Chapter 5 The Working Cell Energy Flow in















- Slides: 70

Chapter 5 The Working Cell Energy Flow in the Life of a Cell

I. Energy is required for life Energy – capacity to do work A. Uses of energy in living things 1. Movement, metabolism, response to stimuli B. Energy and ecosystems

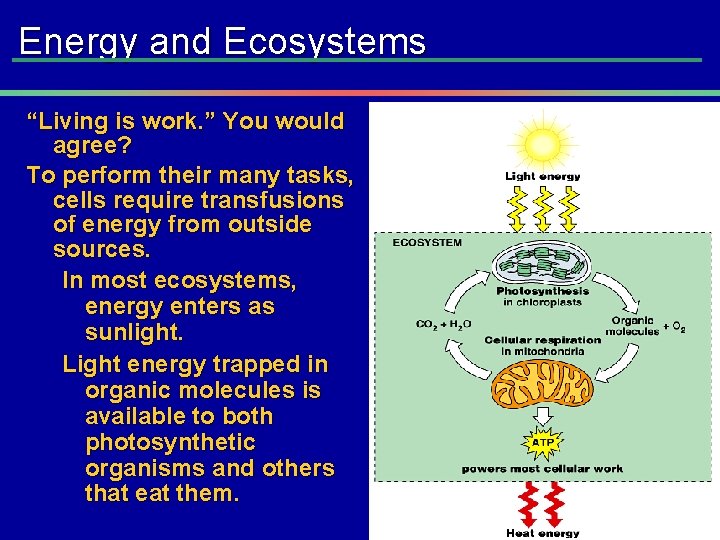
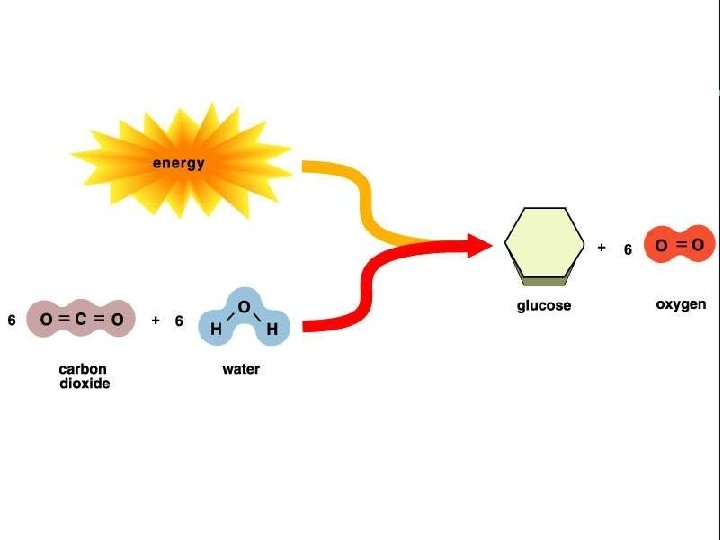
Energy and Ecosystems “Living is work. ” You would agree? To perform their many tasks, cells require transfusions of energy from outside sources. In most ecosystems, energy enters as sunlight. Light energy trapped in organic molecules is available to both photosynthetic organisms and others that eat them.

II. The nature of energy A. Energy undergoes transitions in forms 1. Potential energy—stored energy 2. Kinetic energy—energy of movement 3. Forms of energy - chemical, mechanical, electrical, radiant (light and heat), nuclear


II. The nature of energy B. Properties of energy 1. First law of thermodynamics a. Total energy remains constant in a closed system b. Energy cannot be created or destroyed

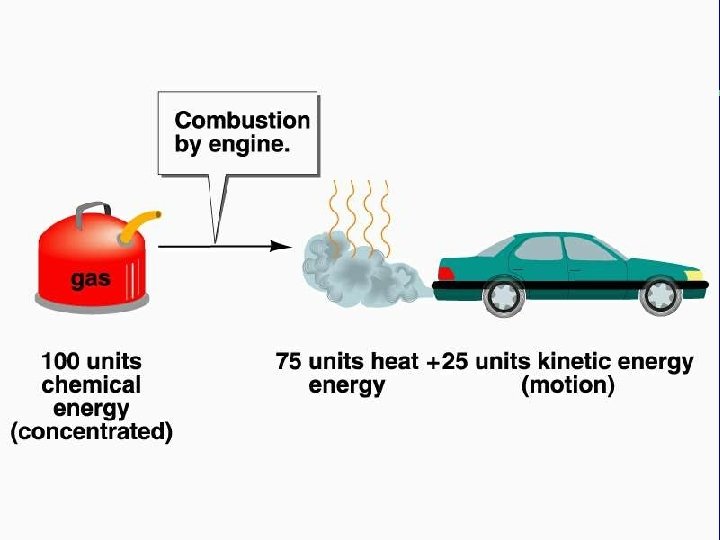
II. The nature of energy B. Properties of energy (cont. ) 2. Second law of thermodynamics a. In an isolated system, any change causes the quantity of concentrated, useful energy to decrease b. Energy is converted from more useful to less useful forms

II. The nature of energy B. Properties of energy (cont. ) 2. Second law of thermodynamics (cont. ) c. Organization of matter and energy 1) Concentrated energy is more ordered (complex) chemically 2) Entropy—all processes in an isolated system result in an increase in randomness and disorder


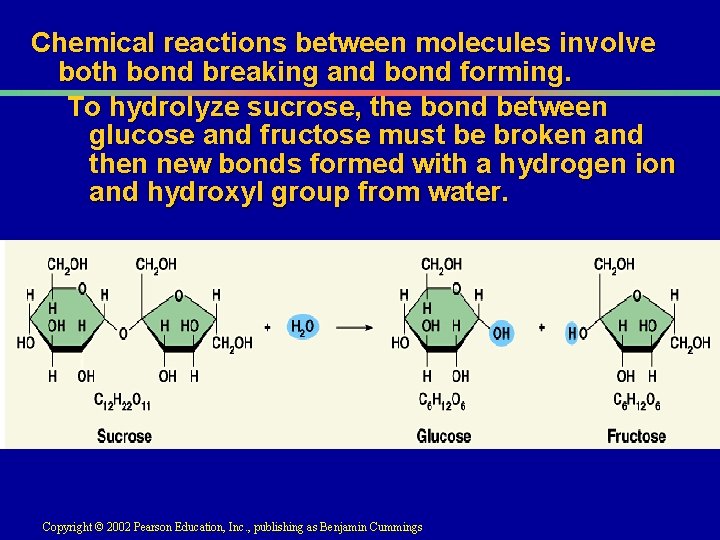
III. Energy use in living things A. Energy is stored in the chemical bonds of biological molecules 1. Review dehydration synthesis B. How is this stored energy released so work can be accomplished? 1. Hydrolysis reaction

Chemical reactions between molecules involve both bond breaking and bond forming. To hydrolyze sucrose, the bond between glucose and fructose must be broken and then new bonds formed with a hydrogen ion and hydroxyl group from water. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

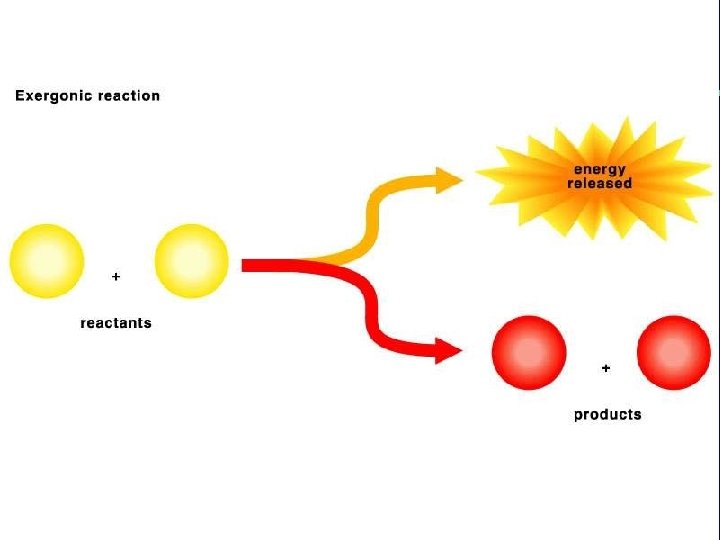
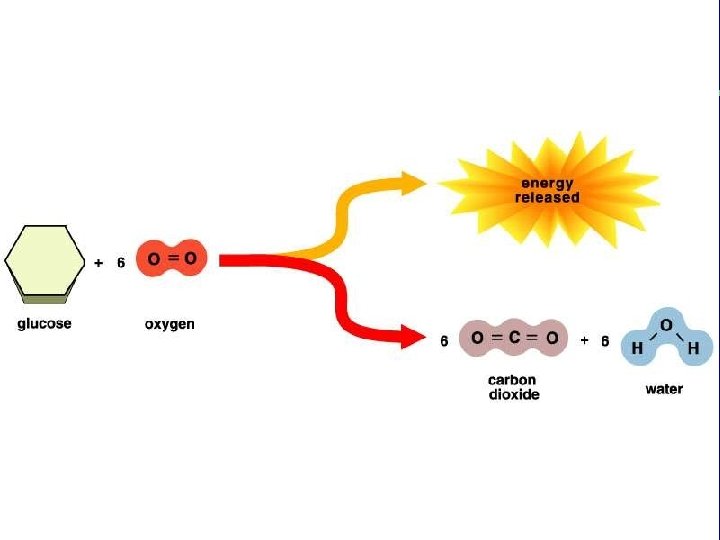
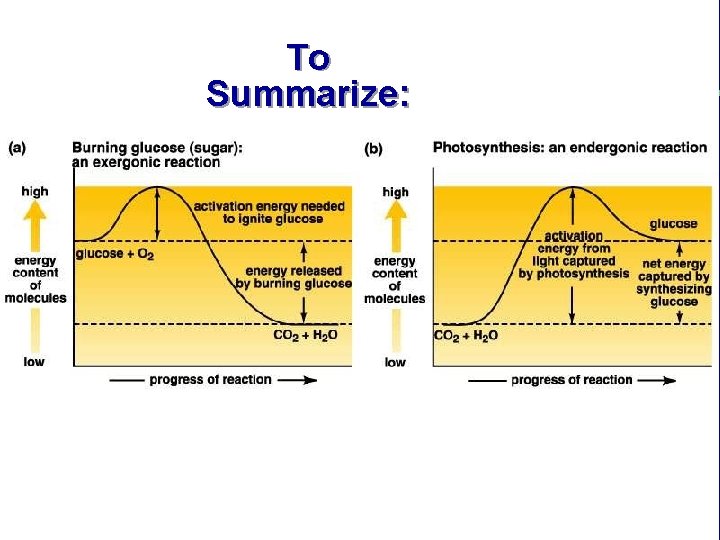
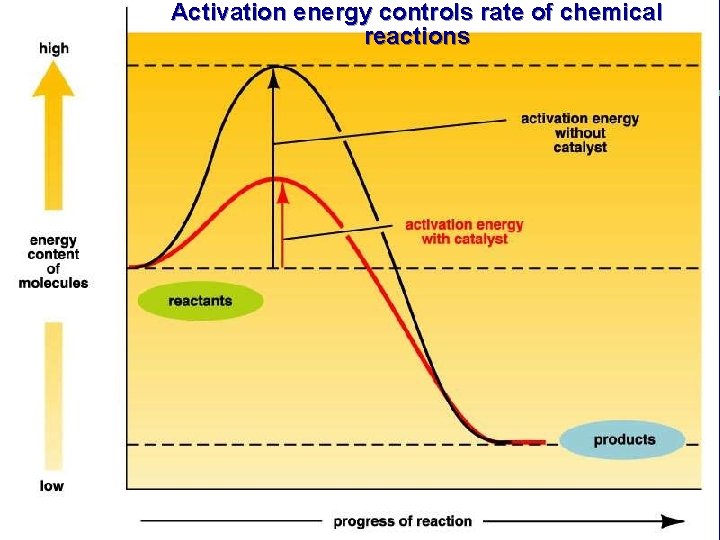
III. Energy use in living things C. Energy releasing chemical reactions are exergonic 1. High energy reactants → Low energy products D. Chemical reactions and activation energy 1. Why does a match not spontaneously burn? 2. Energy, temperature and moving particles



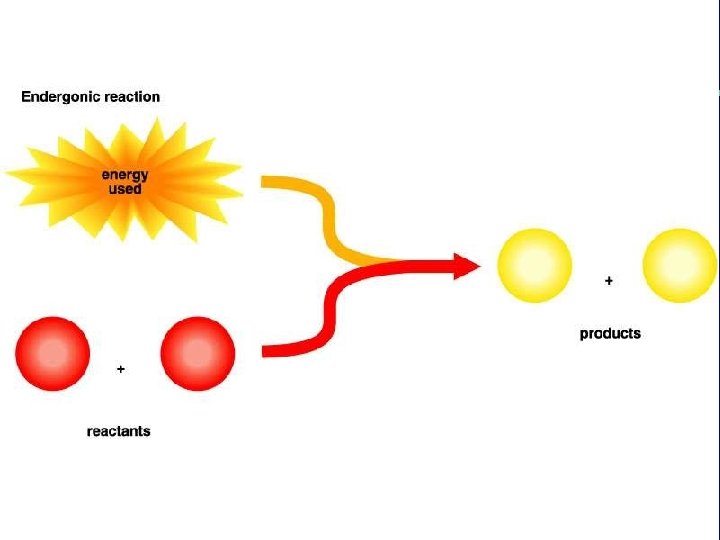
III. Energy use in living things E. Endergonic reactions 1. Low energy reactants → high energy products 2. Do endergonic reactions create energy? 3. How can the product(s) of an endergonic reaction have more energy than the reactants?

III. Energy use in living things E. Endergonic reactions (cont. ) 4. Is anything gained by using energy to make high energy products in an endergonic reaction (energy is converted from more useful to less useful forms in the process)?



To Summarize:

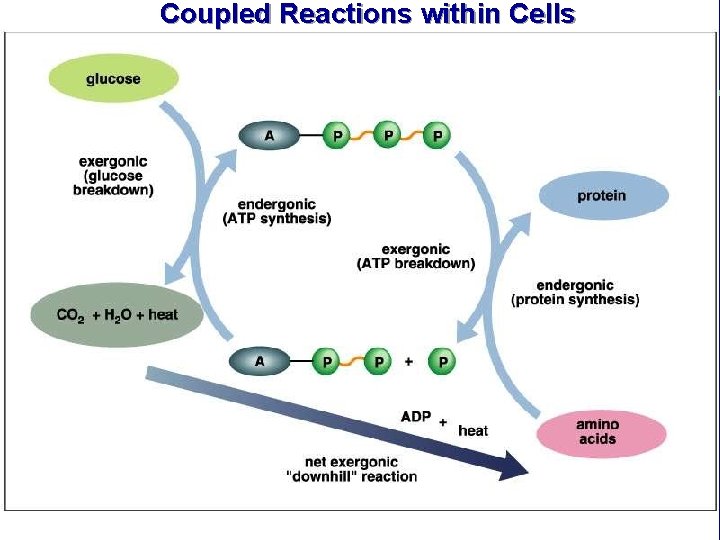
III. Energy use in living things F. Coupling of exergonic and endergonic reactions 1. Exergonic reaction provides energy to drive endergonic reactions 2. How is the energy that is released from an exergonic reaction harnessed and directed to drive its related endergonic reaction?

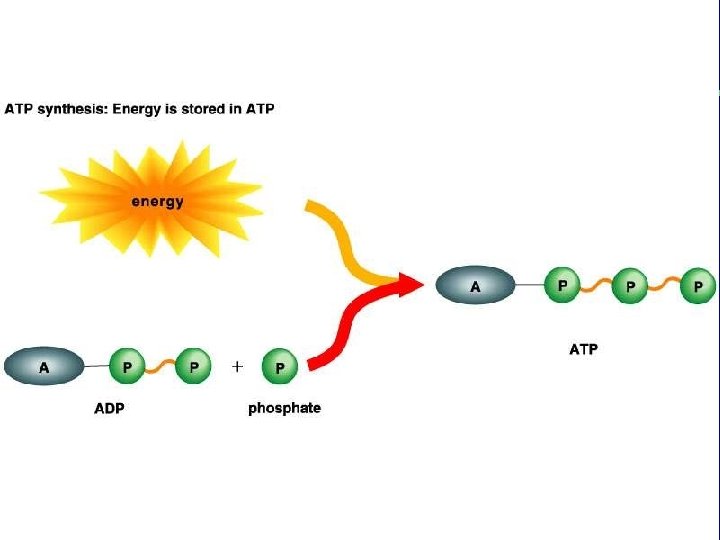
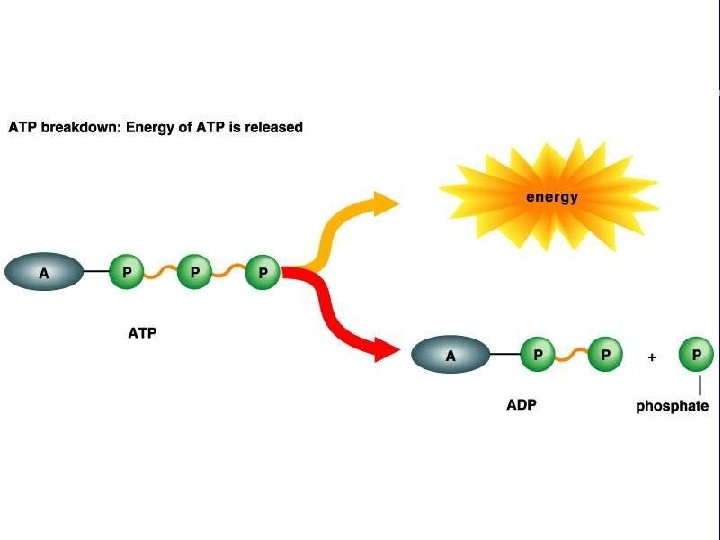
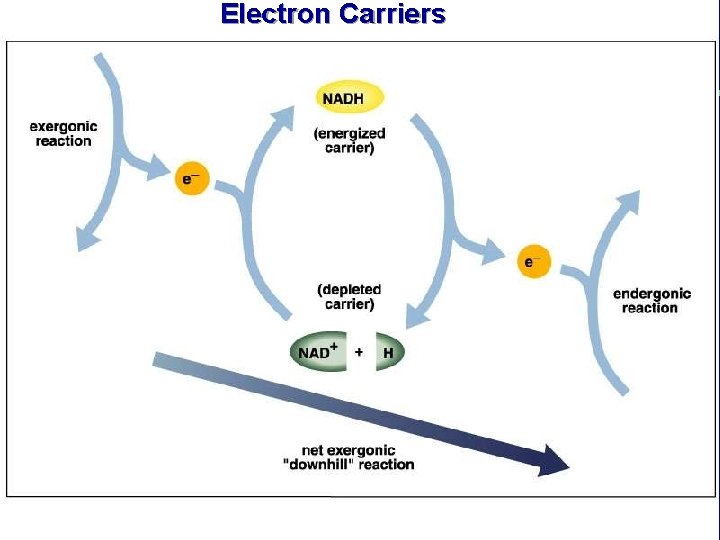
III. Energy use in living things F. Coupling of exergonic and endergonic reactions (cont. ) 3. Energy carrier molecules (energy taxis) a. ATP and electron carrier molecules

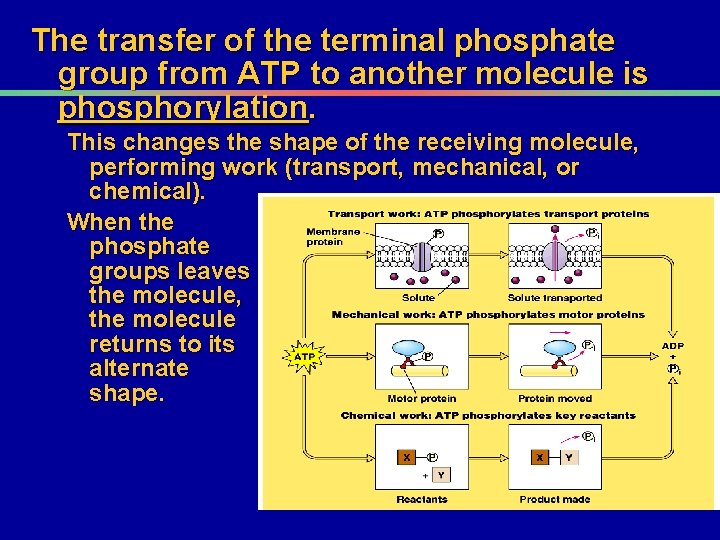
The transfer of the terminal phosphate group from ATP to another molecule is phosphorylation. This changes the shape of the receiving molecule, performing work (transport, mechanical, or chemical). When the phosphate groups leaves the molecule, the molecule returns to its alternate shape.

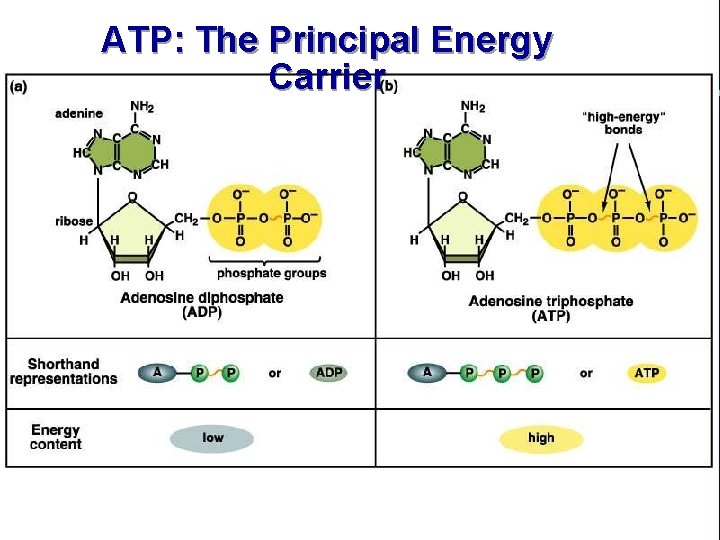
ATP: The Principal Energy Carrier



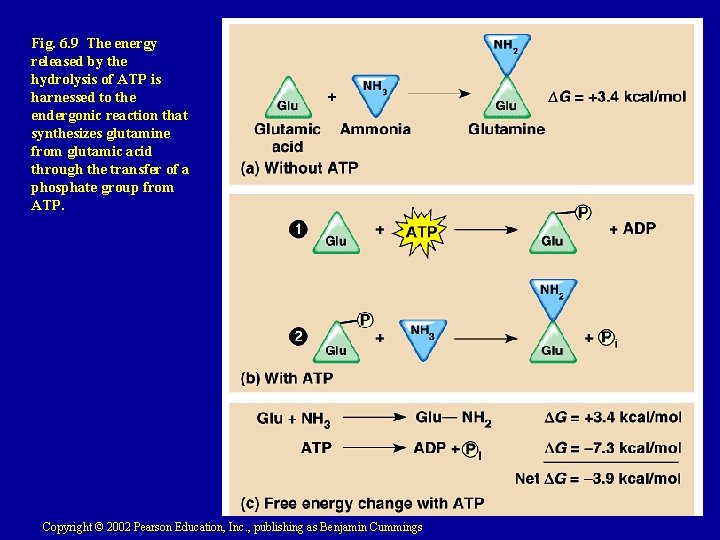
Fig. 6. 9 The energy released by the hydrolysis of ATP is harnessed to the endergonic reaction that synthesizes glutamine from glutamic acid through the transfer of a phosphate group from ATP. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

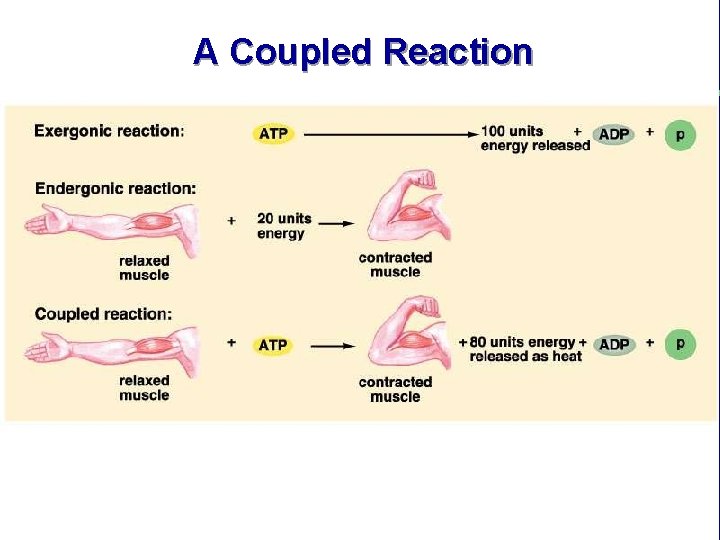
A Coupled Reaction

Coupled Reactions within Cells

Electron Carriers

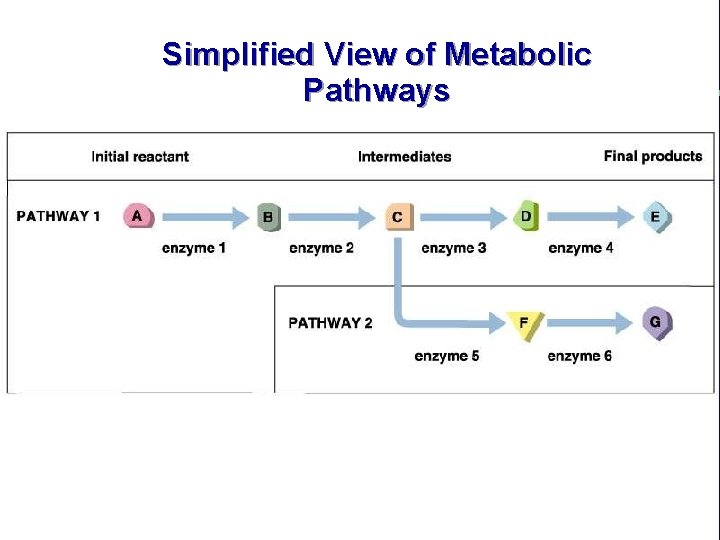
Simplified View of Metabolic Pathways

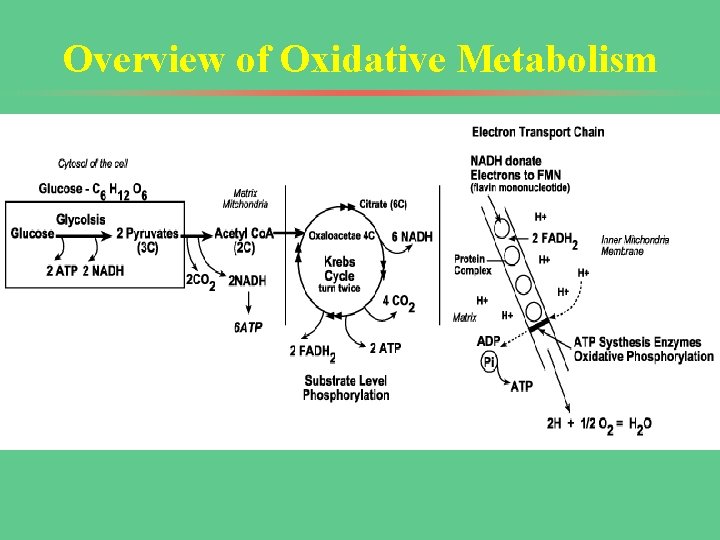
Overview of Oxidative Metabolism

IV. Chemical reactions in living organisms need to be catalyzed A. Activation energy requirements for all the chemical reactions occurring in an organism are too high B. Catalysts reduce activation energy requirements

Activation energy controls rate of chemical reactions

IV. Chemical reactions in living organisms need to be catalyzed C. Enzymes catalyze reactions in living organisms (Biological Catalysts) 1. Bring reactant molecules close together 2. Make bonds easier to break

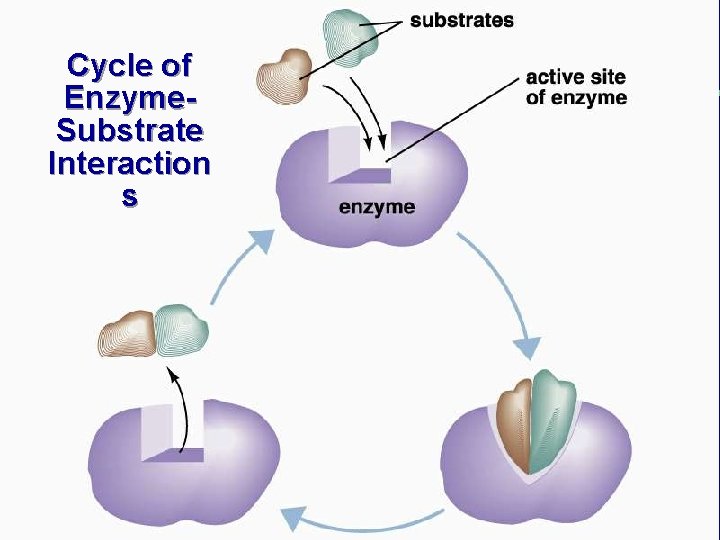
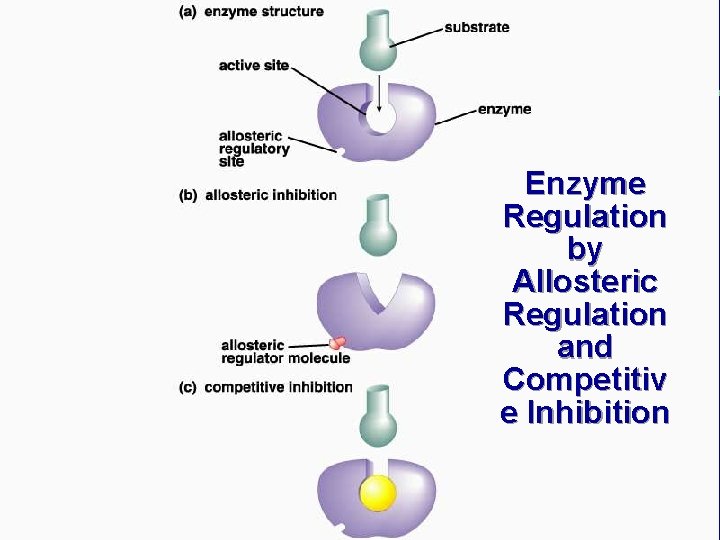
IV. Chemical reactions in living organisms need to be catalyzed C. Enzymes catalyze reactions in living organisms (cont. ) 3. Enzymes are very specific a. Specificity is based on shape of enzyme and substrate b. Lock and key analogy

Cycle of Enzyme. Substrate Interaction s

IV. Chemical reactions in living organisms need to be catalyzed C. Enzymes catalyze reactions in living organisms (cont. ) 4. Most enzymes function as parts of complex, regulated biochemical pathways 5. Most enzymes are proteins a. Mutation and loss of enzyme function

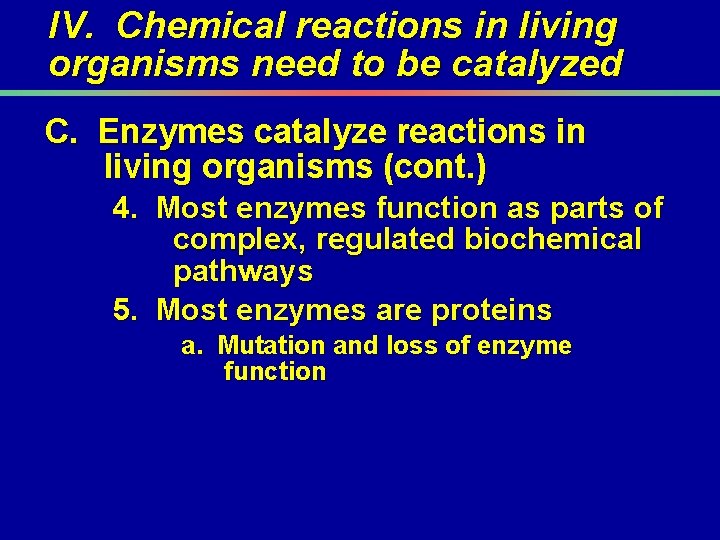
The inset shows the first two steps in the catabolic pathway that breaks down glucose. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

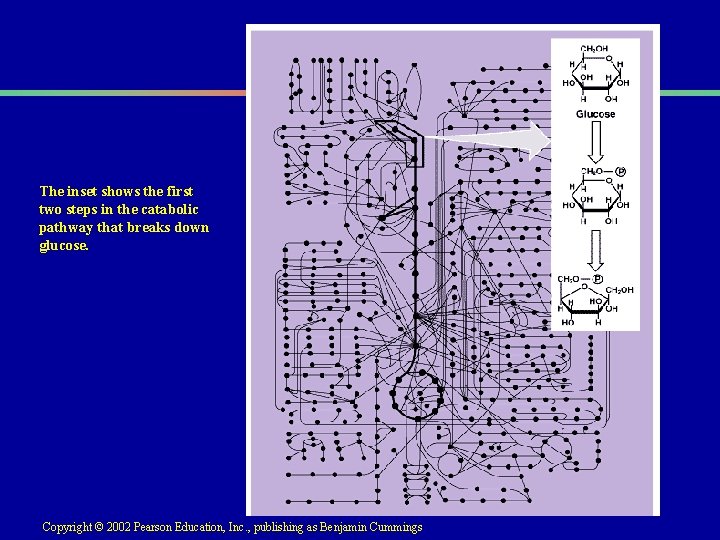
Enzyme Regulation by Feedback Inhibition

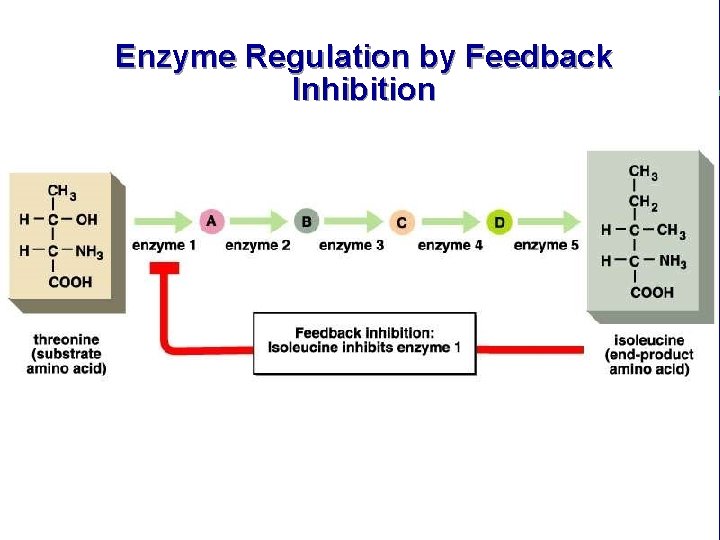
Enzyme Regulation by Allosteric Regulation and Competitiv e Inhibition

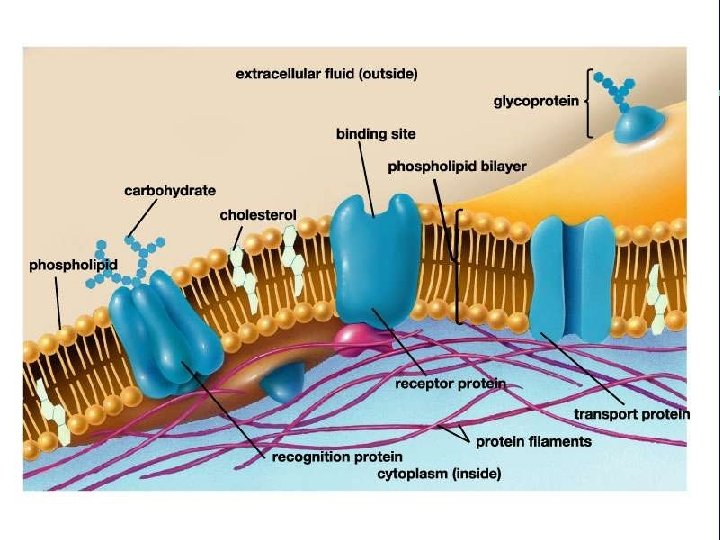
Major Categories of Membrane Proteins • Transport Proteins • Receptor Proteins • Recognition Proteins


I. Plasma membrane structure and functions A. Transport proteins and regulation 1. Controls exchange of watersoluble molecules 2. Energy production and ion pumps

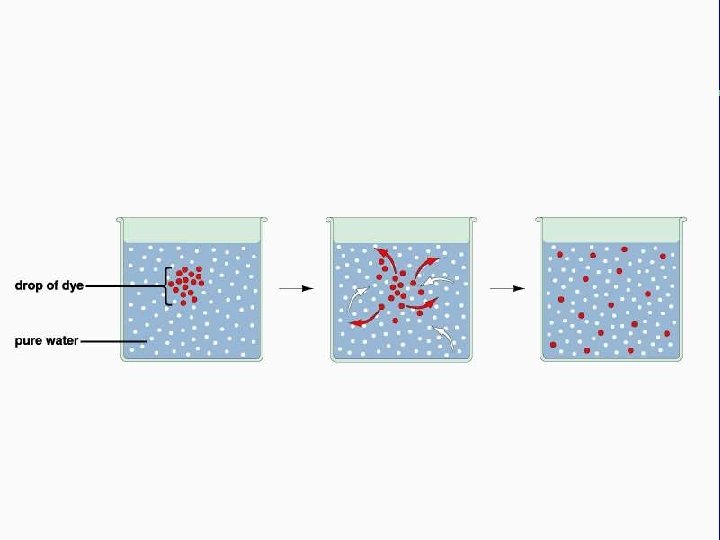
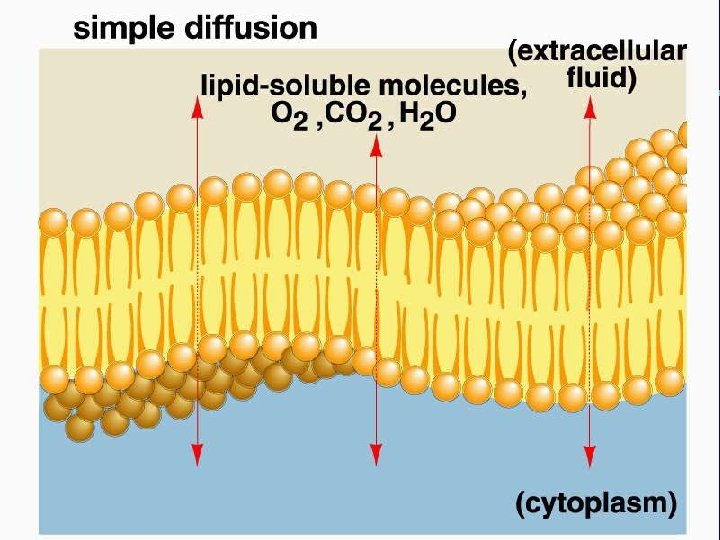
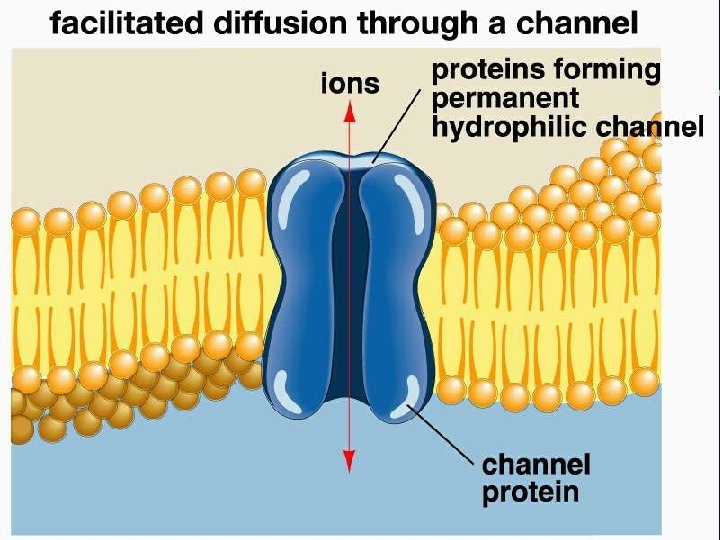
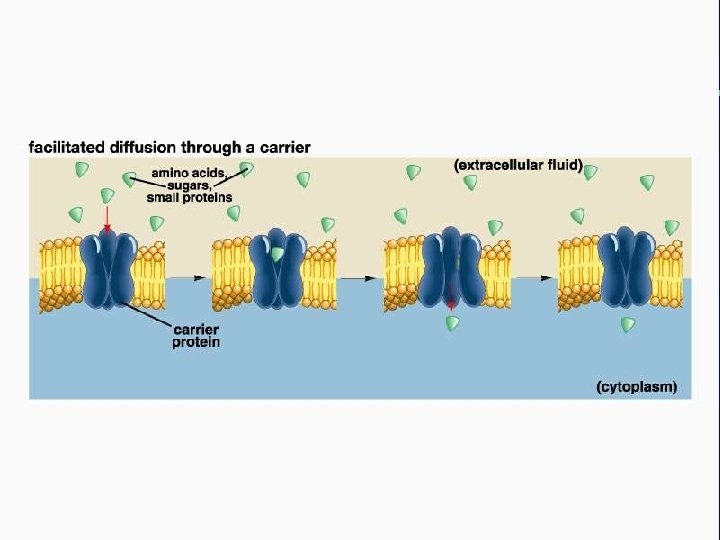
II. Transport across membranes (How substances move across membranes) A. Passive transport is a function of molecular size, lipid solubility, and size of the concentration gradient 1. Simple diffusion 2. Facilitated diffusion





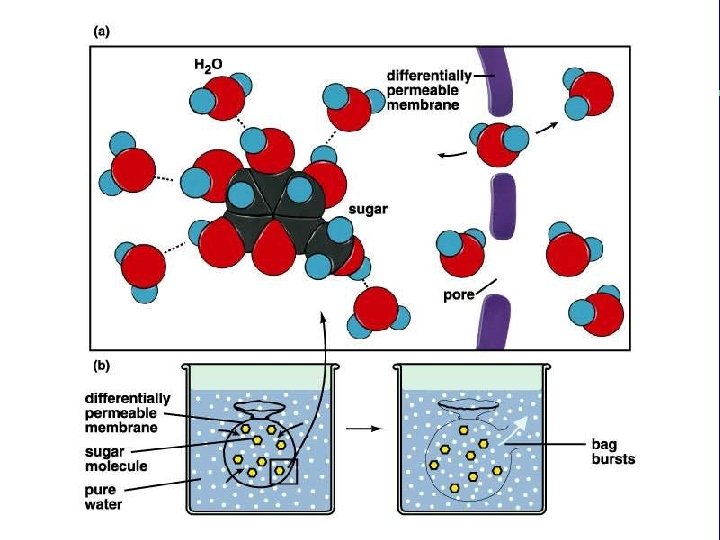
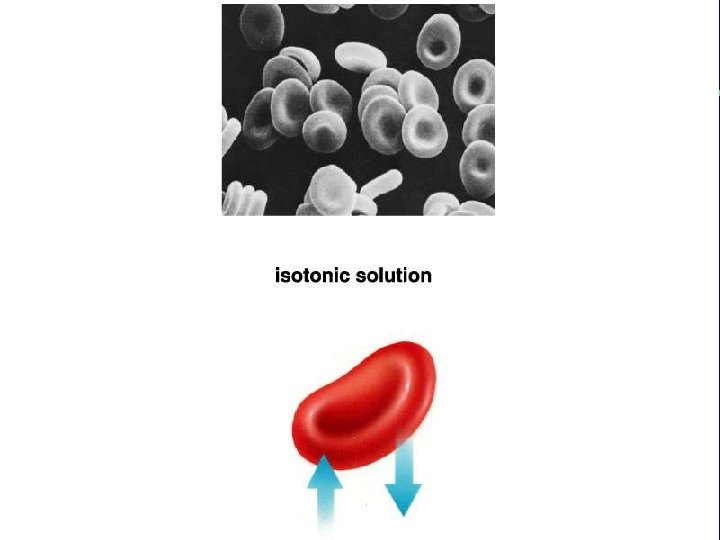
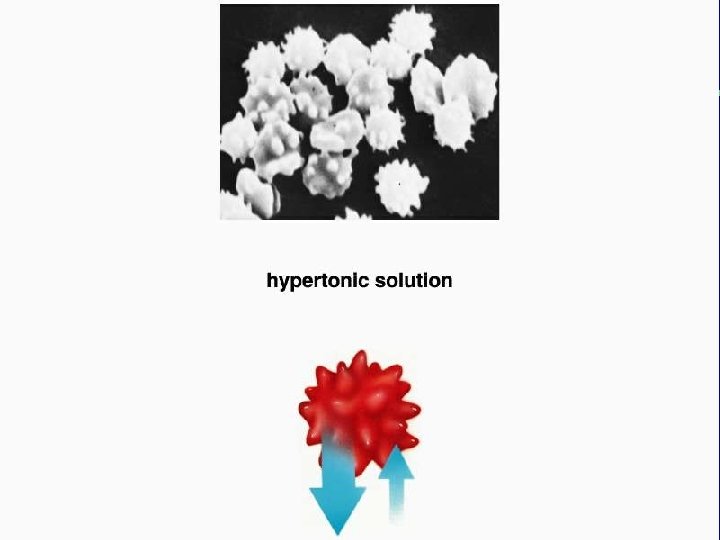
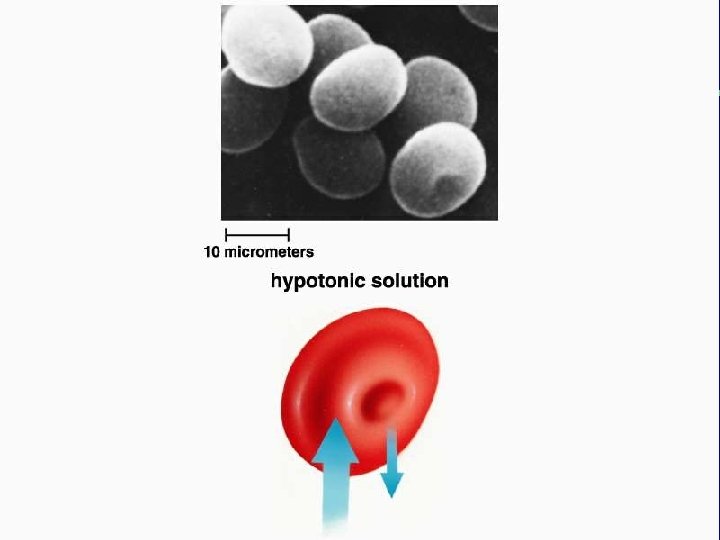
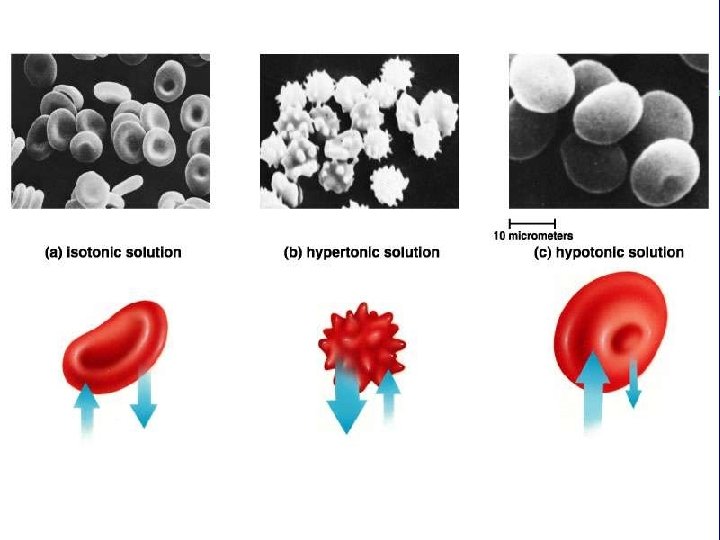
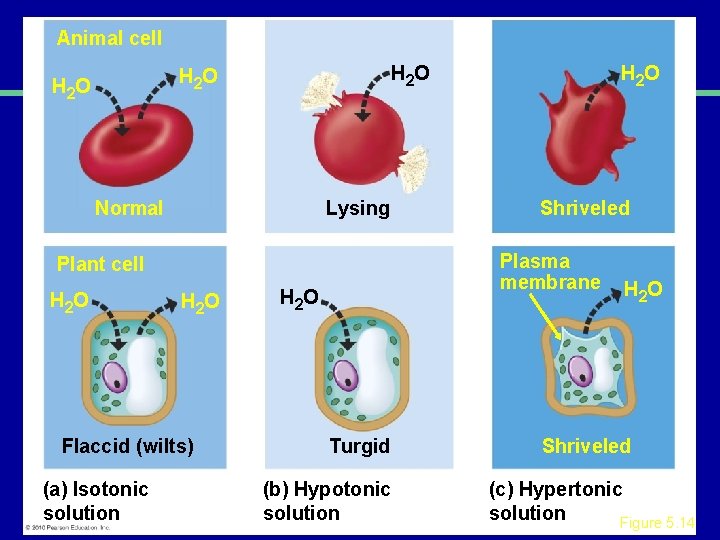
III. Transport across membranes A. Passive transport (cont. ) 3. Osmosis-the diffusion of water a. Isotonic b. Hypertonic c. Hypotonic






Animal cell H 2 O Normal Lysing H 2 O Flaccid (wilts) (a) Isotonic solution Shriveled Plasma membrane Plant cell H 2 O Turgid (b) Hypotonic solution H 2 O Shriveled (c) Hypertonic solution Figure 5. 14

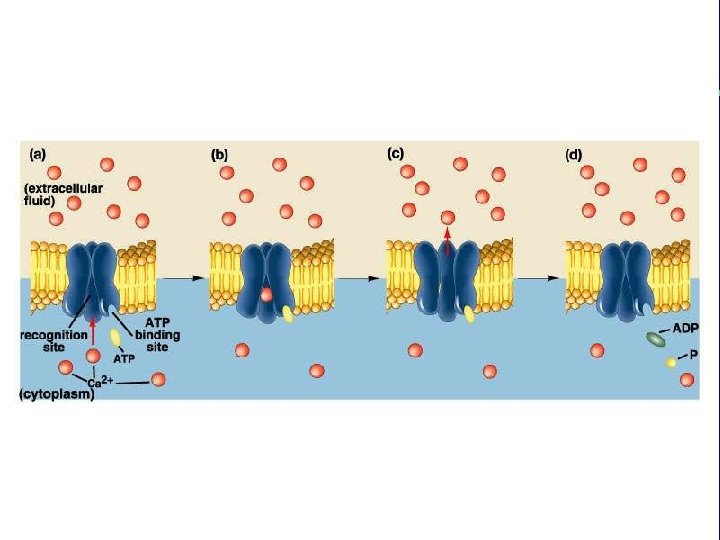
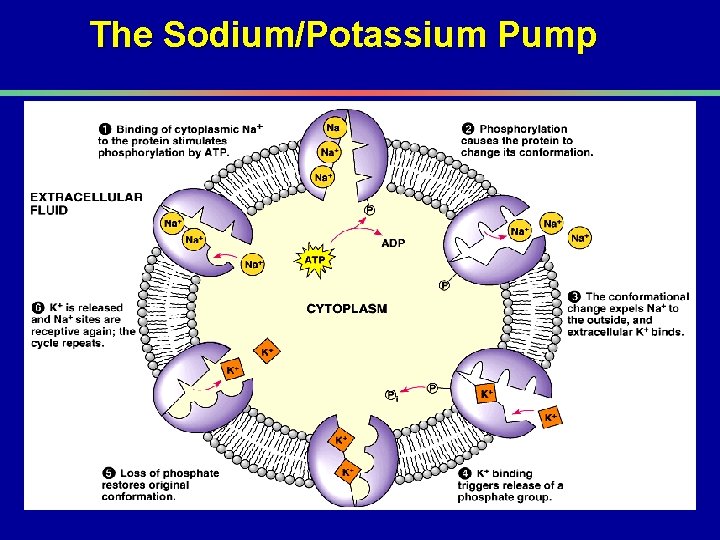
II. Transport across membranes B. Energy-requiring transport 1. Active transport a. Ion gradients and energy production


The Sodium/Potassium Pump

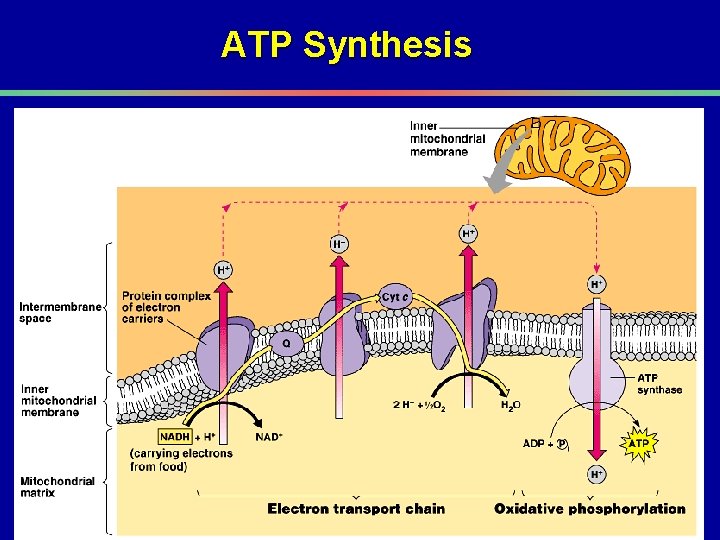
ATP Synthesis

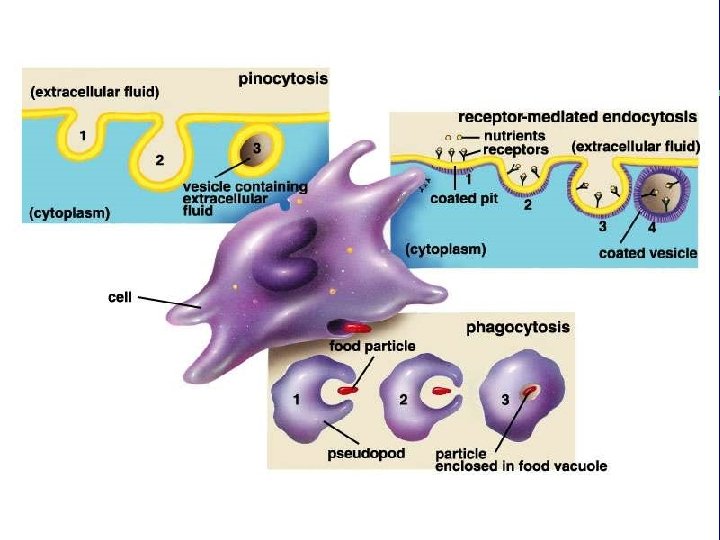
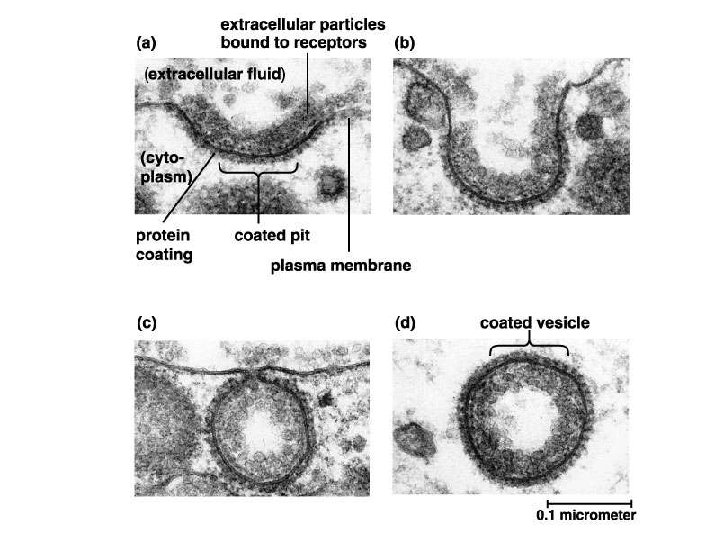
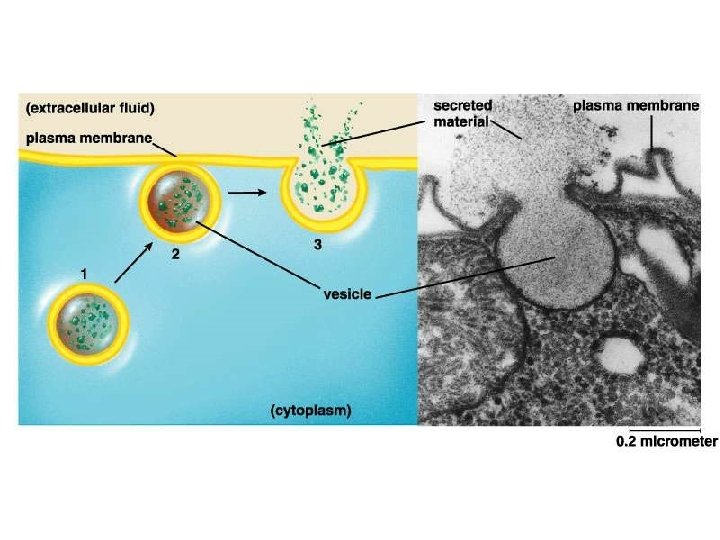
II. Transport across membranes B. Energy-requiring transport of large substances 2. Endocytosis a. b. c. Pinocytosis Receptor-mediated endocytosis Phagocytosis 3. Exocytosis




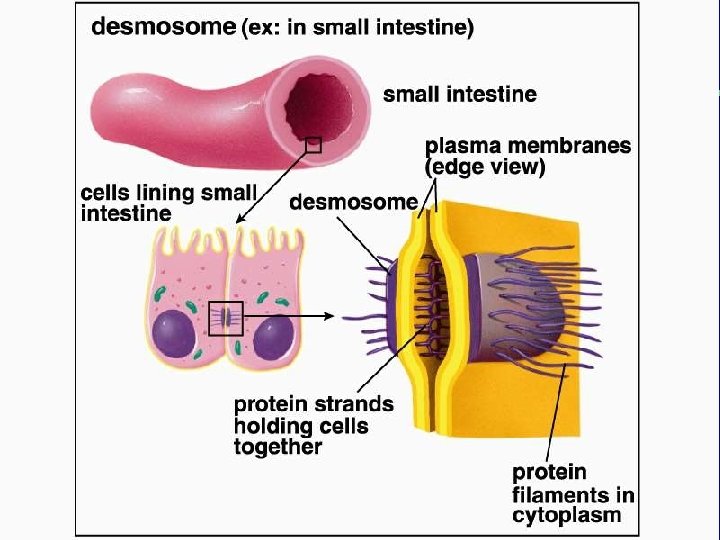
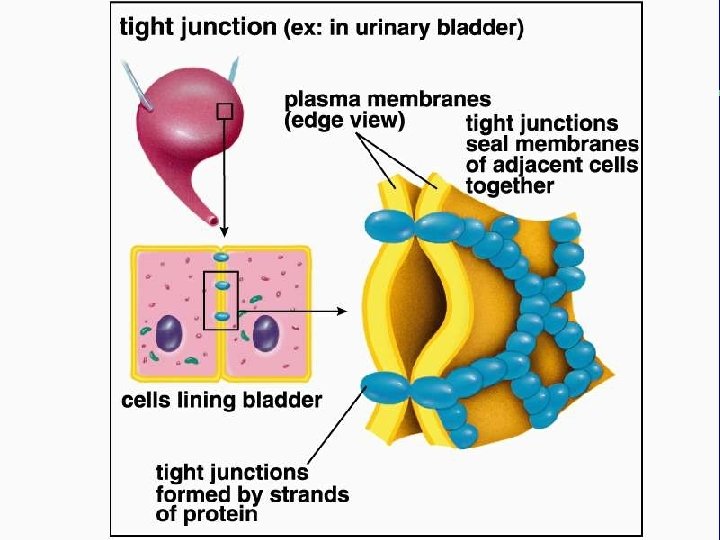
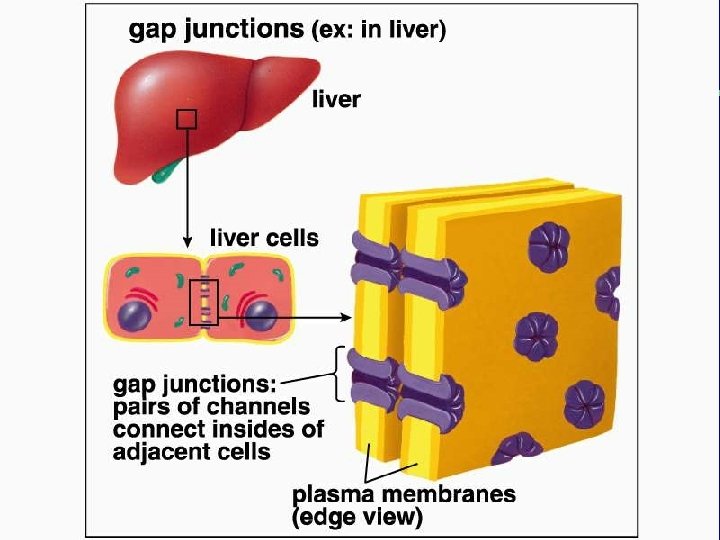
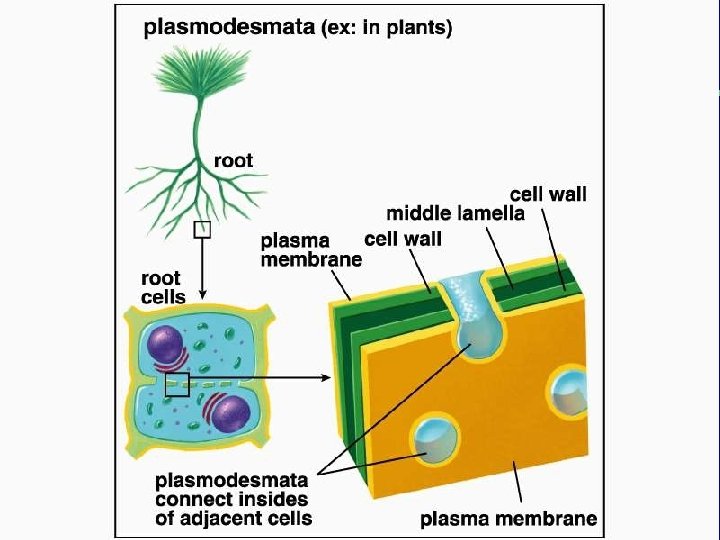
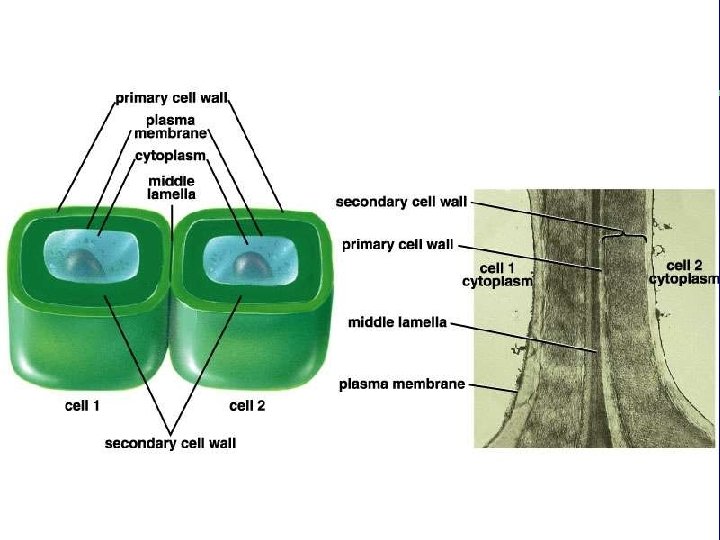
IV. Cell connections A. Desmosomes and tight junctions B. Gap junctions and plasmodesmata






Have a nice week! Read Chap. 6