Chapter 6 1 Percentage Composition Percent Composition Percent


- Slides: 13

Chapter 6. 1 Percentage Composition

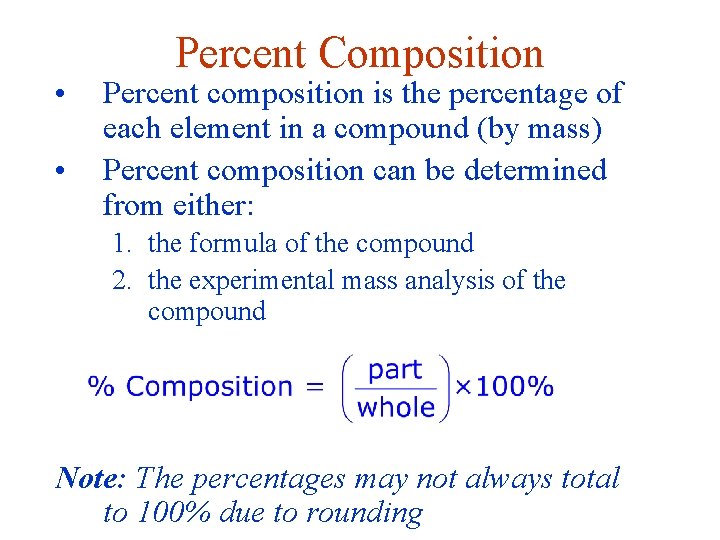
• • Percent Composition Percent composition is the percentage of each element in a compound (by mass) Percent composition can be determined from either: 1. the formula of the compound 2. the experimental mass analysis of the compound Note: The percentages may not always total to 100% due to rounding

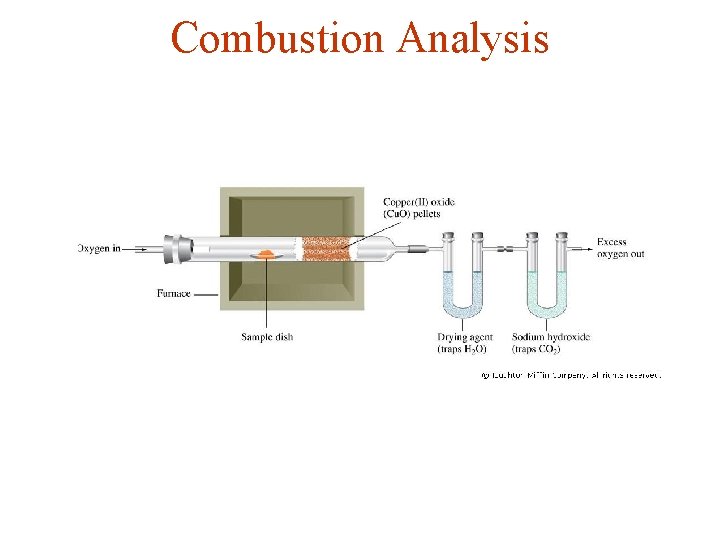
Combustion Analysis

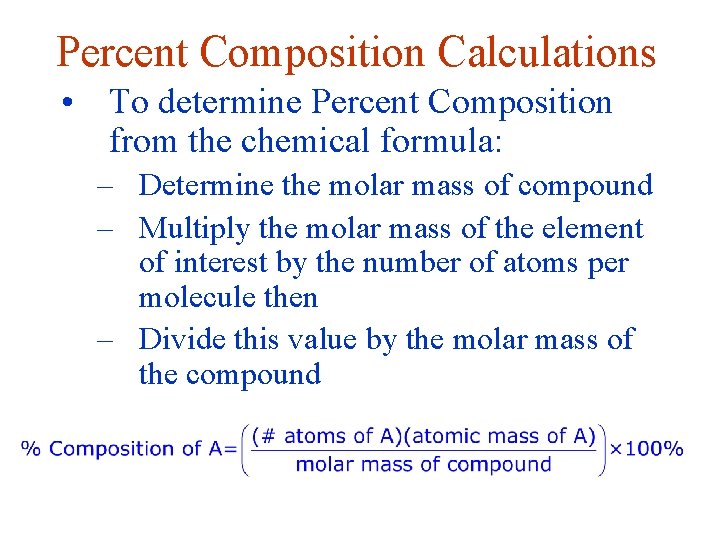
Percent Composition Calculations • To determine Percent Composition from the chemical formula: – Determine the molar mass of compound – Multiply the molar mass of the element of interest by the number of atoms per molecule then – Divide this value by the molar mass of the compound

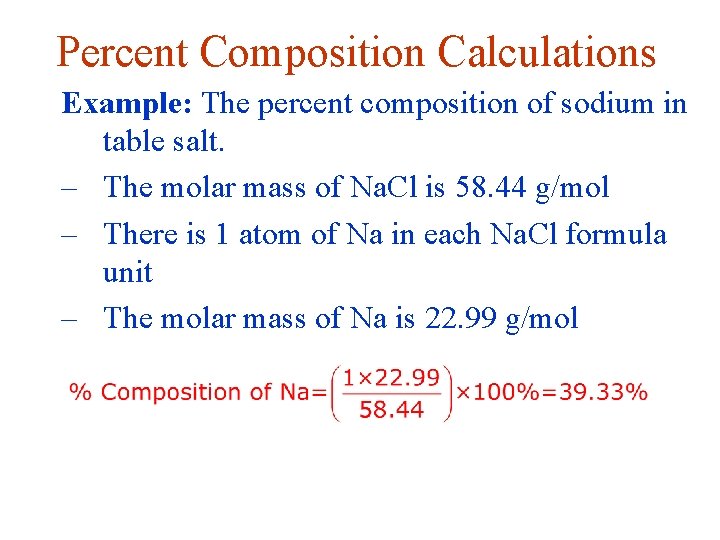
Percent Composition Calculations Example: The percent composition of sodium in table salt. – The molar mass of Na. Cl is 58. 44 g/mol – There is 1 atom of Na in each Na. Cl formula unit – The molar mass of Na is 22. 99 g/mol

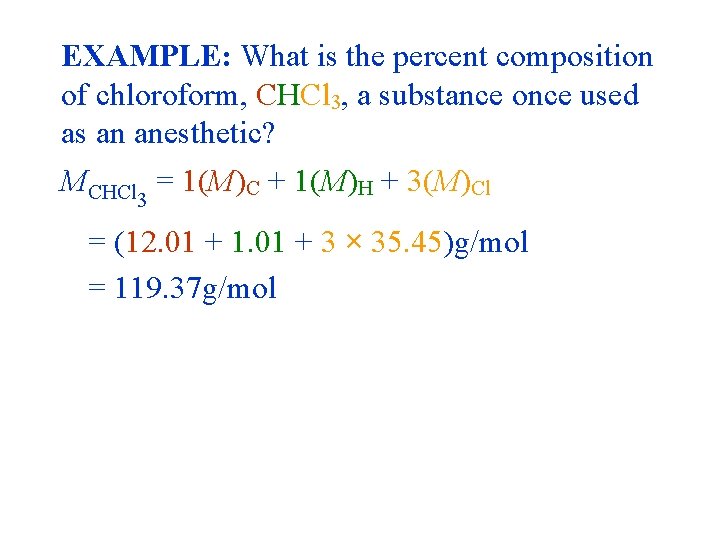
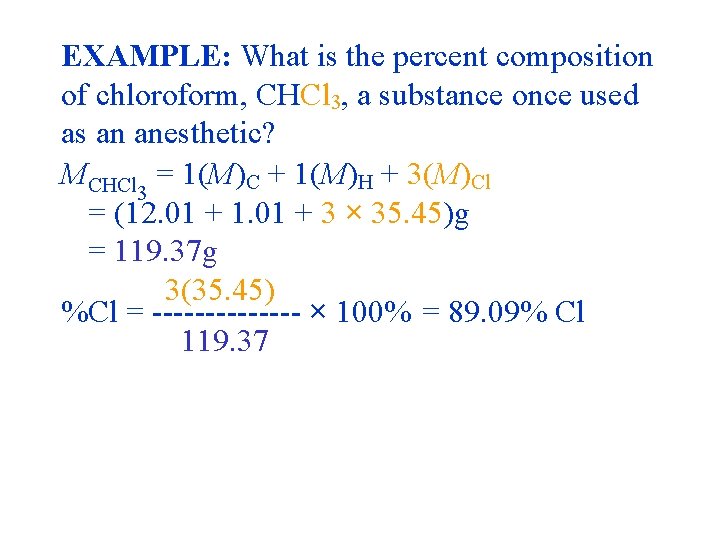
EXAMPLE: What is the percent composition of chloroform, CHCl 3, a substance once used as an anesthetic? MCHCl 3 = 1(M)C + 1(M)H + 3(M)Cl = (12. 01 + 1. 01 + 3 × 35. 45)g/mol = 119. 37 g/mol

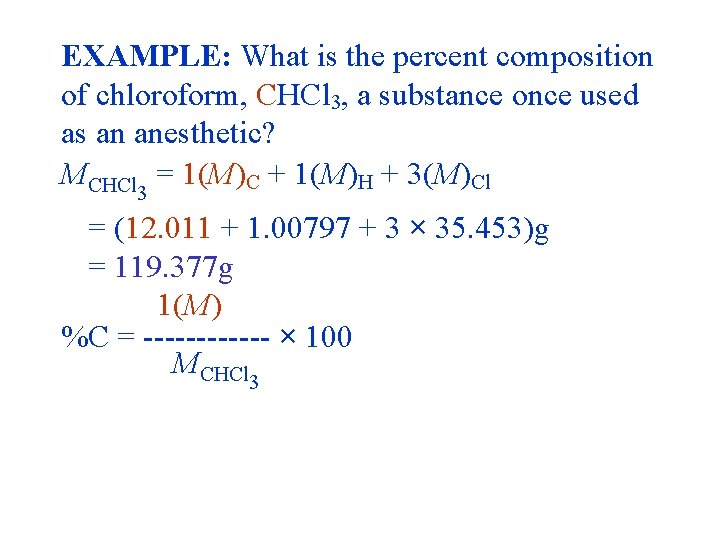
EXAMPLE: What is the percent composition of chloroform, CHCl 3, a substance once used as an anesthetic? MCHCl 3 = 1(M)C + 1(M)H + 3(M)Cl = (12. 011 + 1. 00797 + 3 × 35. 453)g = 119. 377 g 1(M) %C = ------ × 100 MCHCl 3

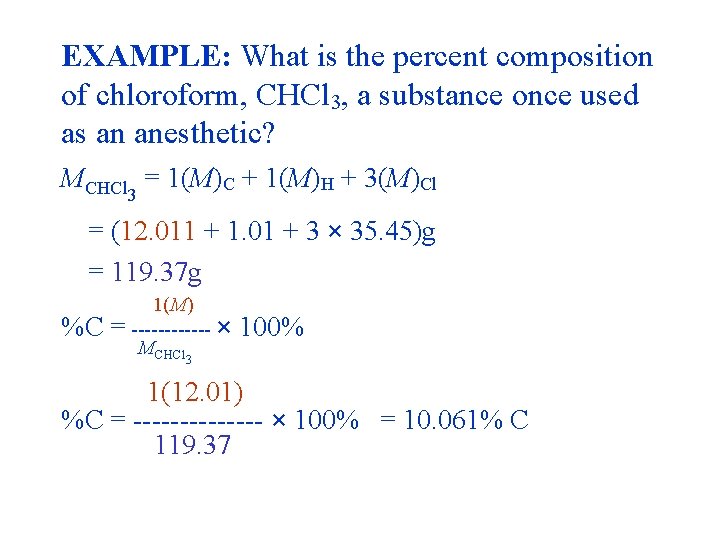
EXAMPLE: What is the percent composition of chloroform, CHCl 3, a substance once used as an anesthetic? MCHCl = 1(M)C + 1(M)H + 3(M)Cl 3 = (12. 011 + 1. 01 + 3 × 35. 45)g = 119. 37 g %C 1(M) = ------ × MCHCl 3 100% 1(12. 01) %C = ------- × 100% = 10. 061% C 119. 37

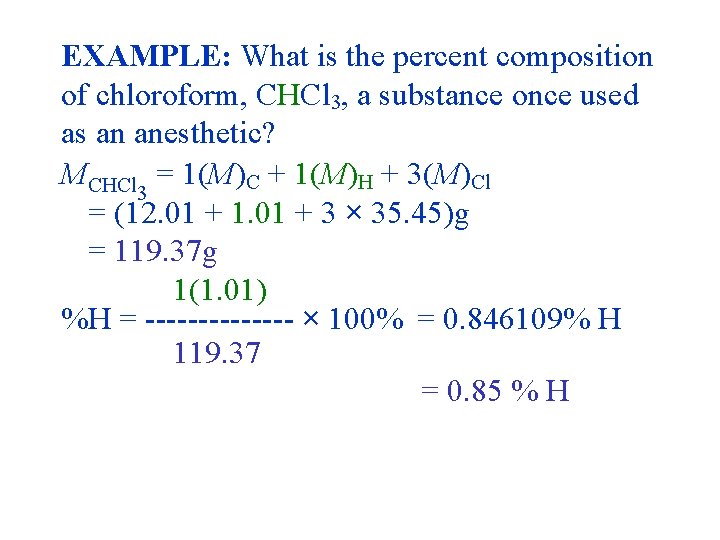
EXAMPLE: What is the percent composition of chloroform, CHCl 3, a substance once used as an anesthetic? MCHCl 3 = 1(M)C + 1(M)H + 3(M)Cl = (12. 01 + 1. 01 + 3 × 35. 45)g = 119. 37 g 1(1. 01) %H = ------- × 100% = 0. 846109% H 119. 37 = 0. 85 % H

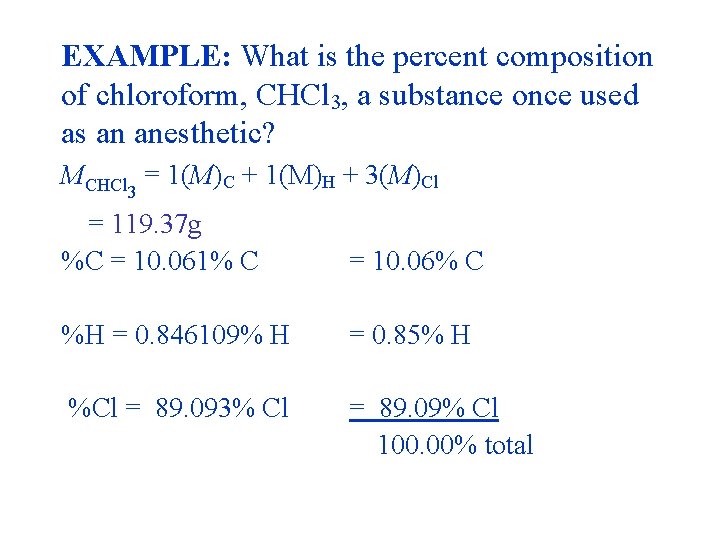
EXAMPLE: What is the percent composition of chloroform, CHCl 3, a substance once used as an anesthetic? MCHCl 3 = 1(M)C + 1(M)H + 3(M)Cl = (12. 01 + 1. 01 + 3 × 35. 45)g = 119. 37 g 3(35. 45) %Cl = ------- × 100% = 89. 09% Cl 119. 37

EXAMPLE: What is the percent composition of chloroform, CHCl 3, a substance once used as an anesthetic? MCHCl = 1(M)C + 1(M)H + 3(M)Cl 3 = 119. 37 g %C = 10. 061% C = 10. 06% C %H = 0. 846109% H = 0. 85% H %Cl = 89. 093% Cl = 89. 09% Cl 100. 00% total

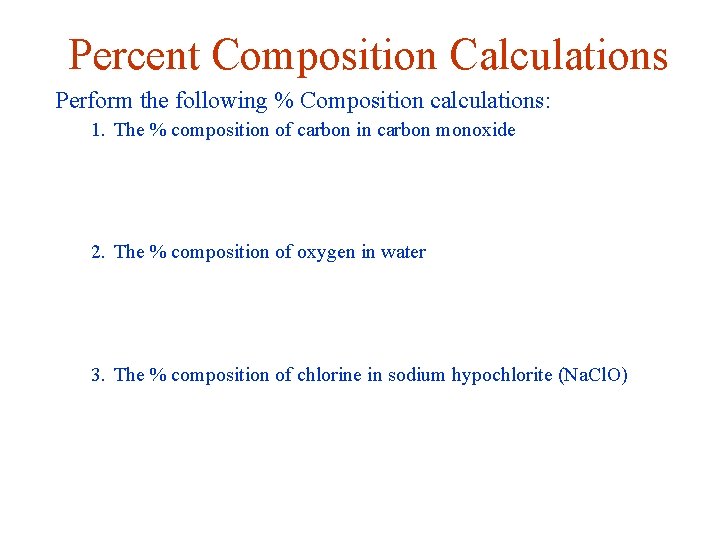
Percent Composition Calculations Perform the following % Composition calculations: 1. The % composition of carbon in carbon monoxide 2. The % composition of oxygen in water 3. The % composition of chlorine in sodium hypochlorite (Na. Cl. O)

One Mole of each Substance Clockwise from top left: 1 -Octanol, C 8 H 17 OH; Mercury(II) iodide, Hg. I 2; Methanol, CH 3 OH; and Sulfur, S 8.