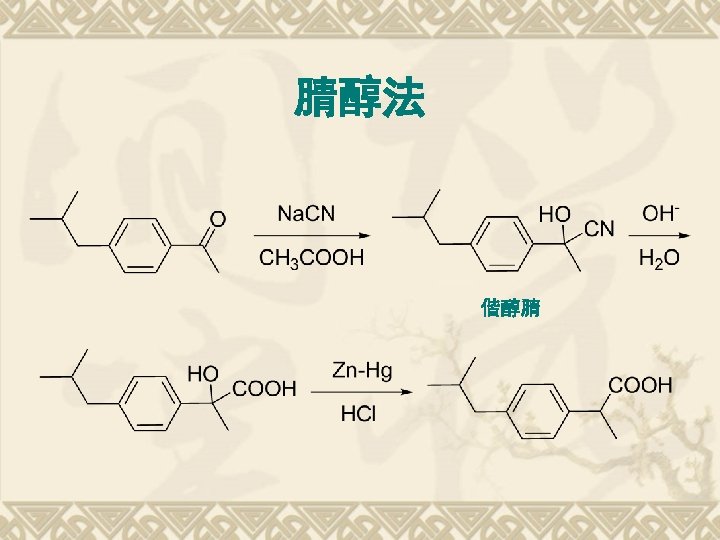
Retrosynthesis of Ibuprofen
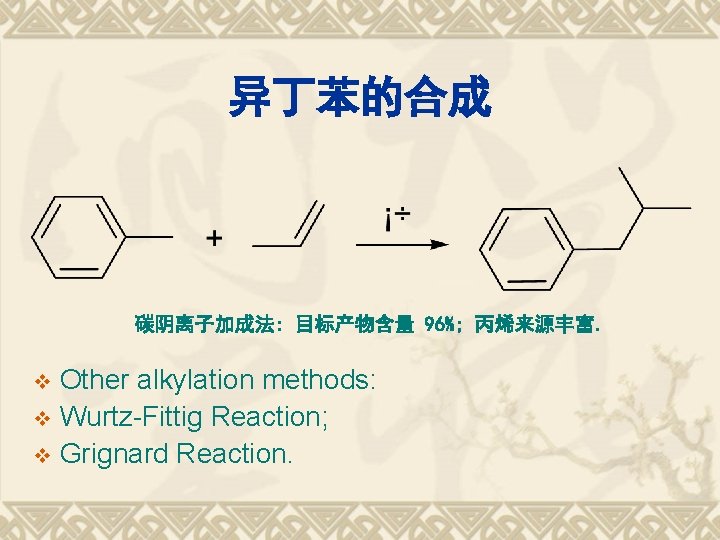
异丁苯的合成 碳阴离子加成法: 目标产物含量 96%; 丙烯来源丰富. Other alkylation methods: v Wurtz-Fittig Reaction; v Grignard Reaction. v
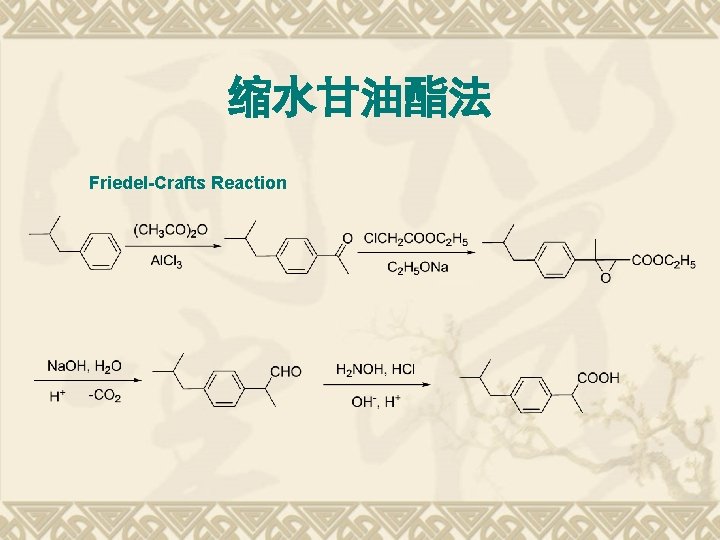
缩水甘油酯法 Friedel-Crafts Reaction

磺酸乳酸酯法 v Friedel-Crafts Reaction
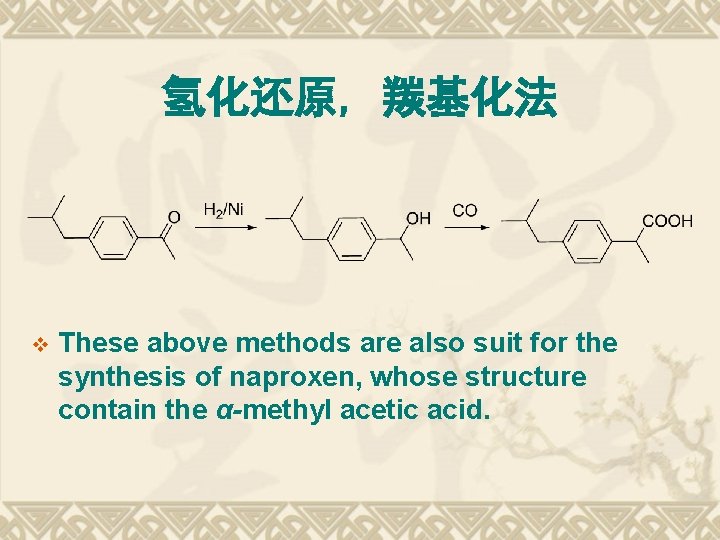
氢化还原, 羰基化法 v These above methods are also suit for the synthesis of naproxen, whose structure contain the α-methyl acetic acid.
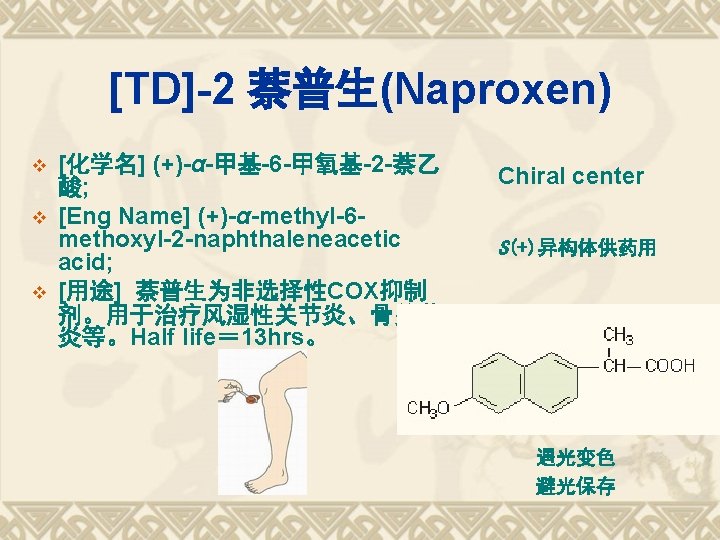

Solubility v Soluble in methanol, chloroform, etc. v Slightly soluble in ether, v Not soluble in water. v M. p. 153 -158.
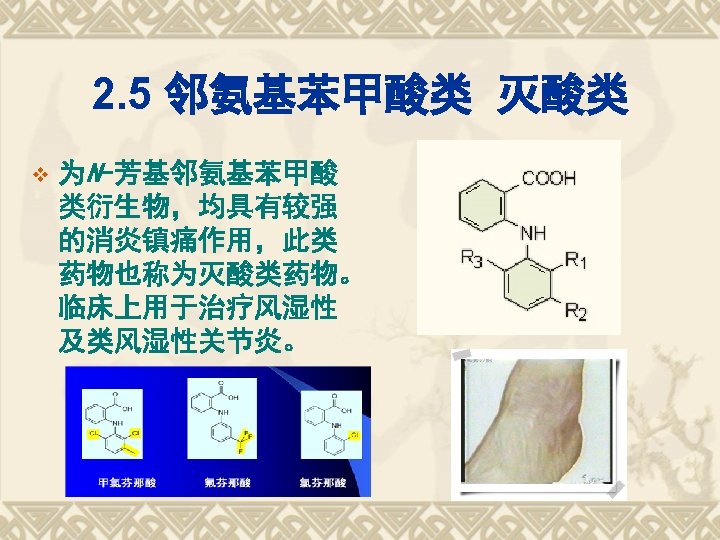
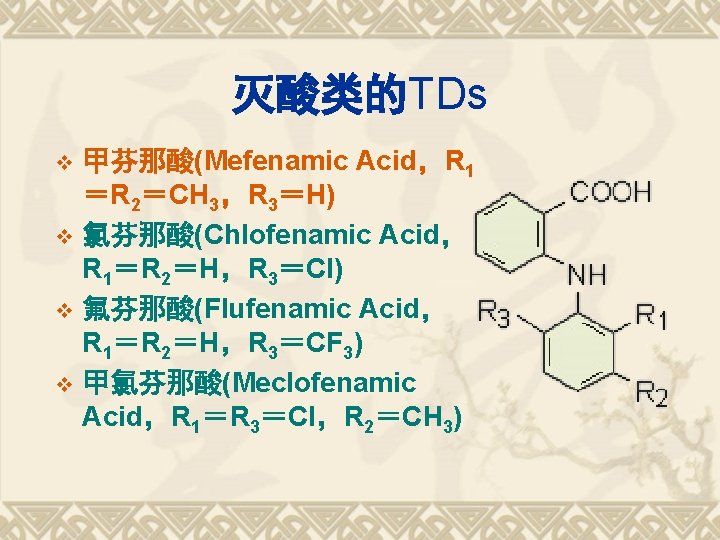
灭酸类的TDs 甲芬那酸(Mefenamic Acid,R 1 =R 2=CH 3,R 3=H) v 氯芬那酸(Chlofenamic Acid, R 1=R 2=H,R 3=Cl) v 氟芬那酸(Flufenamic Acid, R 1=R 2=H,R 3=CF 3) v 甲氯芬那酸(Meclofenamic Acid,R 1=R 3=Cl,R 2=CH 3) v

![甲芬那酸 Meclofena v 结构和化学名: v 2 -[(2, 3 -二甲基苯基)氨基]苯甲酸 v 2 -[(2, 3 -Dimethylphenyl)amino]benzoic 甲芬那酸 Meclofena v 结构和化学名: v 2 -[(2, 3 -二甲基苯基)氨基]苯甲酸 v 2 -[(2, 3 -Dimethylphenyl)amino]benzoic](http://slidetodoc.com/presentation_image_h2/7f5fdf3b217c26b73bec98e7edb0e762/image-115.jpg)
甲芬那酸 Meclofena v 结构和化学名: v 2 -[(2, 3 -二甲基苯基)氨基]苯甲酸 v 2 -[(2, 3 -Dimethylphenyl)amino]benzoic acid

![吡罗昔康(Piroxicam) v [又名] 炎痛喜康; Ten Mel [化学名] 4 -羟基-2 -甲基-N-2 -吡啶基-2 H-1, 2 -苯并噻 吡罗昔康(Piroxicam) v [又名] 炎痛喜康; Ten Mel [化学名] 4 -羟基-2 -甲基-N-2 -吡啶基-2 H-1, 2 -苯并噻](http://slidetodoc.com/presentation_image_h2/7f5fdf3b217c26b73bec98e7edb0e762/image-119.jpg)
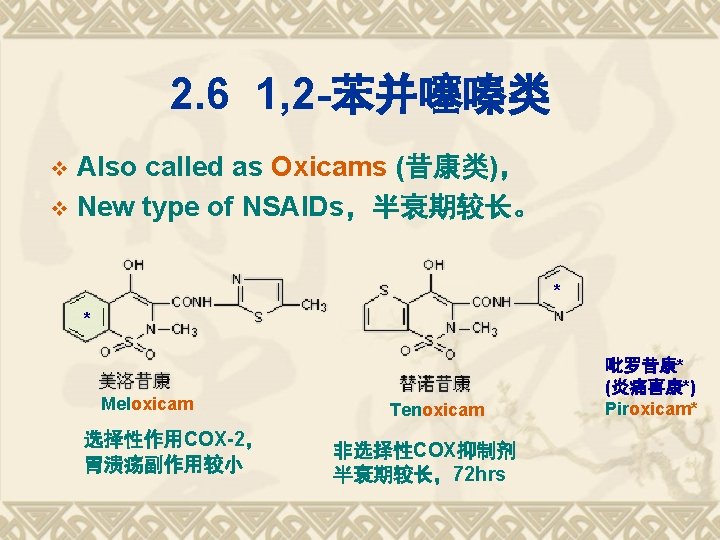
吡罗昔康(Piroxicam) v [又名] 炎痛喜康; Ten Mel [化学名] 4 -羟基-2 -甲基-N-2 -吡啶基-2 H-1, 2 -苯并噻 嗪-3 -甲酰胺-1, 1 -二氧化物; v 4 -hydroxy-2 -methyl-N-2 -pyridinyl-2 H-1, 2 benzothiazine-3 -carboxamide-1, 1 -dioxide. v

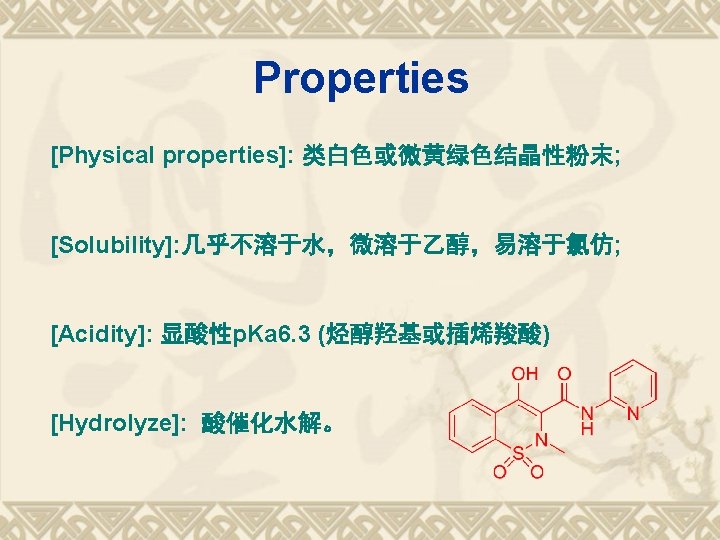
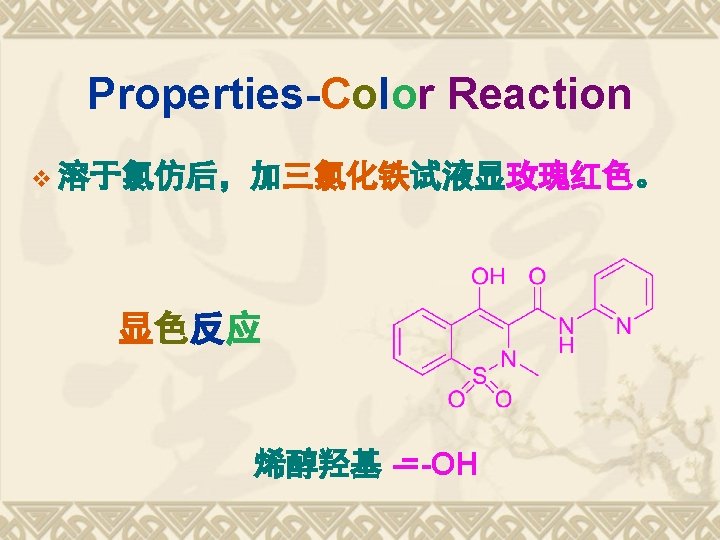

Retrosynthesis analysis of Piroxicam Garbriel-Colman Rearrangement

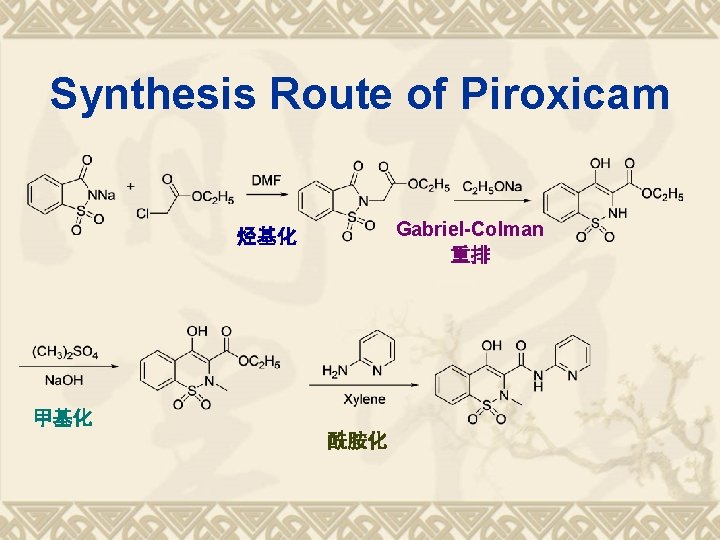
Synthesis Route of Piroxicam Gabriel-Colman 重排 烃基化 甲基化 酰胺化
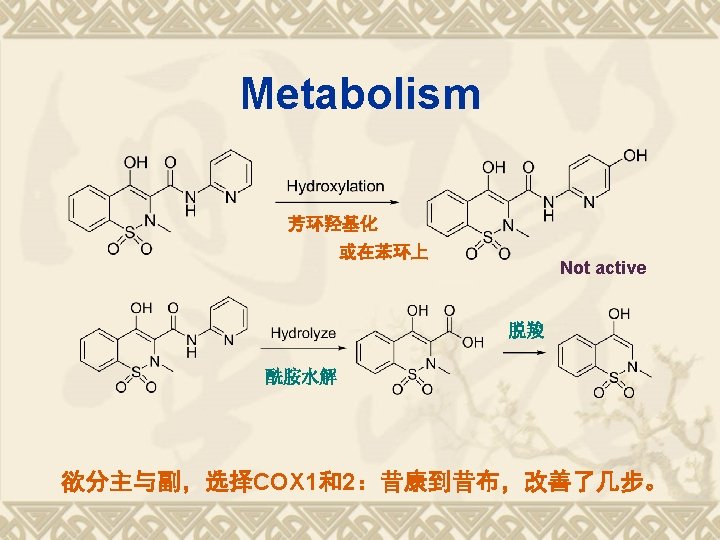
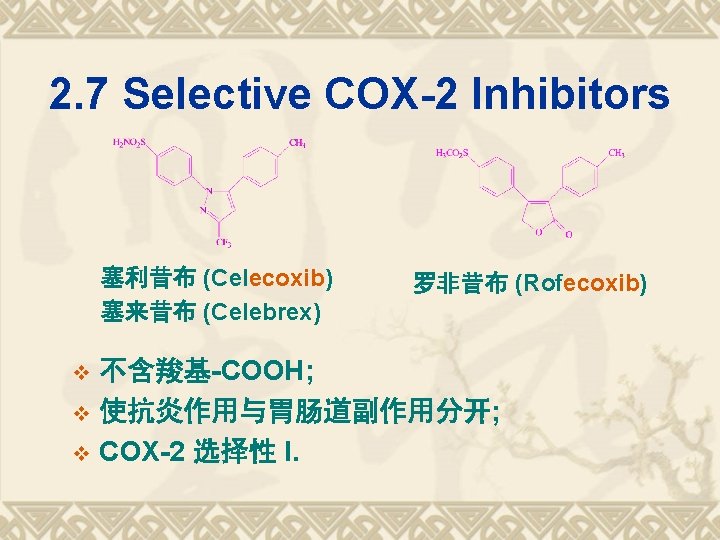
2. 7 Selective COX-2 Inhibitors 塞利昔布 (Celecoxib) 塞来昔布 (Celebrex) 罗非昔布 (Rofecoxib) 不含羧基-COOH; v 使抗炎作用与胃肠道副作用分开; v COX-2 选择性 I. v






Reivew of SAR of Naproxen v 6 -Me. O other position, activity decreases; v 6 -Me. O larger groups: Cl, CH 3, F 2 CHO etc. with less steric hinder; activity remains; ; v 6 -Me. O smaller groups, with more steric hinder activity decreases.
- Slides: 134