Chapter 5 Types of Compounds Ionic Compounds Covalent










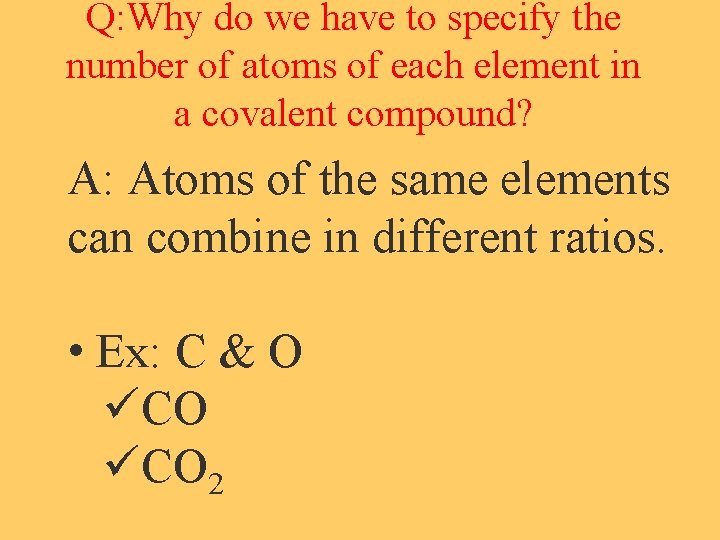


























- Slides: 61

Chapter 5 Types of Compounds • Ionic Compounds • Covalent Compounds Lecture. PLUS Timberlake 1

Electronegativity (EN) Def: The strength with which an atom in a bond pulls on e-s. Lecture. PLUS Timberlake 2

Covalent Bonds (bonds btwn 2 nonmetals) l. Nonmetals have high electronegativity values (REVIEW) l. Electrons are shared single bond shares 1 pair electrons double bond shares 2 pairs electrons triple bond shares 3 pairs electrons Lecture. PLUS Timberlake 3

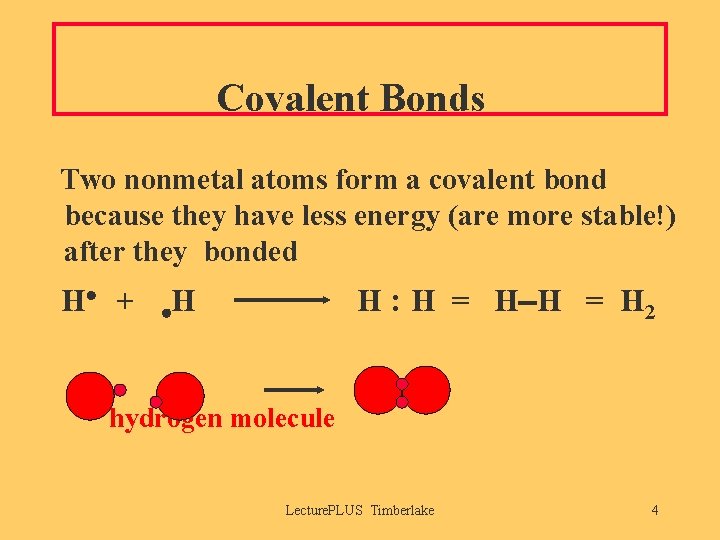
Covalent Bonds Two nonmetal atoms form a covalent bond because they have less energy (are more stable!) after they bonded H + H : H = H 2 H hydrogen molecule Lecture. PLUS Timberlake 4

Learning Check Indicate whether a bond between the following would be 1) Ionic 2) covalent ____ A. sodium & oxygen ____ B. nitrogen & oxygen ____ C. phosphorus & chlorine ____ D. calcium & sulfur ____ E. chlorine & bromine Lecture. PLUS Timberlake 5

Solution Indicate whether a bond between the following would be 1) Ionic 2) covalent 1 A. sodium and oxygen 2 B. nitrogen and oxygen 2 C. phosphorus and chlorine 1 D. calcium and sulfur 2 E. chlorine and bromine Lecture. PLUS Timberlake 6

Types of Covalent (Molecular) Cpds 1. Elements that form diatomic molecules 2. Binary covalent compounds 3. Organic compounds/ Hydrocarbon 4. Acids & Bases • (Common v. Formal Names) Lecture. PLUS Timberlake 7

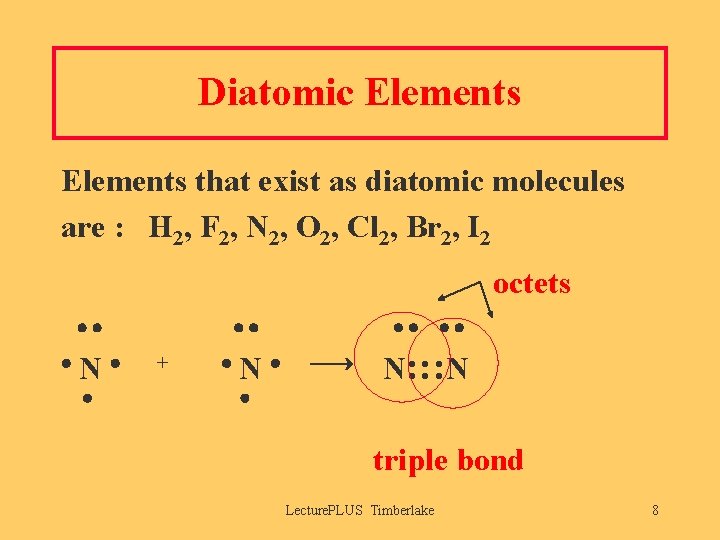
Diatomic Elements that exist as diatomic molecules are : H 2, F 2, N 2, O 2, Cl 2, Br 2, I 2 octets N + N N: : : N triple bond Lecture. PLUS Timberlake 8

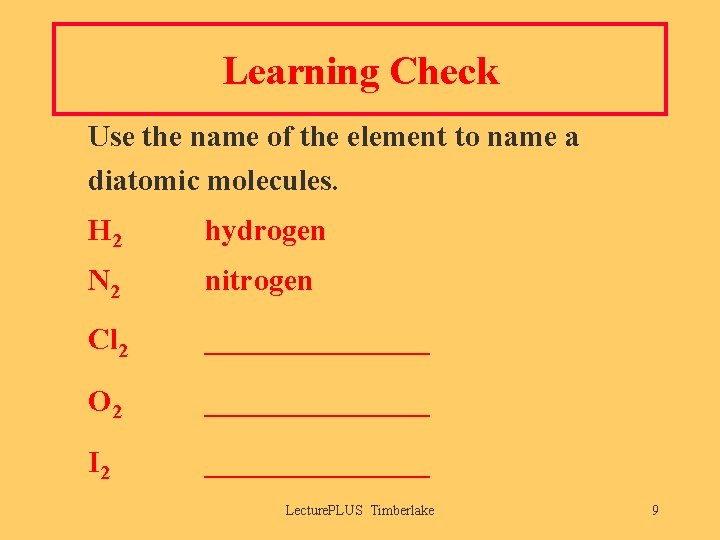
Learning Check Use the name of the element to name a diatomic molecules. H 2 hydrogen N 2 nitrogen Cl 2 ________ O 2 ________ I 2 ________ Lecture. PLUS Timberlake 9

Solution Use the name of the element to name the following diatomic molecules. H 2 hydrogen N 2 nitrogen Cl 2 chlorine O 2 oxygen I 2 iodine Lecture. PLUS Timberlake 10

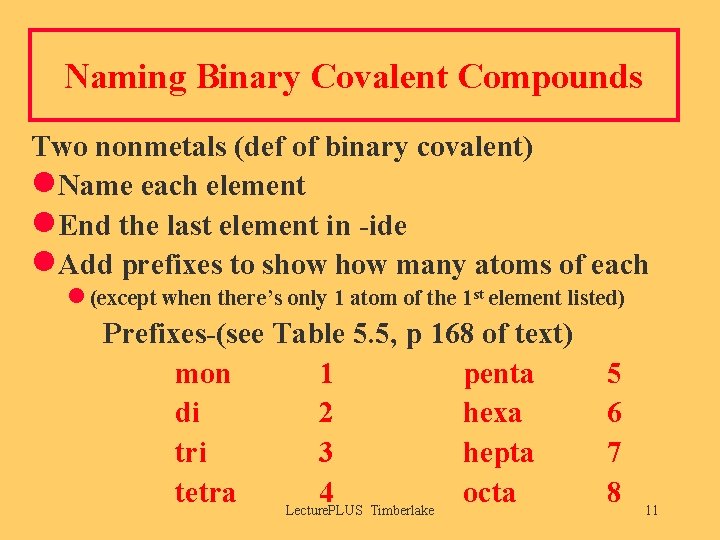
Naming Binary Covalent Compounds Two nonmetals (def of binary covalent) l. Name each element l. End the last element in -ide l. Add prefixes to show many atoms of each l (except when there’s only 1 atom of the 1 st element listed) Prefixes-(see Table 5. 5, p 168 of text) mon 1 penta di 2 hexa tri 3 hepta tetra 4 octa Lecture. PLUS Timberlake 5 6 7 8 11

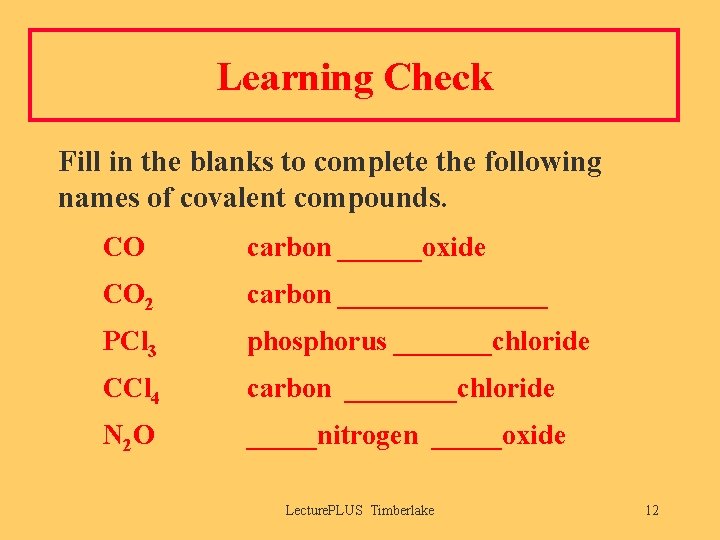
Learning Check Fill in the blanks to complete the following names of covalent compounds. CO carbon ______oxide CO 2 carbon ________ PCl 3 phosphorus _______chloride CCl 4 carbon ____chloride N 2 O _____nitrogen _____oxide Lecture. PLUS Timberlake 12

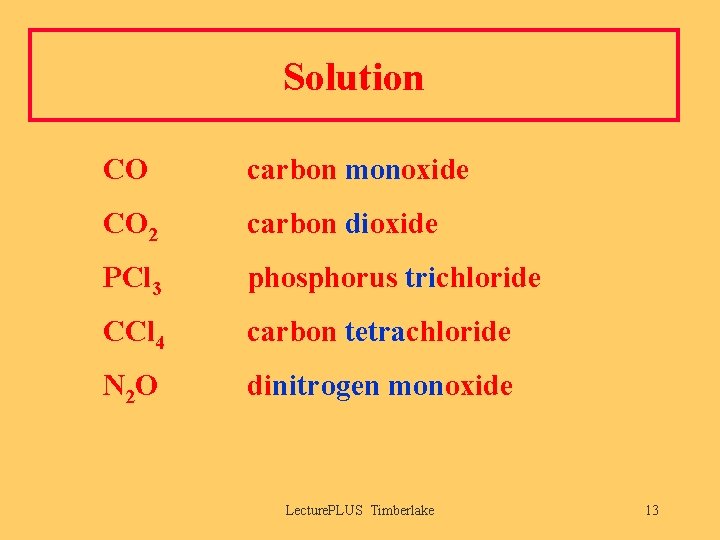
Solution CO carbon monoxide CO 2 carbon dioxide PCl 3 phosphorus trichloride CCl 4 carbon tetrachloride N 2 O dinitrogen monoxide Lecture. PLUS Timberlake 13

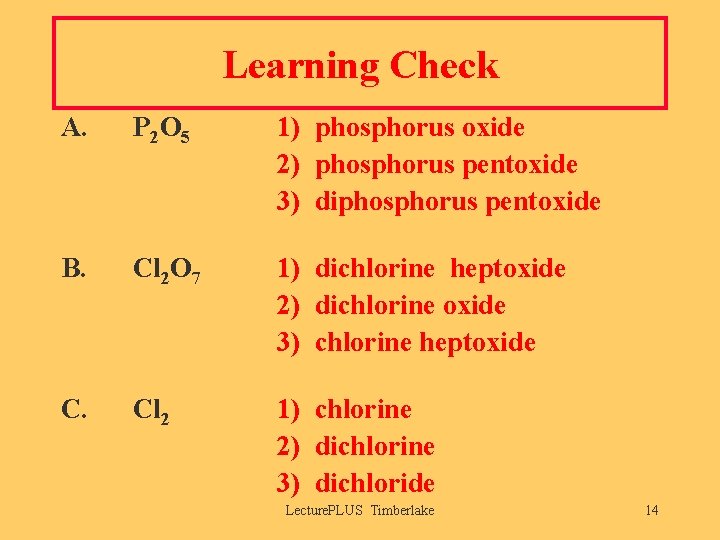
Learning Check A. P 2 O 5 1) phosphorus oxide 2) phosphorus pentoxide 3) diphosphorus pentoxide B. Cl 2 O 7 1) dichlorine heptoxide 2) dichlorine oxide 3) chlorine heptoxide C. Cl 2 1) chlorine 2) dichlorine 3) dichloride Lecture. PLUS Timberlake 14

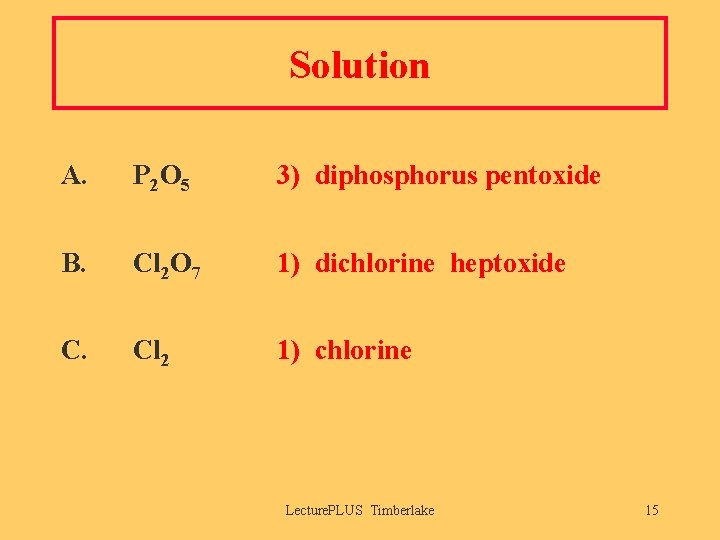
Solution A. P 2 O 5 3) diphosphorus pentoxide B. Cl 2 O 7 1) dichlorine heptoxide C. Cl 2 1) chlorine Lecture. PLUS Timberlake 15

Naming Organic Compounds • Def: organic compounds contain __ atoms hooked together. • (Why do you think this element can hook up with many other atoms, including itself? )

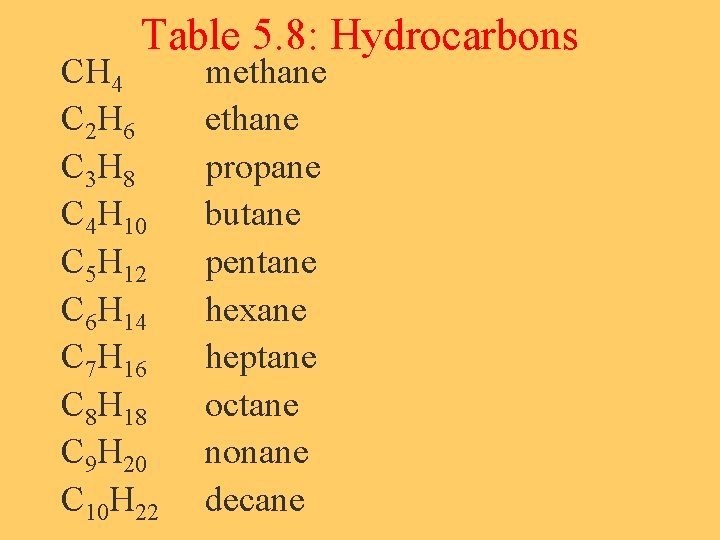
Hydrocarbons-A Type of Organic Compound • Def: hydrocarbons are made of ___ & ___ • They are named by the number of Carbon atoms a molecule contains. • See Table 5. 8, p 183

Table 5. 8: Hydrocarbons CH 4 C 2 H 6 C 3 H 8 C 4 H 10 C 5 H 12 C 6 H 14 C 7 H 16 C 8 H 18 C 9 H 20 C 10 H 22 methane propane butane pentane hexane heptane octane nonane decane

Common v. Formal Names • Formal Names follow the rules we have learned for naming compounds. • Common Names are ones that don’t follow these rules. –Ex: water=

Frequently Used Common Names • Water = H 2 O • Ammonia = NH 3 • Common Acids & Bases

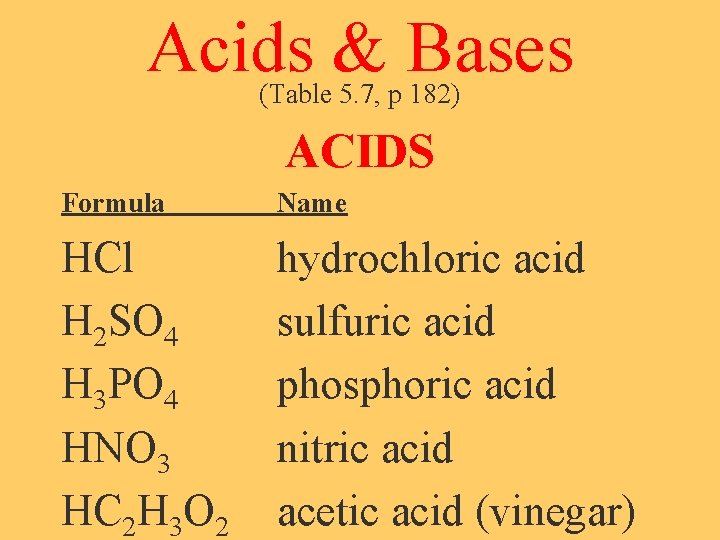
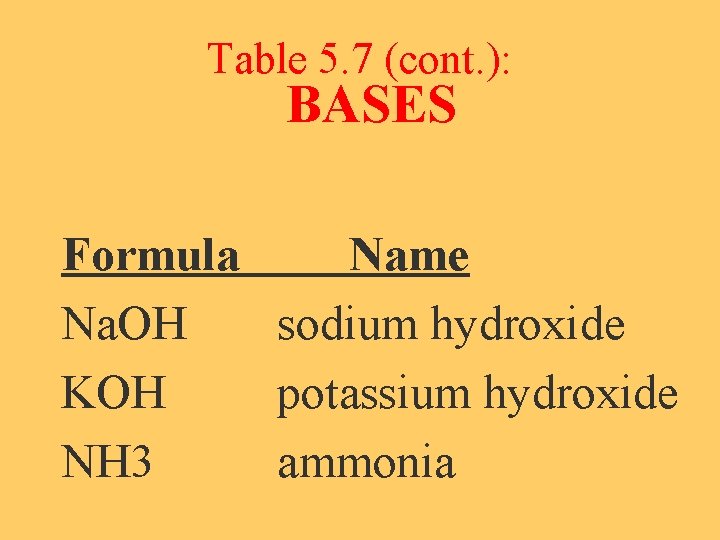
Acids & Bases (Table 5. 7, p 182) ACIDS Formula Name HCl H 2 SO 4 H 3 PO 4 HNO 3 HC 2 H 3 O 2 hydrochloric acid sulfuric acid phosphoric acid nitric acid acetic acid (vinegar)

Table 5. 7 (cont. ): BASES Formula Name Na. OH sodium hydroxide KOH potassium hydroxide NH 3 ammonia

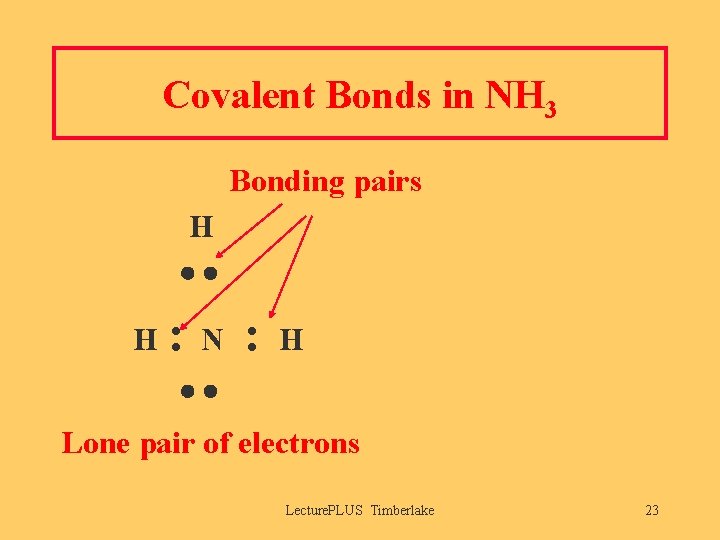
Covalent Bonds in NH 3 Bonding pairs H H : N : H Lone pair of electrons Lecture. PLUS Timberlake 23

Allotropes • Def: molecules of the same element that differ in structure • Ex: Carbon…graphite, charcoal, Buckminsterfullerine (“bucky ball”) - see Fig ___ on p ___ of text • Ex 2: O 2 (oxygen) and O 3 (ozone)
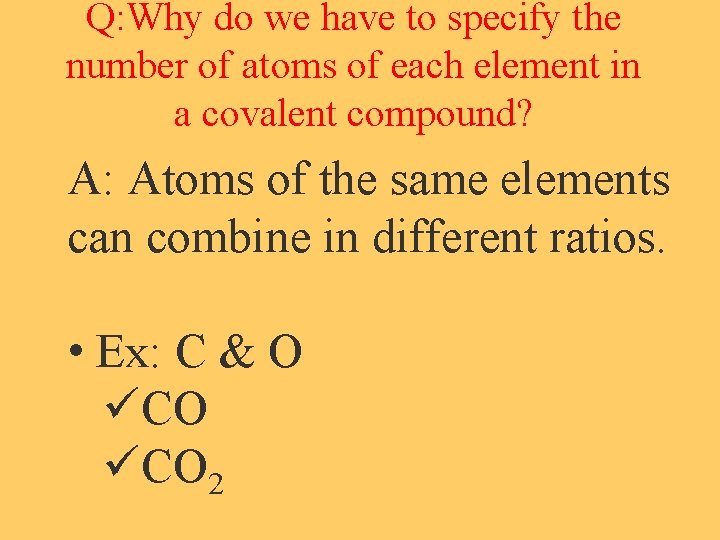
Q: Why do we have to specify the number of atoms of each element in a covalent compound? A: Atoms of the same elements can combine in different ratios. • Ex: C & O üCO 2

Writing Formulas for Covalent Compounds 1. Identify it as a covalent: containing only nonmetals. 2. Determine what type of covalent it is: diatomic element binary hydrocarbon (ends in –ane) acid/base 3. Reverse the naming process. Lecture. PLUS Timberlake 26

Naming Ionic Compounds • Binary Ionic • Ionic Compounds containing Polyatomic Ions. • Ionic Cpds containing Transition Metals Lecture. PLUS Timberlake 27

PLEASE NOTE: • IF YOU ARE UNABLE TO IDENTIFY IONIC & COVALENT COMPOUNDS, YOU WILL BE LOST!!! • PLEASE SEE ME IMMEDIATELY TO GET CAUGHT UP. Lecture. PLUS Timberlake 28

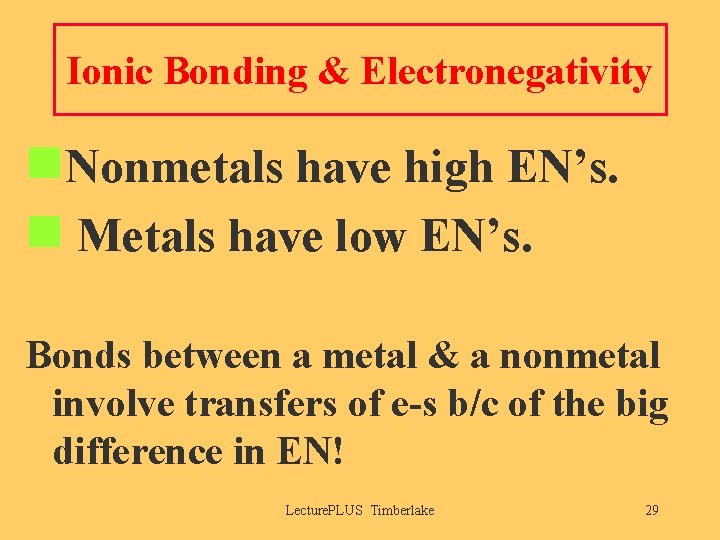
Ionic Bonding & Electronegativity n. Nonmetals have high EN’s. n Metals have low EN’s. Bonds between a metal & a nonmetal involve transfers of e-s b/c of the big difference in EN! Lecture. PLUS Timberlake 29

Binary Ionic Compounds • Binary= 2 elements • Ionic= 1 metal & 1 nonmetal Lecture. PLUS Timberlake 30

Naming Binary Ionic Compounds 1. Identify & name the 2 elements in the compound. 2. Name the cation, which is the given the name of the element. 3. Name the anion, which is given the name of the element, w/the ending changed to “–ide. ” Lecture. PLUS Timberlake 31

PRACTICE Naming Binary Ionic Compounds 1. Na║Cl 2. Na = “sodium” 3. Cl = “chloride” (full name is “sodium chloride”) Lecture. PLUS Timberlake 32

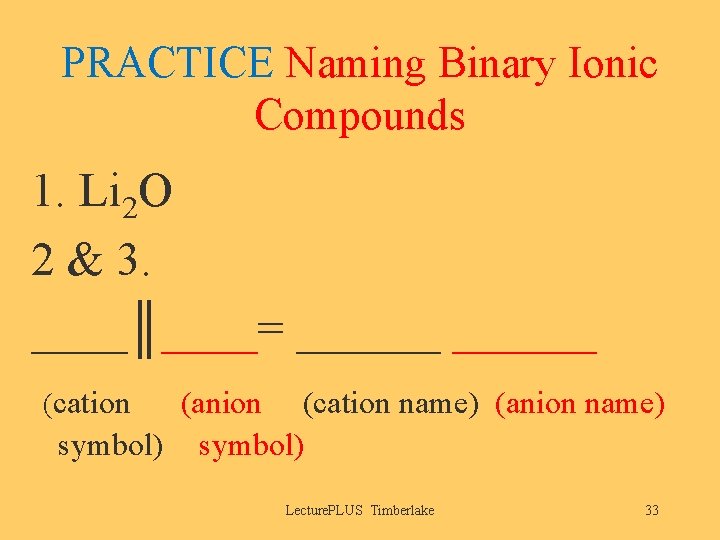
PRACTICE Naming Binary Ionic Compounds 1. Li 2 O 2 & 3. ____║____= ______ (cation (anion (cation name) (anion name) symbol) Lecture. PLUS Timberlake 33

MORE PRACTICE Naming Binary Ionic Compounds-p __ of I. N. 1. KF 2. Ca. F 2 3. Al 2 O 3 Lecture. PLUS Timberlake 34

Naming Ionic Compounds w/ Polyatomic Ions • DEF: Charged particles containing more than 1 type 2 of atom. Ex: SO 4 Lecture. PLUS Timberlake 35

Naming Ionic Compounds w/ Polyatomic Ions 1. Identify the cation & the anion. (Draw a line between the 2 ions) 2. Name the cation, then the anion (find polyatomics on Table 5. 3, p 159 of text). That’s it! Lecture. PLUS Timberlake 36

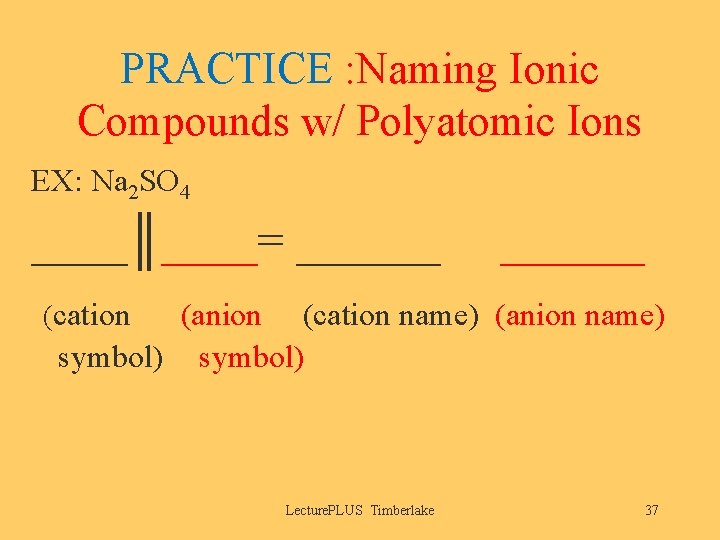
PRACTICE : Naming Ionic Compounds w/ Polyatomic Ions EX: Na 2 SO 4 ____║____= ______ (cation (anion (cation name) (anion name) symbol) Lecture. PLUS Timberlake 37

Naming Ionic Compounds w/ Polyatomic Ions • Most polyatomic ions are anions. Lecture. PLUS Timberlake 38

A Couple of Important Exceptions w/Polyatomic Ions Important Exception #1: there are 2 cations that contain NO METALS: NH 4+ (ammonium) H 3 O+ (hydronium) (this can be tricky b/c we have always identified ionic compounds because they start with a metal cation. ) Lecture. PLUS Timberlake 39

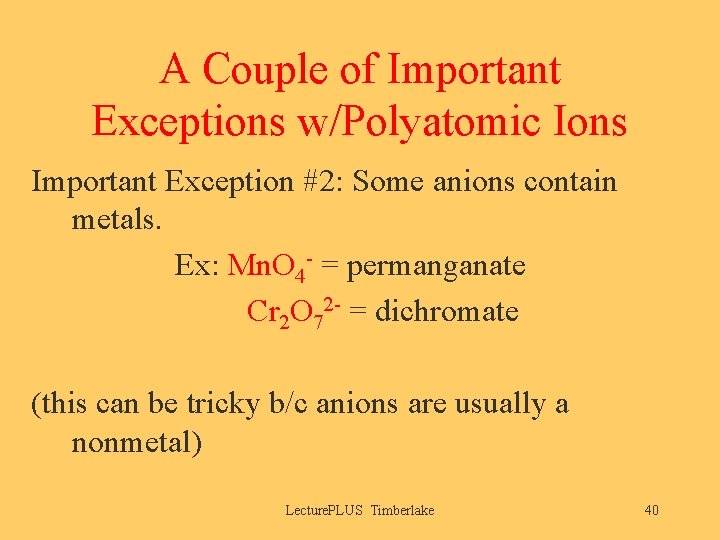
A Couple of Important Exceptions w/Polyatomic Ions Important Exception #2: Some anions contain metals. Ex: Mn. O 4 - = permanganate Cr 2 O 72 - = dichromate (this can be tricky b/c anions are usually a nonmetal) Lecture. PLUS Timberlake 40

Copy Table 5. 2 into Notes Lecture. PLUS Timberlake 41

Naming Ionic Cpds Containing Ex: Ni. O 2 Transition Metals 1. Determine the total # of negative charges in a unit of the compound: Ex: O 2 - & O 2= 4 total - charges 2. Determine the charge on the cation that will give you 4 total + charges Ex: Ni 4+ 3. Write the cation & anion names. Write cation with the oxidation # written as a Roman numeral in parentheses: Ex: nickel (IV) oxide Lecture. PLUS Timberlake 42

Writing Formulas for Binary Ionic Compounds 1. Identify the ionic charge (“oxidation number”) on the cation & anion. Lecture. PLUS Timberlake 43

Writing Formulas for Binary Ionic Compounds ELEMENT • Group 1 • Group 2 • Group 13 • Group 14 • Group 15 • Group 16 • Group 17 OXIDATION # 1+ 2+ 3+ 4+ or 4321 -Timberlake Lecture. PLUS 44

Practice Predicting Oxidation #s • • • Li O Mg F B Lecture. PLUS Timberlake 45

Writing Formulas for Binary Ionic Compounds 2. A compound has NO CHARGE on it, so a formula unit (the smallest ratio of cations to anions) must have equal numbers of + & charges. (use the LCM) Lecture. PLUS Timberlake 46

Cross-Over Method • You can use this to write formulas. • Take the charge on the cation and use it as the subscript on the anion • Take the charge on the anion and use it as the subscript on the cation • Reduce the subscripts, if necessary Lecture. PLUS Timberlake 47

Using the LCM to Write Ionic Formulas • Ex: Li & F • Ex: Li & O • Ex: Al & O Lecture. PLUS Timberlake 48

ANSWERS • Li. F • Li 2 O • Al 2 O 3 Lecture. PLUS Timberlake 49

Writing Formulas for I. Cpds Containing Polyatomic Ions • Determine the cation & anion • Determine the oxidation # on each ion. (oxidation #s for polyatomics are found on Table 5. 2) • Write a balanced formula –If there is more than 1 of an ion, use parentheses, then a subscript Lecture. PLUS Timberlake 50

Writing Formulas for I. Cpds Containing Polyatomic Ions • Ex: see Practice Problems, p 162 3. Write the formula for the compound formed from the following pairs of ions a) ammonium & sulfite ions • IONS: NH 4+ & SO 3 2 NH 4+ • FORMULA: (NH 4)2 SO 3 Lecture. PLUS Timberlake 51

Practice Problems, cont. from p 16 2 of text 3 b) 3 c) 3 d) 4 a) 4 b) 4 c) 4 d) Lecture. PLUS Timberlake 52

Transition Metals • QUESTION: What was strange about the e- configurations of transition metals? ANSWER: Their d sublevels overlap with the other sublevels in the next higher main E. L. Lecture. PLUS Timberlake 53

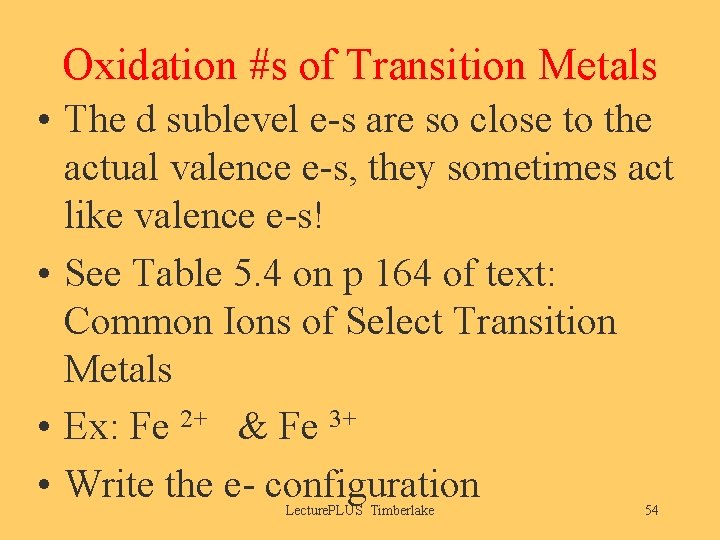
Oxidation #s of Transition Metals • The d sublevel e-s are so close to the actual valence e-s, they sometimes act like valence e-s! • See Table 5. 4 on p 164 of text: Common Ions of Select Transition Metals • Ex: Fe 2+ & Fe 3+ • Write the e- configuration Lecture. PLUS Timberlake 54

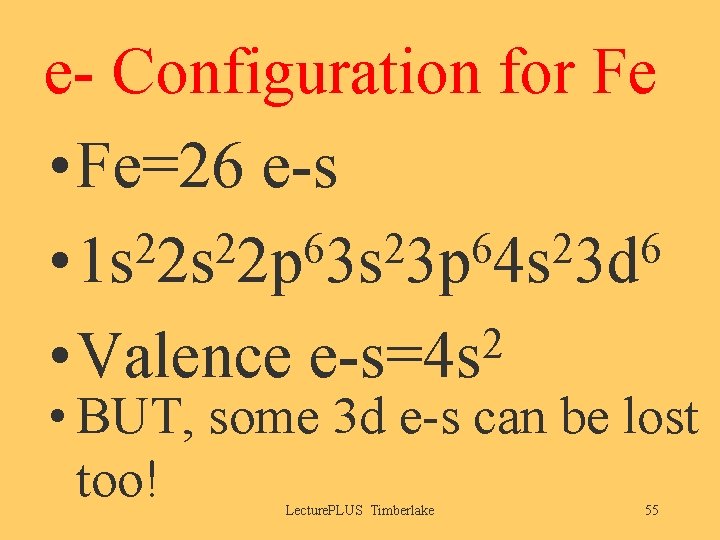
e- Configuration for Fe • Fe=26 e-s 2 2 6 2 6 • 1 s 2 s 2 p 3 s 3 p 4 s 3 d 2 • Valence e-s=4 s • BUT, some 3 d e-s can be lost too! Lecture. PLUS Timberlake 55

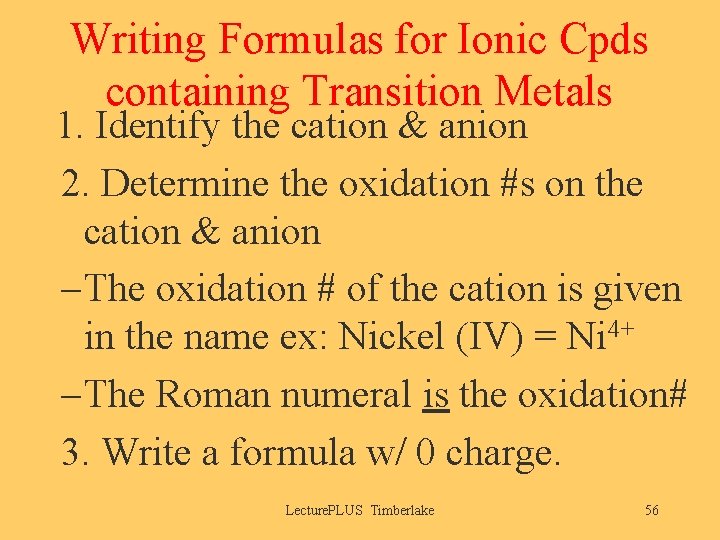
Writing Formulas for Ionic Cpds containing Transition Metals 1. Identify the cation & anion 2. Determine the oxidation #s on the cation & anion – The oxidation # of the cation is given in the name ex: Nickel (IV) = Ni 4+ – The Roman numeral is the oxidation# 3. Write a formula w/ 0 charge. Lecture. PLUS Timberlake 56

Distillation • Def: process of separating ionic & covalent compounds by heating them till the covalent compound evaporates. – The ionic compound remains in the flask – The covalent compound can be cooled & collected in a separate container. – This process is called distillation

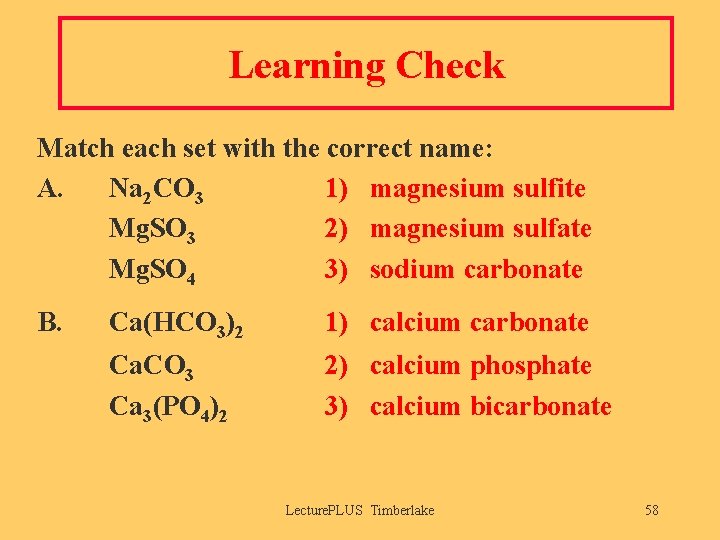
Learning Check Match each set with the correct name: A. Na 2 CO 3 1) magnesium sulfite Mg. SO 3 2) magnesium sulfate Mg. SO 4 3) sodium carbonate B. Ca(HCO 3)2 1) calcium carbonate Ca. CO 3 Ca 3(PO 4)2 2) calcium phosphate 3) calcium bicarbonate Lecture. PLUS Timberlake 58

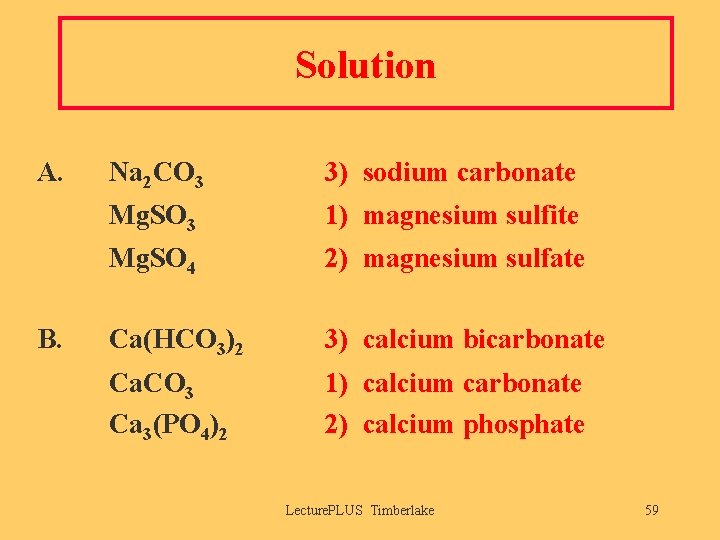
Solution A. B. Na 2 CO 3 3) sodium carbonate Mg. SO 3 1) magnesium sulfite Mg. SO 4 2) magnesium sulfate Ca(HCO 3)2 3) calcium bicarbonate Ca. CO 3 Ca 3(PO 4)2 1) calcium carbonate 2) calcium phosphate Lecture. PLUS Timberlake 59

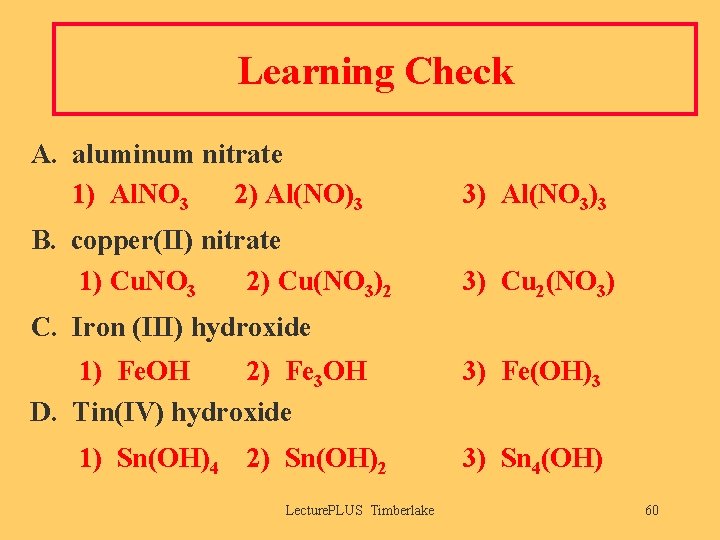
Learning Check A. aluminum nitrate 1) Al. NO 3 2) Al(NO)3 3) Al(NO 3)3 B. copper(II) nitrate 1) Cu. NO 3 2) Cu(NO 3)2 3) Cu 2(NO 3) C. Iron (III) hydroxide 1) Fe. OH 2) Fe 3 OH D. Tin(IV) hydroxide 1) Sn(OH)4 2) Sn(OH)2 Lecture. PLUS Timberlake 3) Fe(OH)3 3) Sn 4(OH) 60

Solution A. aluminum nitrate 3) Al(NO 3)3 B. copper(II) nitrate 2) Cu(NO 3)2 C. Iron (III) hydroxide 3) Fe(OH)3 D. Tin(IV) hydroxide 1) Sn(OH)4 Lecture. PLUS Timberlake 61