CHAPTER 6 1 Solutions and Other Mixtures Classifying








- Slides: 18

CHAPTER 6. 1 Solutions and Other Mixtures

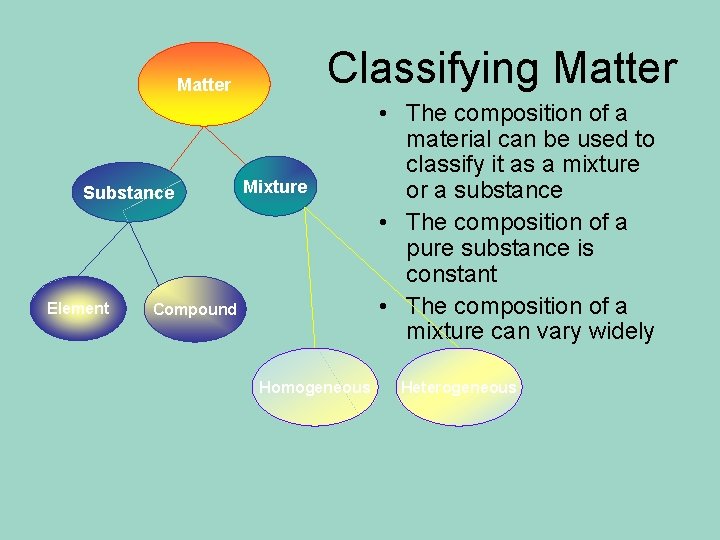
Classifying Matter Substance Element Mixture Compound Homogeneous • The composition of a material can be used to classify it as a mixture or a substance • The composition of a pure substance is constant • The composition of a mixture can vary widely Heterogeneous

Types of Mixtures • Heterogeneous Mixture: The parts of the mixture are noticeably different from one another. – Examples: Sand, Salsa, Chocolate Chip Cookie Dough • Homogeneous Mixture: The substances are so evenly distributed that it is difficult to distinguish one substance in the mixture from another. – Examples: Swimming pool water, Stainless steel fork

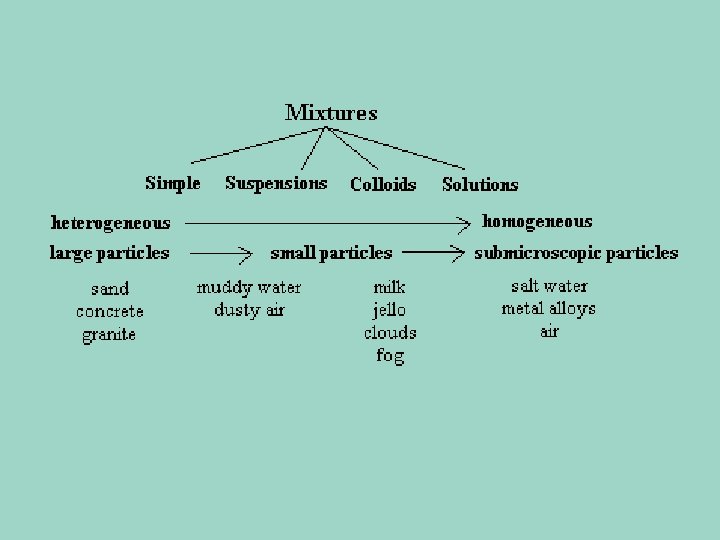
Solutions, Suspensions, & Colloids • Mixtures can be classified based on the size of their largest particles.


Suspension • Suspension – • Settles out or separates into different layers when it is no longer agitated • (no longer moving). • Heterogeneous mixture • Separates into layers over time • Particles can be trapped by filter paper (like a coffee filter) • Are cloudy in appearance • Examples: Sand Water

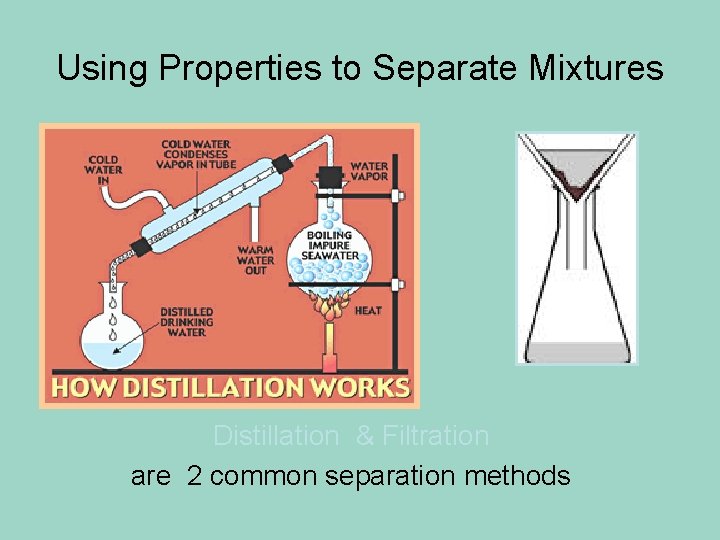
Filtration • Separates materials based on the size of their particles • Examples: – Drip coffee makers – Wire screens at an archaeological site

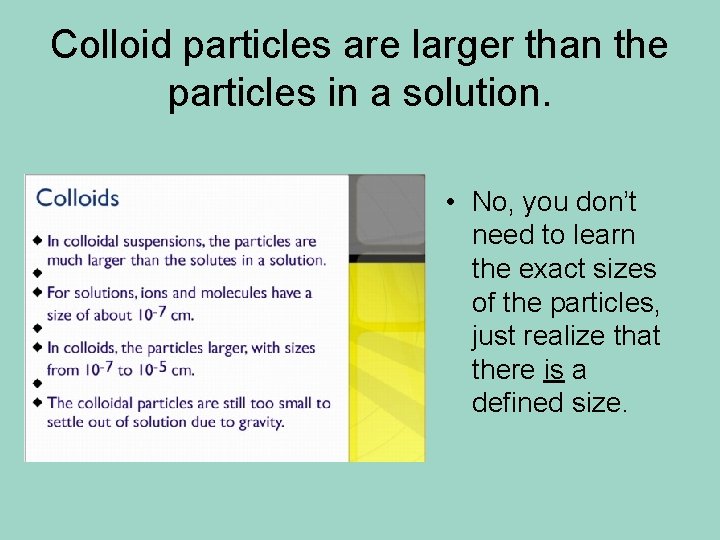
Colloid particles are larger than the particles in a solution. • No, you don’t need to learn the exact sizes of the particles, just realize that there is a defined size.

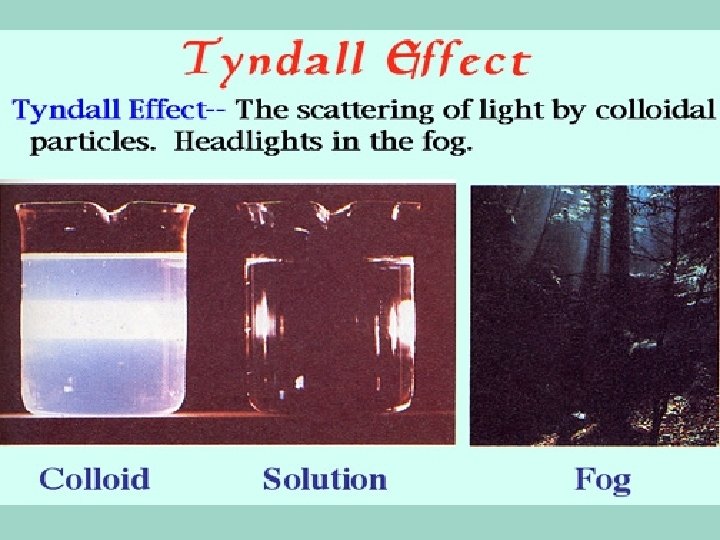
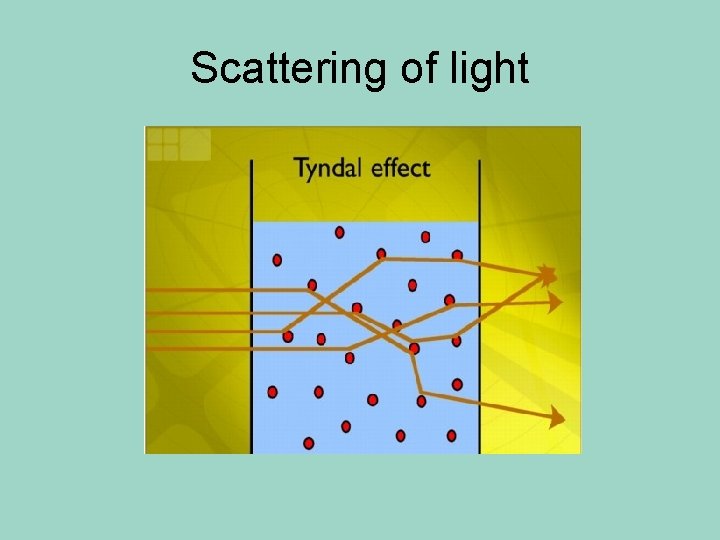
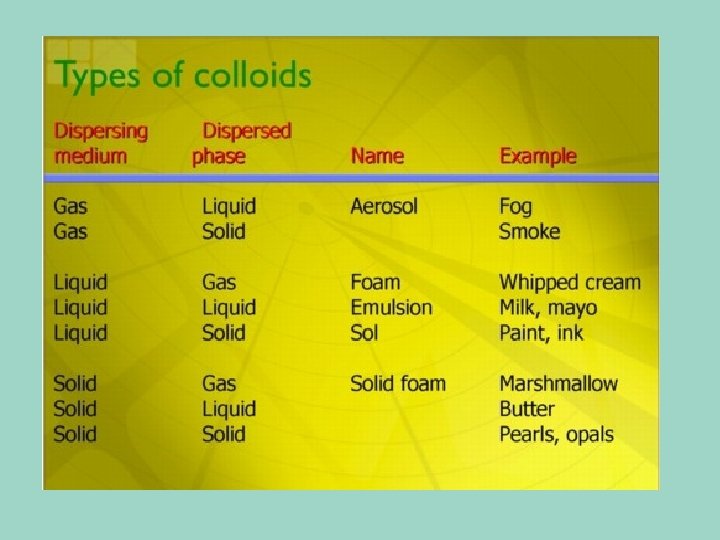
Colloids • Contain some particles that are intermediate in size and are in between the small parts in a solution, and the larger particles in a suspension. • They do not separate in layers • You can’t use a filter to separate the parts of a colloid • The scattering of light property can be used to separate them from other mixtures

Colloids • Particles are large enough to scatter light. • That means that you actually see what we call a “beam of light” – in a solution, which has really small particles, the light passes right through.


Scattering of light


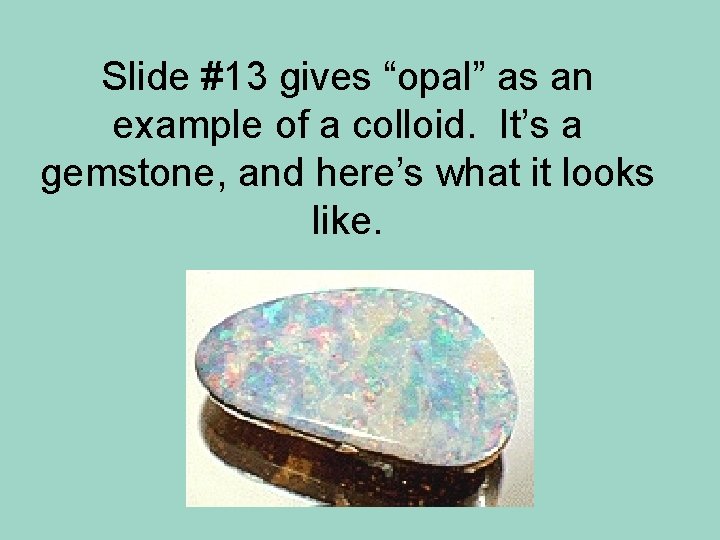
Slide #13 gives “opal” as an example of a colloid. It’s a gemstone, and here’s what it looks like.

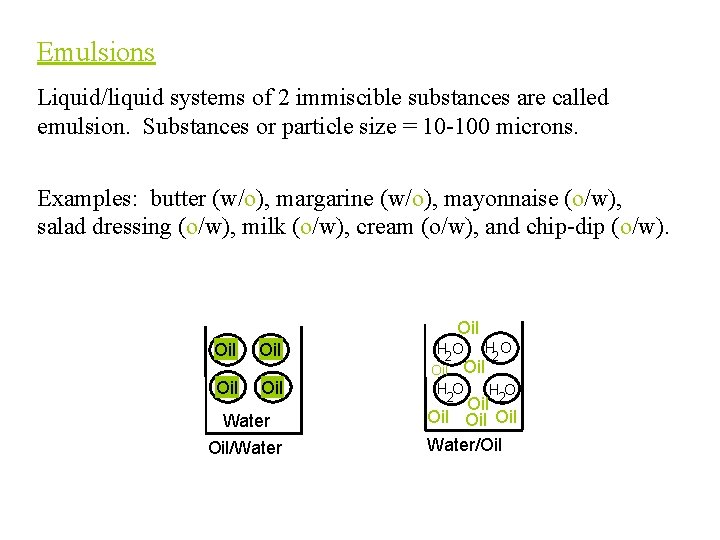
Emulsions Liquid/liquid systems of 2 immiscible substances are called emulsion. Substances or particle size = 10 -100 microns. Examples: butter (w/o), margarine (w/o), mayonnaise (o/w), salad dressing (o/w), milk (o/w), cream (o/w), and chip-dip (o/w). Oil Oil Oil Water Oil/Water H 2 O HO HO H 2 O Oil 2 Oil Oil Water/Oil

Solutions • Formed when substances dissolve and form a homogeneous mixture • Characteristics – Do not separate into distinct layers over time – Will not leave trapped substances when poured through a filter – Most are translucent (clear or see-through)

Solutions • Solvent • Solute • Substance that dissolves the solute, • stuff “doing the dissolving. ” • Water in salt and water • water in “sweet tea” • What you have the most of! • Substance that dissolves in the solution. • Salt in the water • Sugar in the tea • Chemicals put in your fish tank.

Using Properties to Separate Mixtures Distillation & Filtration are 2 common separation methods