Chapter 6 A Chemical Bonding General Chemistry Mr
Chapter 6 A: Chemical Bonding General Chemistry Mr. Mata Cartoon courtesy of Nearing. Zero. net

Standard 2 a • Atoms combine to form molecules by sharing electrons to form covalent or metallic bonds.
Essential Question • How do electrons behave during bonding?
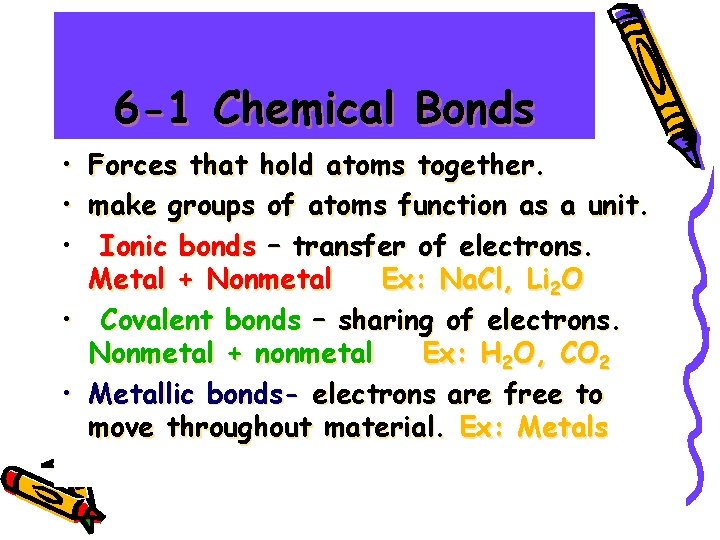
6 -1 Chemical Bonds • • • Forces that hold atoms together. make groups of atoms function as a unit. Ionic bonds – transfer of electrons. Metal + Nonmetal Ex: Na. Cl, Li 2 O • Covalent bonds – sharing of electrons. Nonmetal + nonmetal Ex: H 2 O, CO 2 • Metallic bonds- electrons are free to move throughout material. Ex: Metals
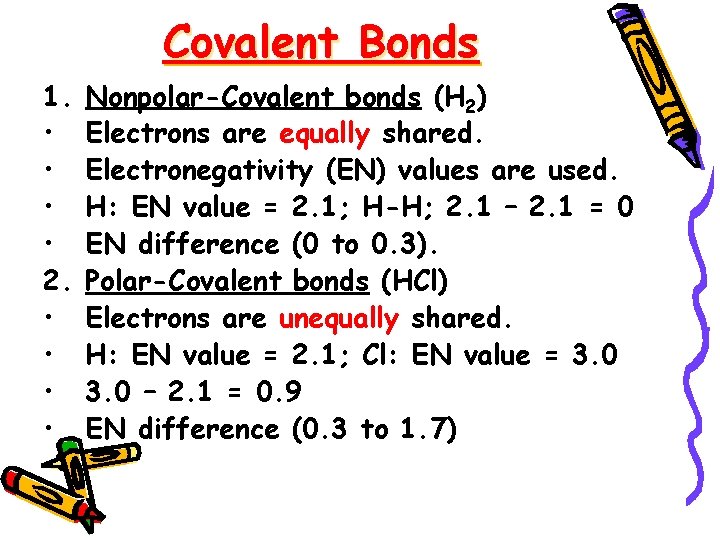
Covalent Bonds 1. • • 2. • • Nonpolar-Covalent bonds (H 2) Electrons are equally shared. Electronegativity (EN) values are used. H: EN value = 2. 1; H-H; 2. 1 – 2. 1 = 0 EN difference (0 to 0. 3). Polar-Covalent bonds (HCl) Electrons are unequally shared. H: EN value = 2. 1; Cl: EN value = 3. 0 – 2. 1 = 0. 9 EN difference (0. 3 to 1. 7)
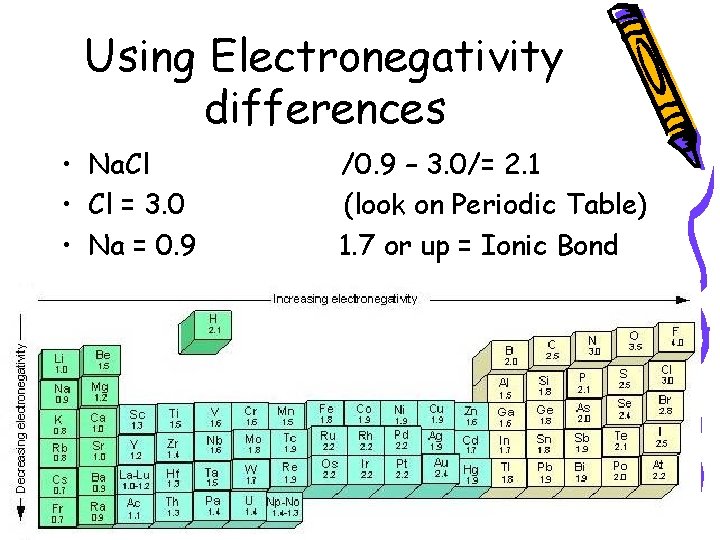
Using Electronegativity differences • Na. Cl • Cl = 3. 0 • Na = 0. 9 /0. 9 – 3. 0/= 2. 1 (look on Periodic Table) 1. 7 or up = Ionic Bond
6 -2 Covalent Bonding • Molecule- smallest unit of matter that can exist & retain all properties of substance. Examples: H 2 O & O 2, C 12 H 22 O 11 • Diatomic molecule- molecule containing 2 identical atoms. (H 2 N 2 O 2 F 2 Cl 2 Br 2 I 2)
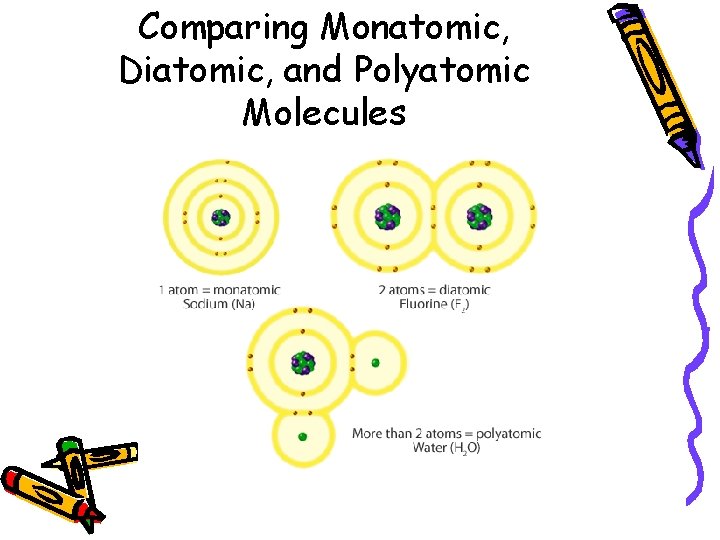
Comparing Monatomic, Diatomic, and Polyatomic Molecules
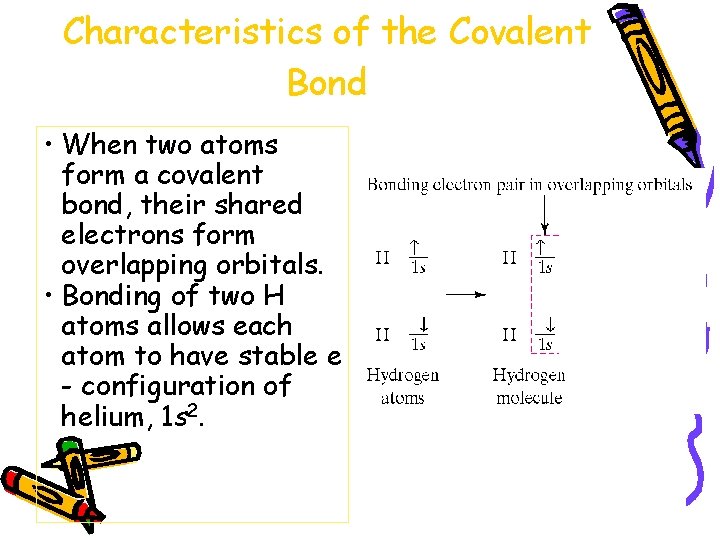
Characteristics of the Covalent Bond • When two atoms form a covalent bond, their shared electrons form overlapping orbitals. • Bonding of two H atoms allows each atom to have stable e - configuration of helium, 1 s 2.
• Chemical Formula- represents relative # of atoms in a chemical compound by using symbols & subscripts. • Example: H 2 O H=2 atoms O=1 atom • Molecular compound (Covalent) - simplest formula unit are molecules. • Have low melting & boiling pts. • Molecular formula- shows types & #’s of atoms combined in a single molecule.

• Bond Length- average distance between 2 bonded atoms. • Bond Energy- energy required to break a bond. • Gives info about strength of bond. • Energy is stored in chemical bonds.

The Octet Rule • Compounds form so that each atom has an octet (8) e -’s outer shell. • By gaining, losing, or sharing e-’s. H He 8 is Great!
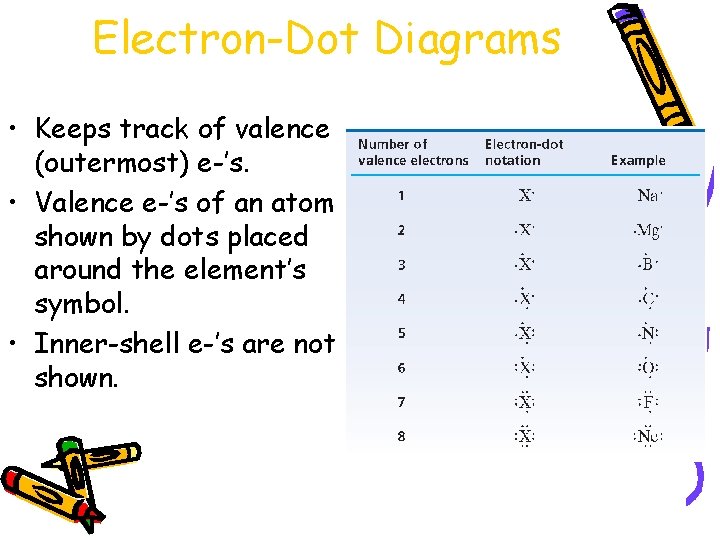
Electron-Dot Diagrams • Keeps track of valence (outermost) e-’s. • Valence e-’s of an atom shown by dots placed around the element’s symbol. • Inner-shell e-’s are not shown.
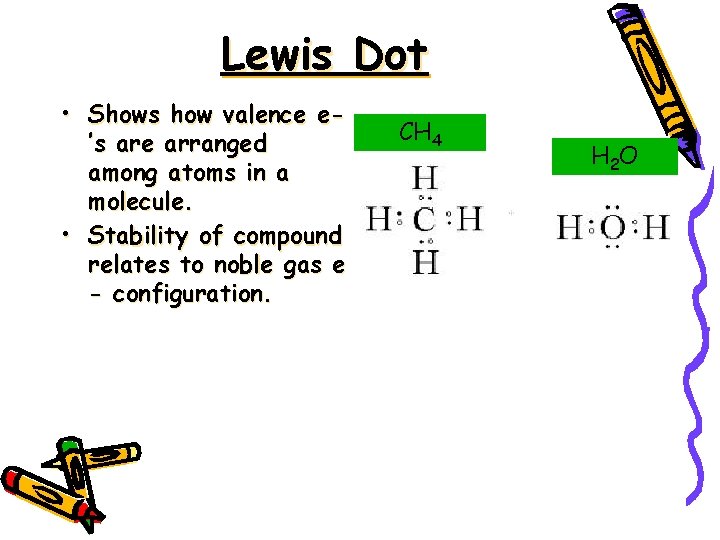
Lewis Dot • Shows how valence e’s are arranged among atoms in a molecule. • Stability of compound relates to noble gas e - configuration. CH 4 H 2 O
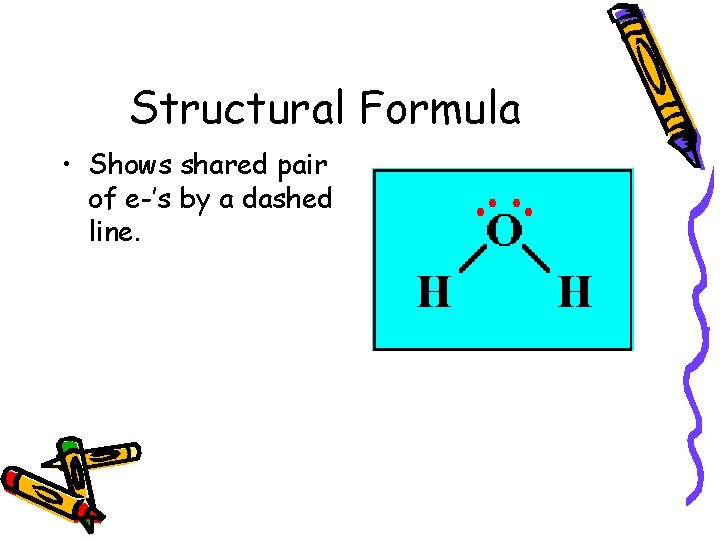
Structural Formula • Shows shared pair of e-’s by a dashed line.
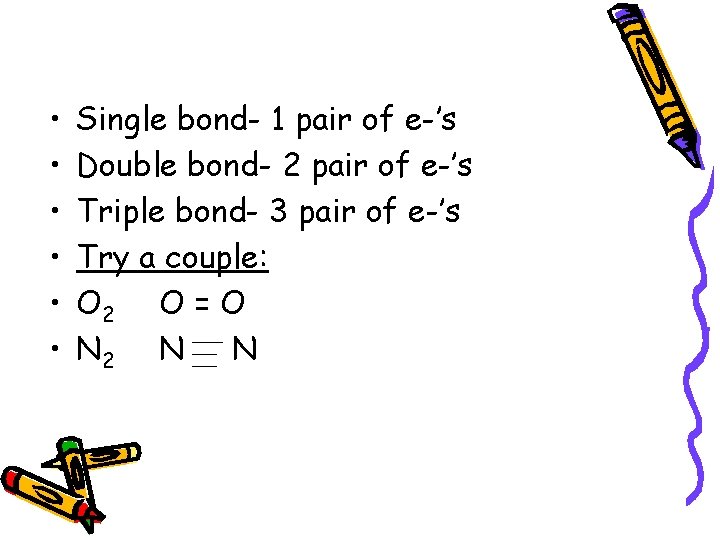
• • • Single bond- 1 pair of e-’s Double bond- 2 pair of e-’s Triple bond- 3 pair of e-’s Try a couple: O 2 O = O N 2 N N
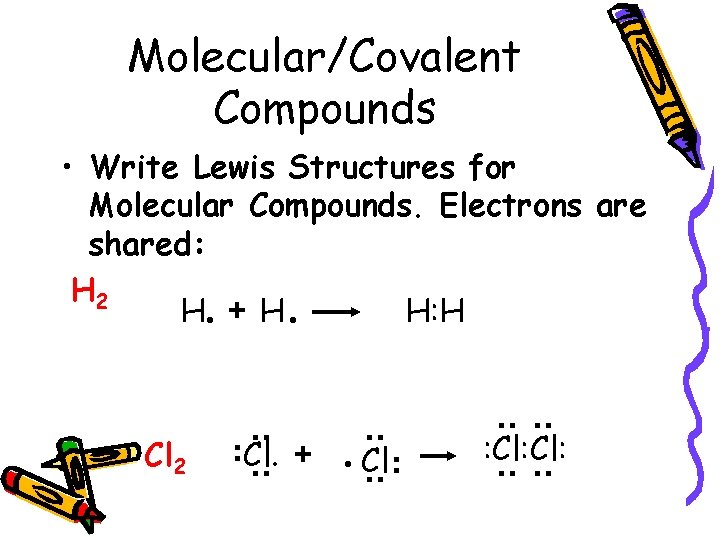
Molecular/Covalent Compounds : : Cl: : : . Cl : : : Cl. + : : Cl 2 : : • Write Lewis Structures for Molecular Compounds. Electrons are shared: H 2 H. + H. H: H
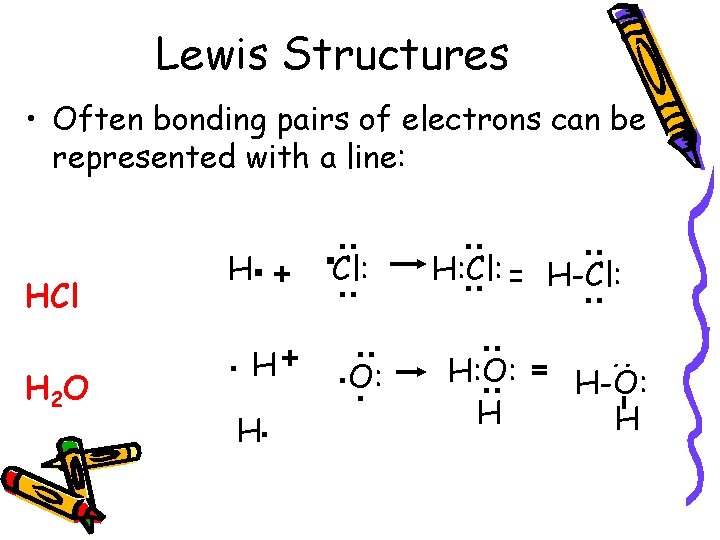
Lewis Structures • Often bonding pairs of electrons can be represented with a line: : H: Cl: = H-Cl: : : H: O: = H-O: H H : . O: . : : H 2 O . H+ . Cl: : HCl H. +
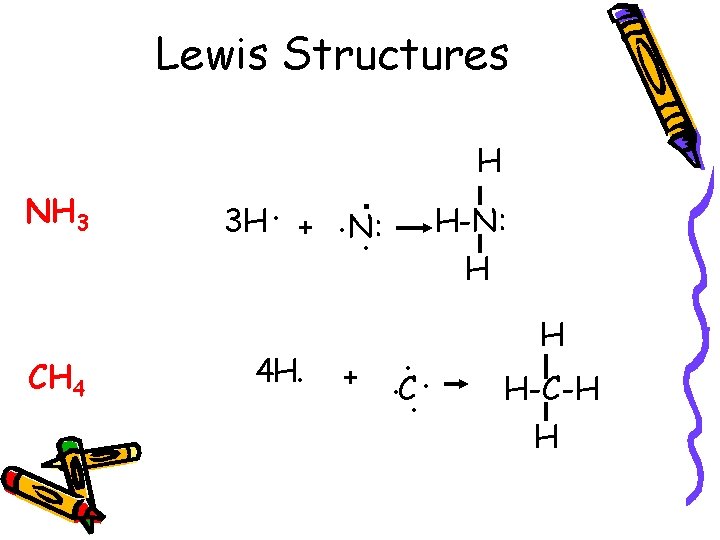
Lewis Structures NH 3 CH 4 3 H. H . +. N: . 4 H. + H-N: H. . C. . H H-C-H H
- Slides: 19