Chapter 5 Thermochemistry relationship between chemical reactions and

Chapter 5 Thermochemistry -relationship between chemical reactions and energy changes energy- capacity to do work or transfer heat work- energy used to cause an object to move against a force heat- the energy used to cause the temp of an object to increase
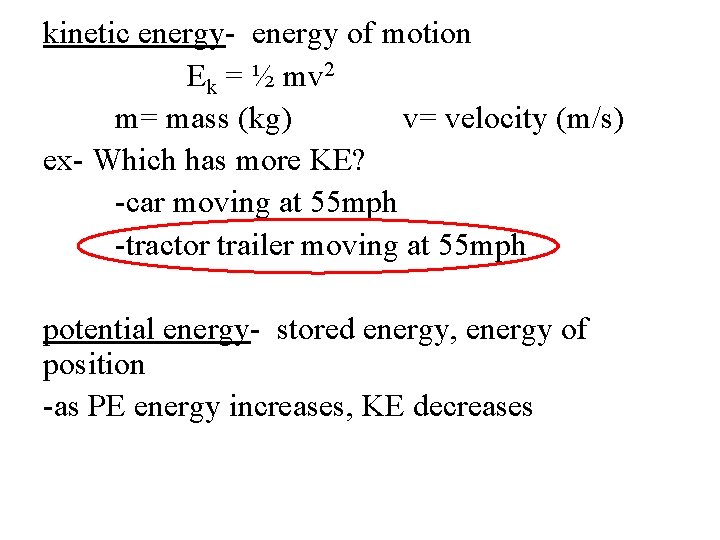
kinetic energy- energy of motion Ek = ½ mv 2 m= mass (kg) v= velocity (m/s) ex- Which has more KE? -car moving at 55 mph -tractor trailer moving at 55 mph potential energy- stored energy, energy of position -as PE energy increases, KE decreases
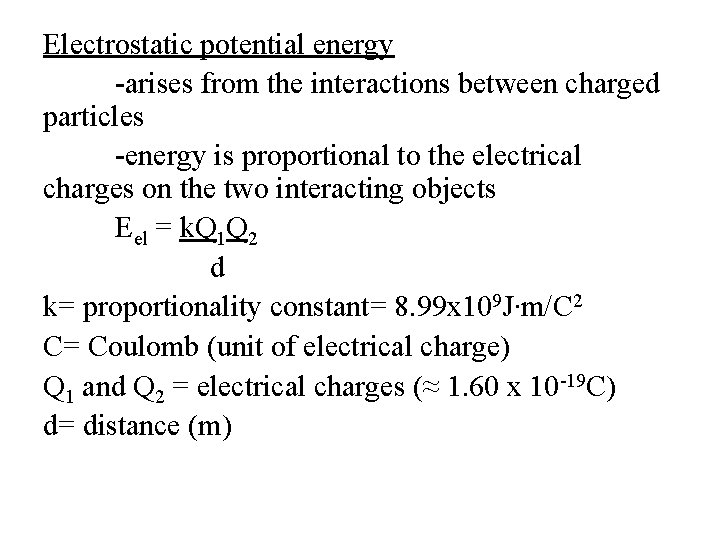
Electrostatic potential energy -arises from the interactions between charged particles -energy is proportional to the electrical charges on the two interacting objects Eel = k. Q 1 Q 2 d k= proportionality constant= 8. 99 x 109 J∙m/C 2 C= Coulomb (unit of electrical charge) Q 1 and Q 2 = electrical charges (≈ 1. 60 x 10 -19 C) d= distance (m)

-when Q 1 and Q 2 have the same sign, the particles repel each other -Eel is positive and PE decreases -when Q 1 and Q 2 have opposite signs, the particles attract each other -Eel is negative and PE increases **FIGURE 5. 3 page 161**

Units of Energy Joule (J) SI unit for energy/heat -use k. J often b/c J is small 1 J = 1 kg∙m 2/s 2 calorie (cal) amount of energy needed to raise the temp of 1 g of water 1°C 1 cal = 4. 184 J 0. 2390 cal = 1 J Calorie (Cal) = 1000 cal

system- what is being studied surroundings- everything else but the system universe- system and surroundings together

Systems may be: open- matter and energy can be exchanged with surroundings ex- boiling pot of water with no lid closed- can exchange energy but not matter with surroundings ex- page 162 fig 5. 4 isolated- energy or matter cannot be exchanged with surroundings ex- thermos
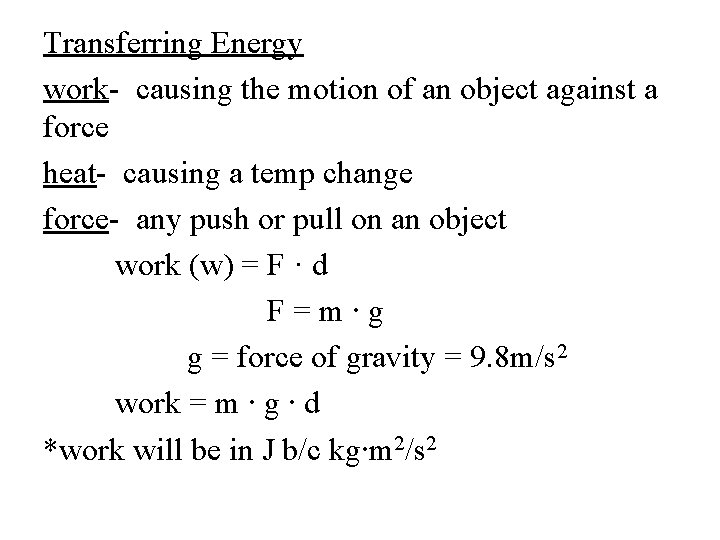
Transferring Energy work- causing the motion of an object against a force heat- causing a temp change force- any push or pull on an object work (w) = F · d F=m∙g g = force of gravity = 9. 8 m/s 2 work = m ∙ g ∙ d *work will be in J b/c kg∙m 2/s 2
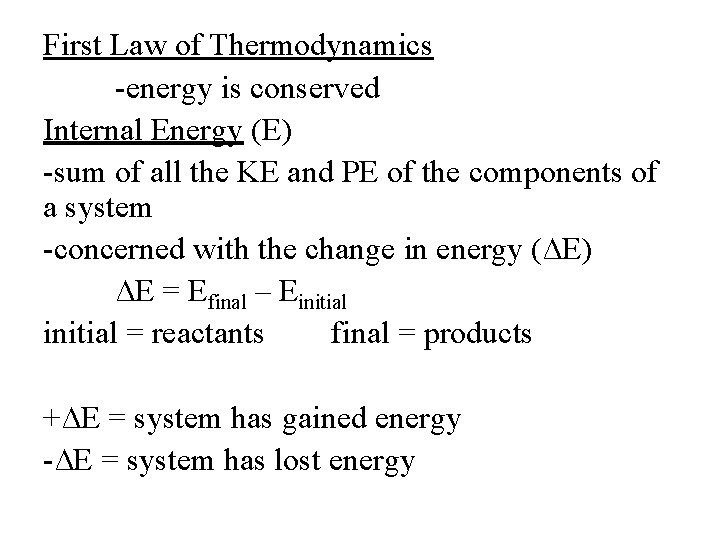
First Law of Thermodynamics -energy is conserved Internal Energy (E) -sum of all the KE and PE of the components of a system -concerned with the change in energy (∆E) ∆E = Efinal – Einitial = reactants final = products +∆E = system has gained energy -∆E = system has lost energy

Relating ∆E to Heat and Work ∆E = q + w q= heat w= work done For q : For w: For ∆E: + if system gains heat - if system loses heat + if work done on system - if work done by system + if net gain of energy by system - if net loss of energy by system *if volume is constant, then w= 0 b/c w= -P∆V

endothermic- system absorbs heat from surroundings ex- melting of ice exothermic- system loses heat to surroundings ex- burning gasoline
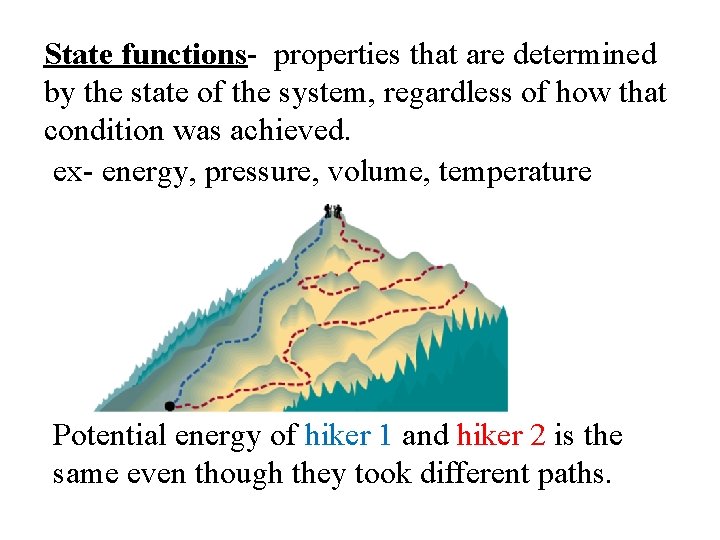
State functions- properties that are determined by the state of the system, regardless of how that condition was achieved. ex- energy, pressure, volume, temperature Potential energy of hiker 1 and hiker 2 is the same even though they took different paths.
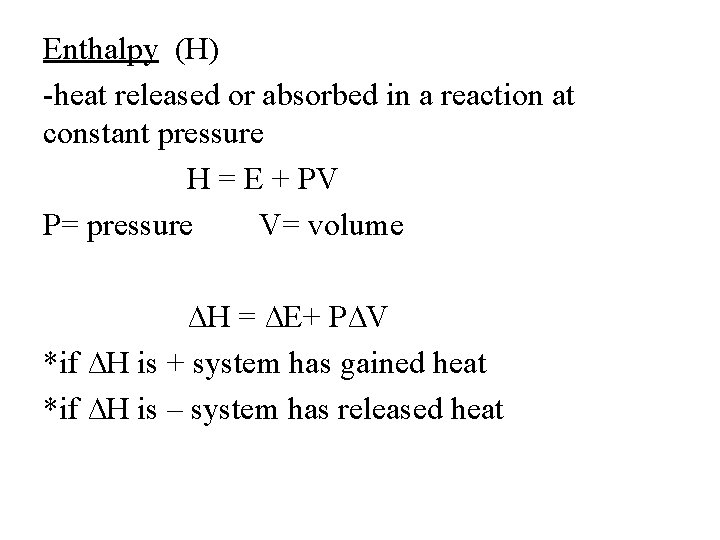
Enthalpy (H) -heat released or absorbed in a reaction at constant pressure H = E + PV P= pressure V= volume ∆H = ∆E+ P∆V *if ∆H is + system has gained heat *if ∆H is – system has released heat
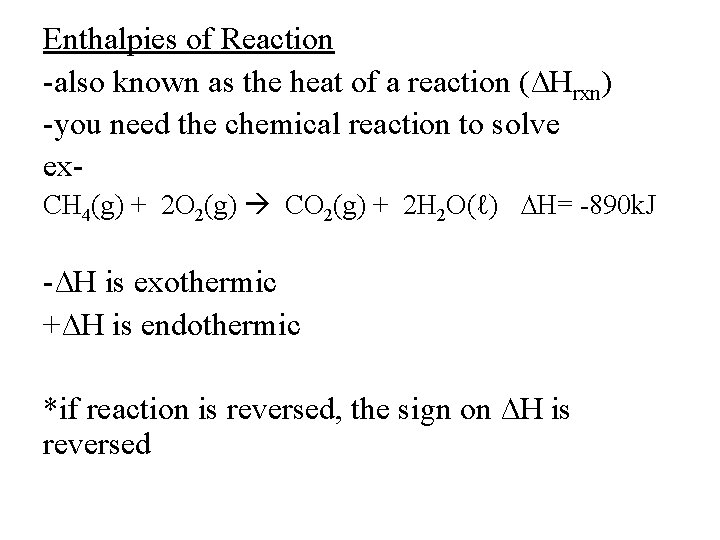
Enthalpies of Reaction -also known as the heat of a reaction (∆Hrxn) -you need the chemical reaction to solve ex. CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(ℓ) ∆H= -890 k. J -∆H is exothermic +∆H is endothermic *if reaction is reversed, the sign on ∆H is reversed
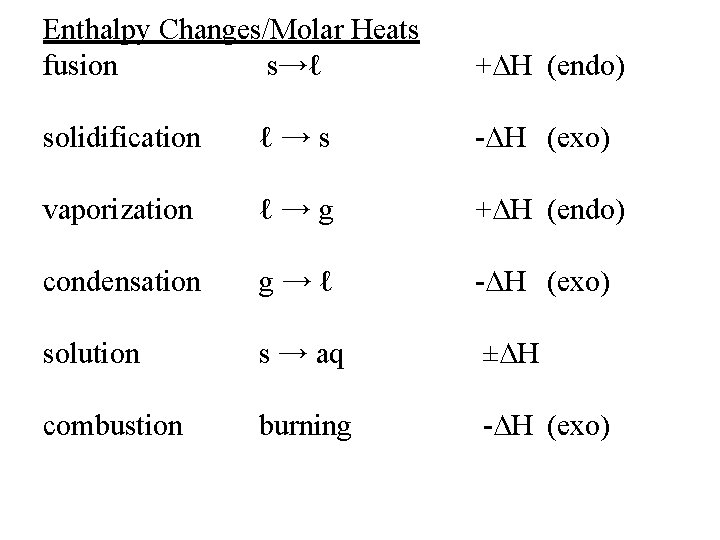
Enthalpy Changes/Molar Heats fusion s→ℓ +∆H (endo) solidification ℓ→s -∆H (exo) vaporization ℓ→g +∆H (endo) condensation g→ℓ -∆H (exo) solution s → aq ±∆H combustion burning -∆H (exo)
Calorimetry -measurement of heat flow calorimeter- used to measure heat flow -insulated container heat capacity- amount of heat required to raise the temp of an object 1°C (J/°C or J/K) specific heat (C) - amount of heat needed to raise the temp of 1 g of a substance 1°C (J/g·°C or J/g·K) molar heat capacity- heat capacity of one mole of a substance (J/mol·°C or J/mol·K)

Specific heat of water = 4. 18 J/g∙K q = (m)(C)(ΔT) q = heat (J or cal) m = mass (g) C = specific heat capacity (J/g∙K) *will be given to you if not solving for ΔT = temp change Tfinal – Tinitial (K) m = q/C∆T C = q/m∆T ∆T = q/m. C
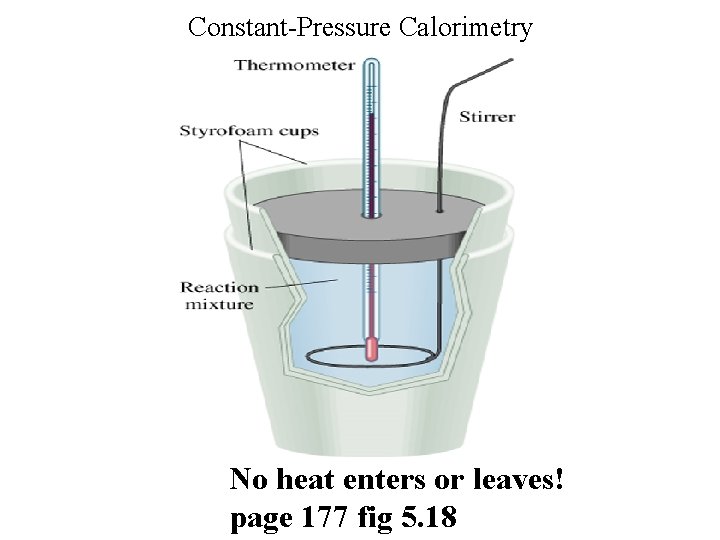
Constant-Pressure Calorimetry No heat enters or leaves! page 177 fig 5. 18
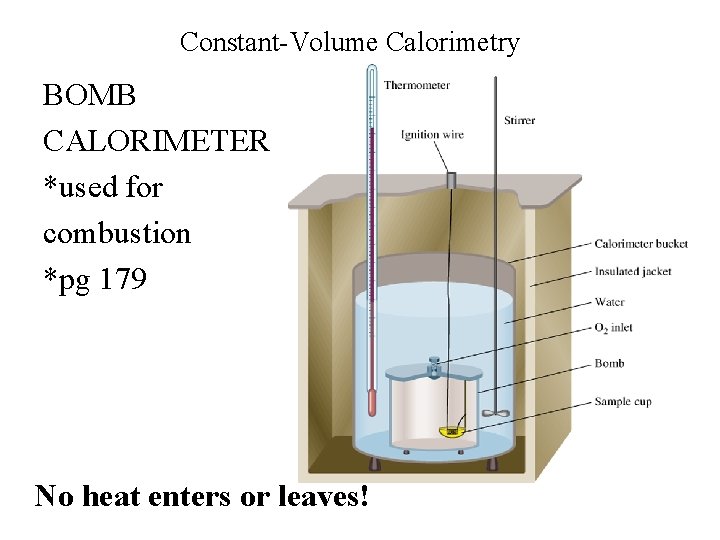
Constant-Volume Calorimetry BOMB CALORIMETER *used for combustion *pg 179 No heat enters or leaves!
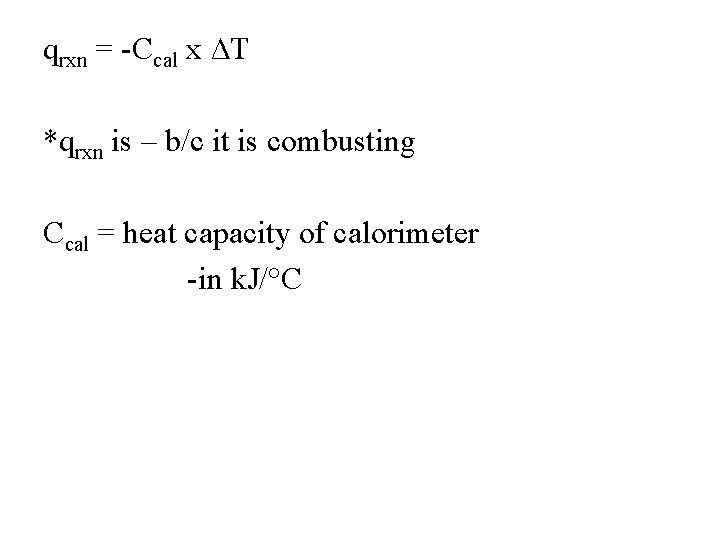
qrxn = -Ccal x ∆T *qrxn is – b/c it is combusting Ccal = heat capacity of calorimeter -in k. J/°C

Hess’s Law -if a reaction is carried out in a series of steps, ∆H of the overall reaction equals the sum of the enthalpy changes for each step *∆H is a state function so it will be the same whether the reaction takes place in one step or a series of steps
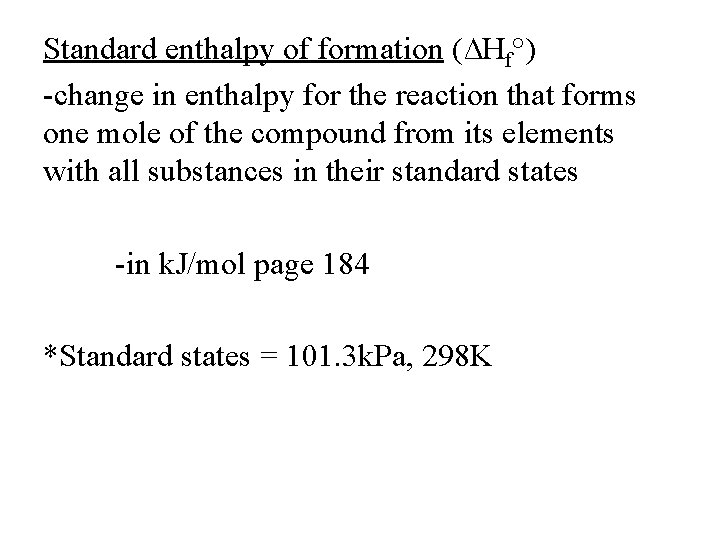
Standard enthalpy of formation (∆Hf°) -change in enthalpy for the reaction that forms one mole of the compound from its elements with all substances in their standard states -in k. J/mol page 184 *Standard states = 101. 3 k. Pa, 298 K
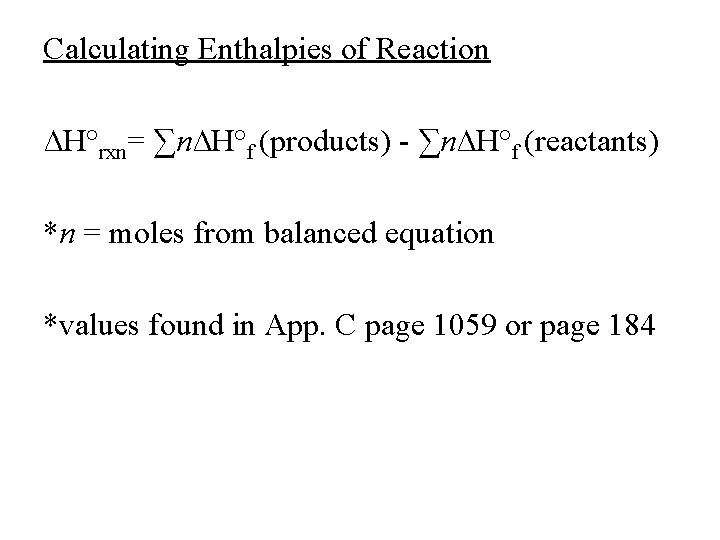
Calculating Enthalpies of Reaction ∆H°rxn= ∑n∆H°f (products) - ∑n∆H°f (reactants) *n = moles from balanced equation *values found in App. C page 1059 or page 184
- Slides: 23