Chapter 5 Thermochemistry Section 5 1 The Nature















- Slides: 29

Chapter 5 Thermochemistry

Section 5. 1 The Nature of Energy

Objectives • Understand the nature of energy and the forms it takes (kinetic and potential). • Apply the units used to measure energy • Demonstrate energy through work and heat transfer • Study energy changes in the system and surroundings

Key Terms • • • Thermodynamics Thermochemistry Kinetic energy Potential energy Joule Calorie • • • System Surroundings Work Force Heat Energy

What is Thermodynamics? • The study of energy and its transformations • Greek: “heat power”

Thermochemistry • Portion of thermodynamics • Relationships between chemical reactions and energy changes involving heat

Energy • The capacity to do work or transfer heat – Work: Energy used to cause an object that has mass to move. – Heat: Energy used to cause the temperature of an object to rise.

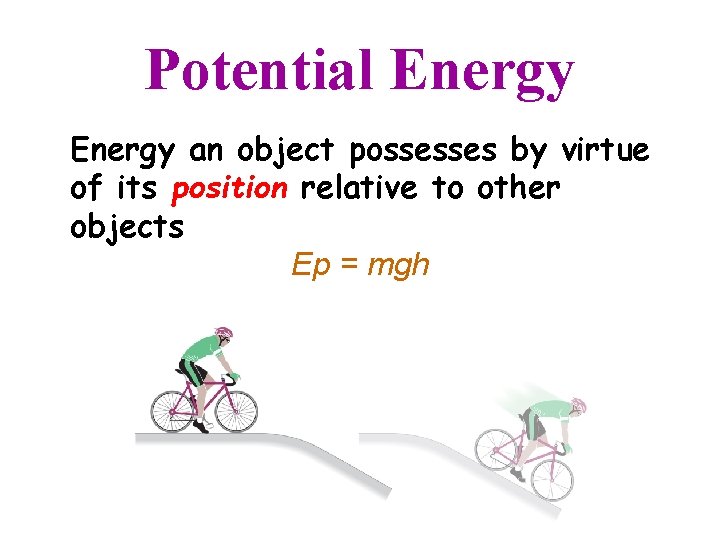
Potential Energy an object possesses by virtue of its position relative to other objects Ep = mgh

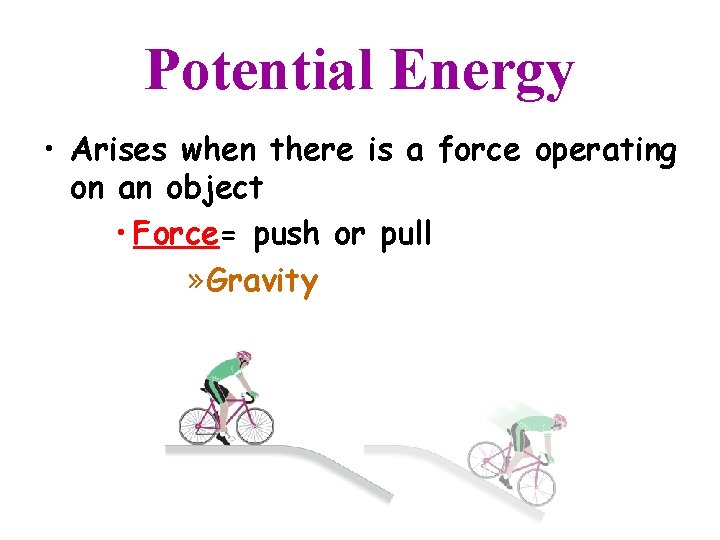
Potential Energy • Arises when there is a force operating on an object • Force= push or pull » Gravity

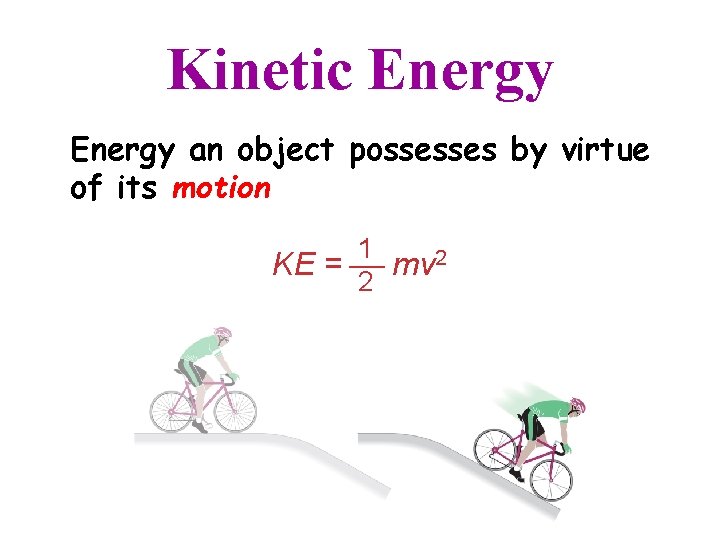
Kinetic Energy an object possesses by virtue of its motion 1 KE = mv 2 2

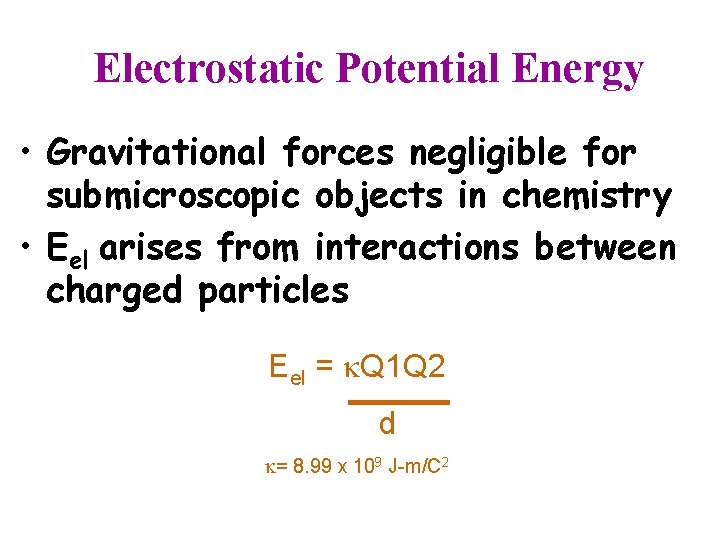
Electrostatic Potential Energy • Gravitational forces negligible for submicroscopic objects in chemistry • Eel arises from interactions between charged particles Eel = Q 1 Q 2 d = 8. 99 x 109 J-m/C 2

Chemical Energy • Due to the potential energy stored in the arrangements of the atoms of the substance

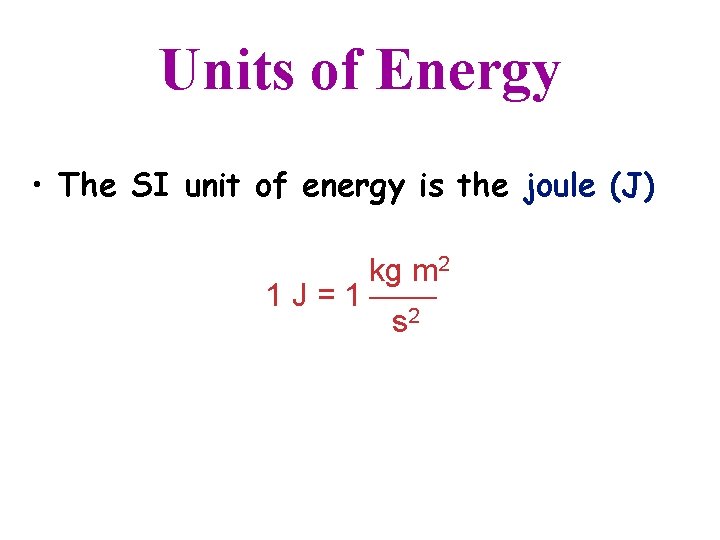
Units of Energy • The SI unit of energy is the joule (J) kg m 2 1 J = 1 s 2

Calorie • An older, non-SI unit • Amount of energy required to raise the temperature of 1 g of water from 14. 5 °C to 15. 5 °C 1 cal = 4. 184 J

System and Surroundings • The system includes the molecules we want to study (here, the hydrogen and oxygen molecules). • The surroundings are everything else (here, the cylinder and piston).

System and Surroundings • System does not exchange matter with surroundings • Exchanges energy in the form of work and heat

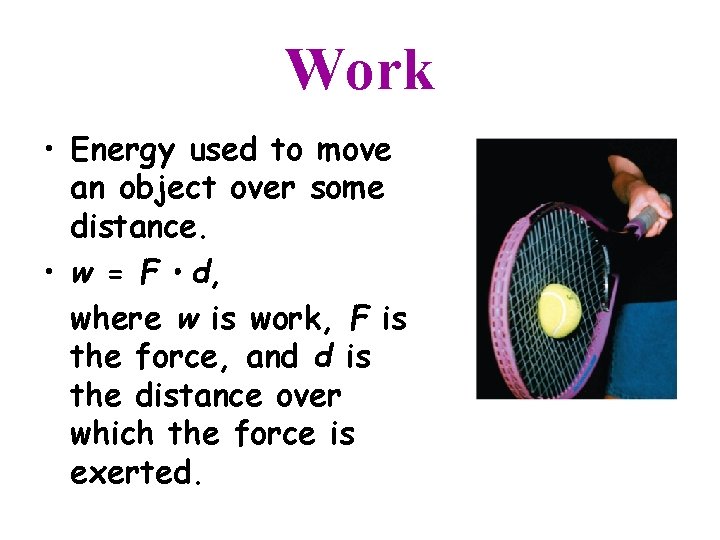
Work • Energy used to move an object over some distance. • w = F d, where w is work, F is the force, and d is the distance over which the force is exerted.

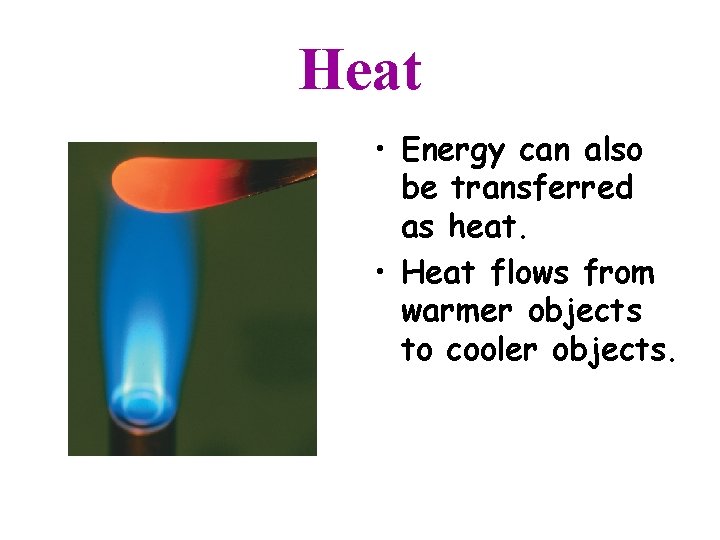
Heat • Energy can also be transferred as heat. • Heat flows from warmer objects to cooler objects.

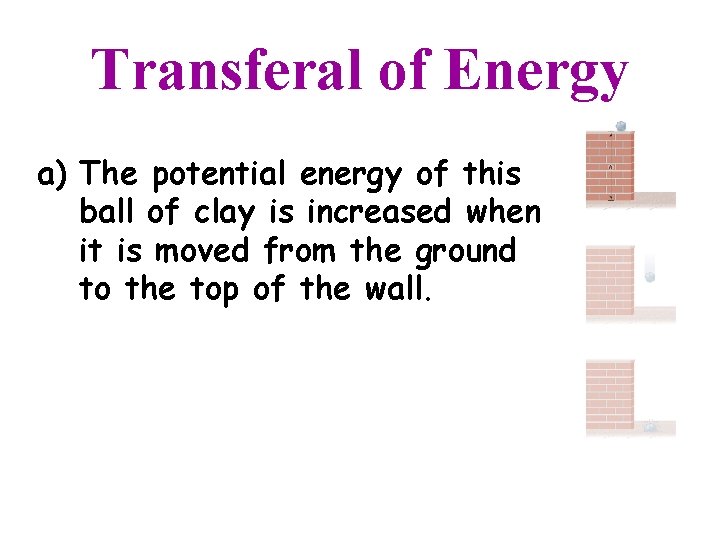
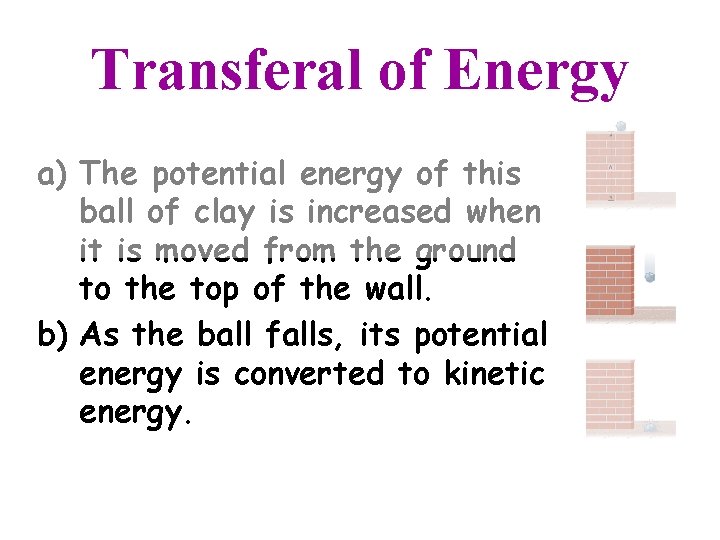
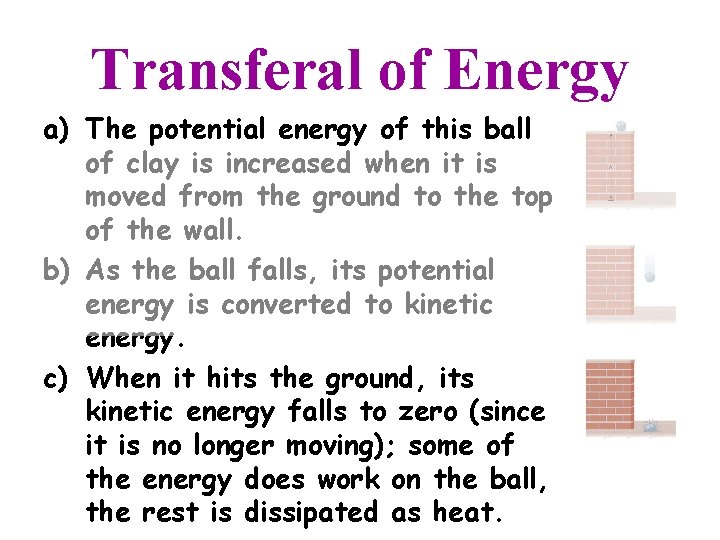
Transferal of Energy a) The potential energy of this ball of clay is increased when it is moved from the ground to the top of the wall.

Transferal of Energy a) The potential energy of this ball of clay is increased when it is moved from the ground to the top of the wall. b) As the ball falls, its potential energy is converted to kinetic energy.

Transferal of Energy a) The potential energy of this ball of clay is increased when it is moved from the ground to the top of the wall. b) As the ball falls, its potential energy is converted to kinetic energy. c) When it hits the ground, its kinetic energy falls to zero (since it is no longer moving); some of the energy does work on the ball, the rest is dissipated as heat.

a. 1. 2. 3. 4. frictional energy electrical energy kinetic energy potential energy

a. 1. 2. 3. 4. frictional energy electrical energy kinetic energy potential energy

b. 1. 2. 3. 4. potential energy kinetic energy heat energy electrical energy

b. 1. 2. 3. 4. potential energy kinetic energy heat energy electrical energy

c. 1. 2. 3. 4. transference heat transduction work

c. 1. 2. 3. 4. transference heat transduction work

d. 1. 2. 3. 4. work transference transduction heat

d. 1. 2. 3. 4. work transference transduction heat