Chapter 5 THE STRUCTURE OF MATTER ELECTRON DOT






















- Slides: 26

Chapter 5 THE STRUCTURE OF MATTER

ELECTRON DOT DIAGRAMS � Short-hand way to show valence electrons

STEPS TO DRAWING ELECTRON DOT DIAGRAMS � Write out chemical symbol � Find the number of valence electrons � Draw the valence electrons in the configuration

DRAW ELECTRON DOT STRUCTURES FOR ELEMENTS 1 -18

COMPOUNDS � 2 or more elements that are chemically combined � Examples: H 20 � Held Na. Cl C 6 H 12 O 6 together by chemical bonds � Have different properties that the elements that make them up � Always has same chemical formula no matter which state of matter


CHEMICAL BONDING

CHEMICAL BONDS � Ionic � Covalent � Metallic

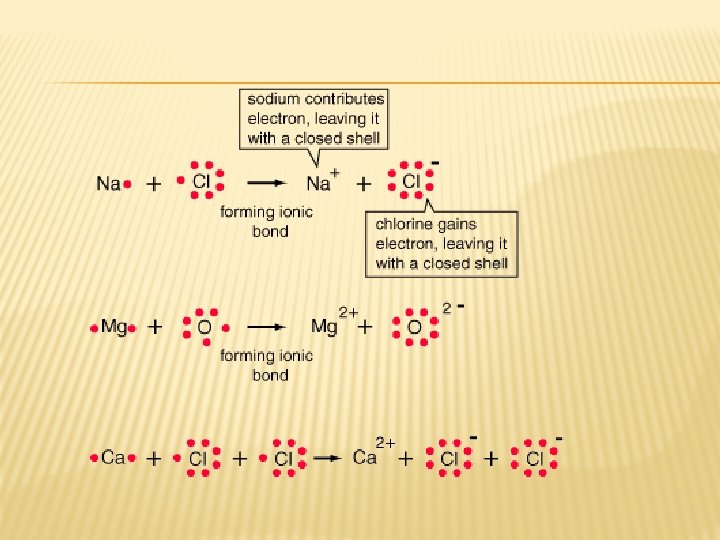
IONIC BONDING � Electrons are gained or lost. One atom gains what another atom has lost. � Formed between opposite charged ions � Positive metals (cations) and negative nonmetals (anions) � Examples: Ca. Cl 2 Na. Cl Mg. O


� Compounds � Conduct � (b/c � Have Mg. O that have ionic bonds: electricity when dissolved in water ions are then free to move) high melting points

NAMING IONIC COMPOUNDS � Includes names of all elements that are in the compound � Cations (+) uses same name � Example: Na. Cl (sodium chloride) � Anion (-) have altered names; drop ending and add “ide” � Example: Ca. Cl 2 (calcium chloride)

PRACTICE: NAME THE FOLLOWING IONIC COMPOUNDS. � Ca. F 2 � KCl � Be. S � K 2 S � Mg. S � Ca. I 2

WRITING IONIC FORMULAS � Identify type of bond that has occurred � Identify charges of ions � Balance charges using subscripts

PRACTICE: WRITE THE FORMULAS FOR THE FOLLOWING COMPOUNDS. � Sodium flouride � Calcium oxide � Potassium sulfide � Lithium oxide � Beryllium chloride � Barium phosphide

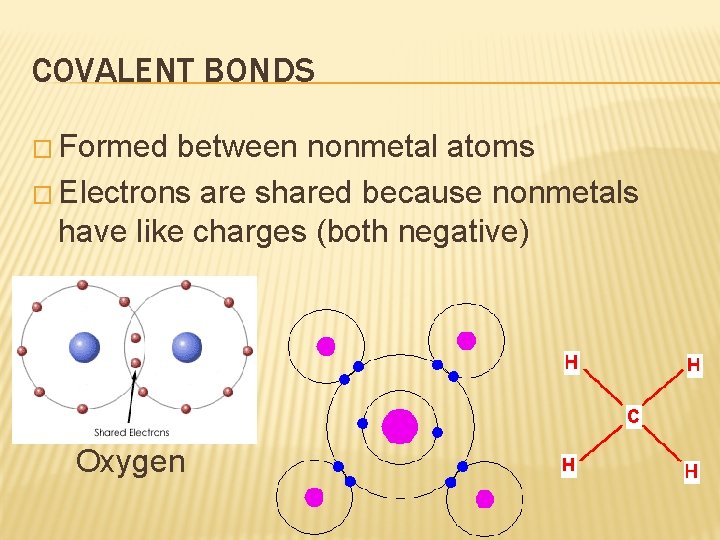
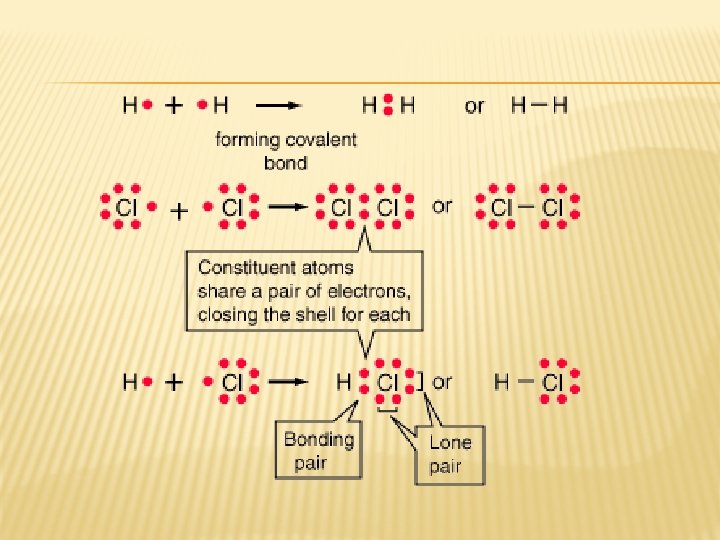
COVALENT BONDS � Formed between nonmetal atoms � Electrons are shared because nonmetals have like charges (both negative) Oxygen



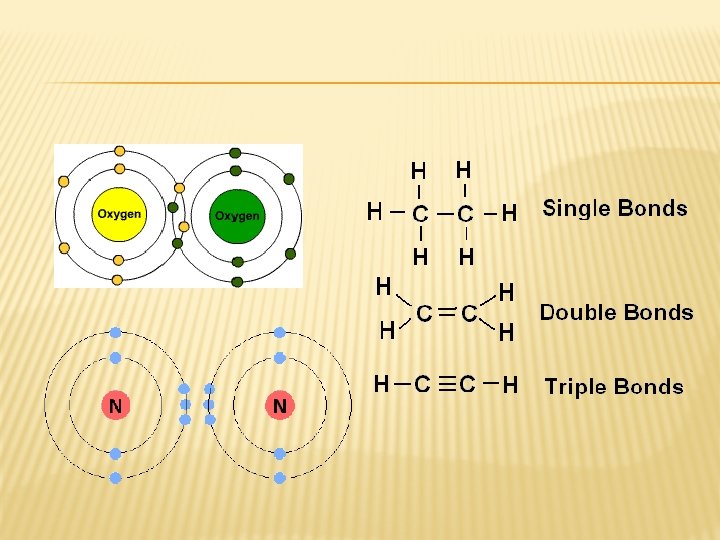
PROPERTIES OF COVALENT BONDS � Low melting points & Do NOT conduct electricity � More energy is needed to break triple bond than double, thus it’s stronger.

� When electrons are shared equally called nonpolar covalent bonds � When atoms aren’t shared equally, they are attracted more to one nucleus than another, polar covalent bond

NAMING COVALENT COMPOUNDS � 1 st element � has no prefix, if there is only 1 atom � If more than 1, use prefix to tell number of atoms � 2 nd element – � Changes ending to ‘ide’ suffix � Prefix tells number of atoms

NAME THESE COVALENT COMPOUNDS � CO 2 � BF 3 � N 2 O 4

WRITE THE FORMULA FOR THE FOLLOWING COMPOUNDS. � Sulfur dioxide � Silicon tetriodide � Tetraphophorus decoxide � Phosphorus trichloride

POLYATOMIC IONS � Covalently bonded atoms that have lost or gained electrons � They can bond with compounds � Example: � They KNO 3 can bond with other polyatomic ions � Example: NH 4 NO 3 � Parenthesis show that it acts as one compound � Examples: (NH 4)2 SO 4

PRACTICE WRITING THE FORMULA NAME � Na. NO 3 � Na. HCO 3 � (NH 4)2 SO 4

WRITE THE FORMULAS FROM THE COMPOUND NAME. � Sodium nitrate � Lithium hydroxide � Ammonium chloride � Sodium carbonate � Potassium phosphate