CHAPTER 5 THE STRUCTURE AND FUNCTION OF MACROMOLECULES












- Slides: 53

CHAPTER 5 THE STRUCTURE AND FUNCTION OF MACROMOLECULES Section B: Carbohydrates - Fuel and Building Material Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

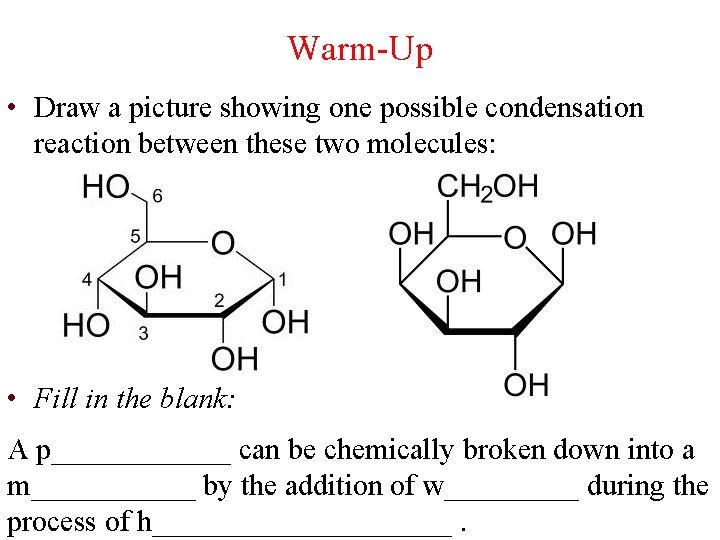
Warm-Up • Draw a picture showing one possible condensation reaction between these two molecules: • Fill in the blank: A p______ can be chemically broken down into a m______ by the addition of w_____ during the process of h__________.

Day 3 – Carbohydrates and Lipids • DWBAT draw, label and identify the properties of carbohydrates and lipids • Homework: Flashcards 13 -23. Packet pgs. 7 -10.

Introduction • Carbohydrates include both sugars and polymers. • The simplest carbohydrates are monosaccharides or simple sugars. • Disaccharides, double sugars, consist of two monosaccharides joined by a condensation reaction. • Polysaccharides are polymers of monosaccharides. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

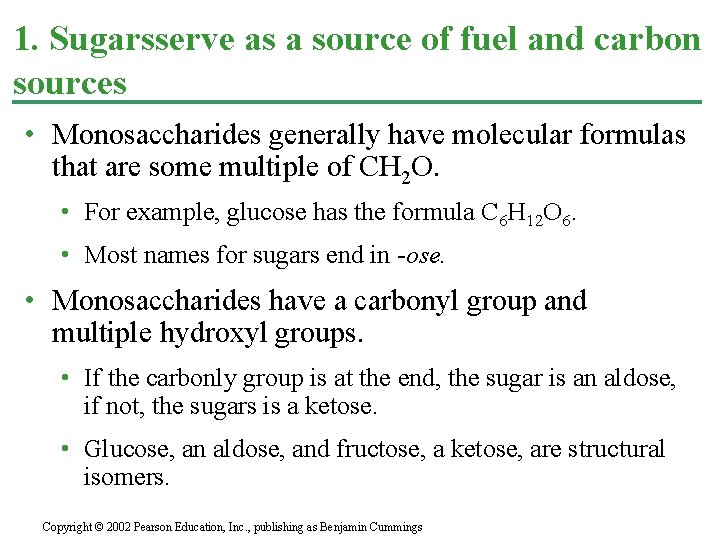
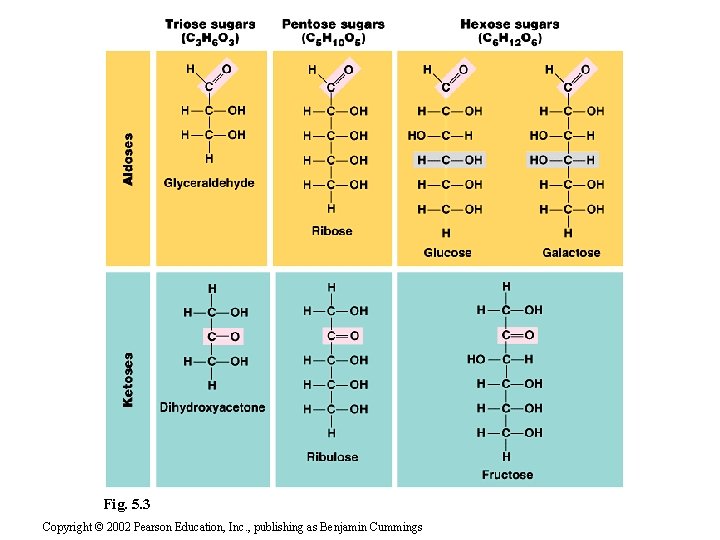
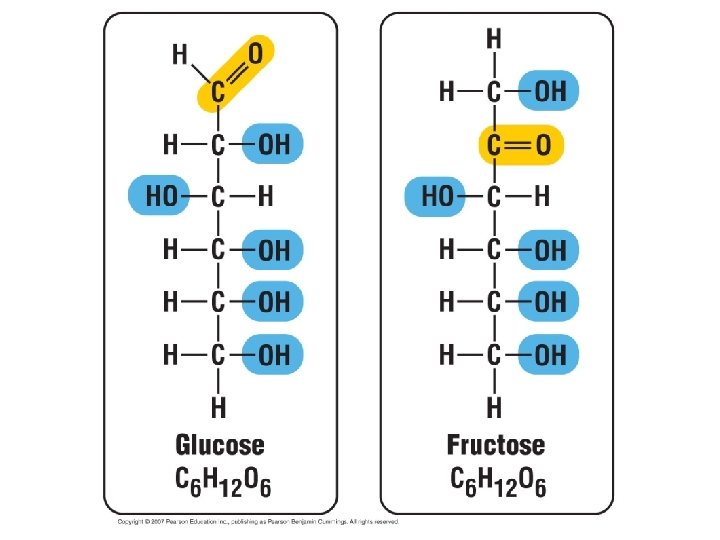
1. Sugarsserve as a source of fuel and carbon sources • Monosaccharides generally have molecular formulas that are some multiple of CH 2 O. • For example, glucose has the formula C 6 H 12 O 6. • Most names for sugars end in -ose. • Monosaccharides have a carbonyl group and multiple hydroxyl groups. • If the carbonly group is at the end, the sugar is an aldose, if not, the sugars is a ketose. • Glucose, an aldose, and fructose, a ketose, are structural isomers. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

• Monosaccharides are also classified by the number of carbons in the backbone. • Glucose and other six carbon sugars are hexoses. • Five carbon backbones are pentoses and three carbon sugars are trioses. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

Fig. 5. 3 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

Figure 3. 8

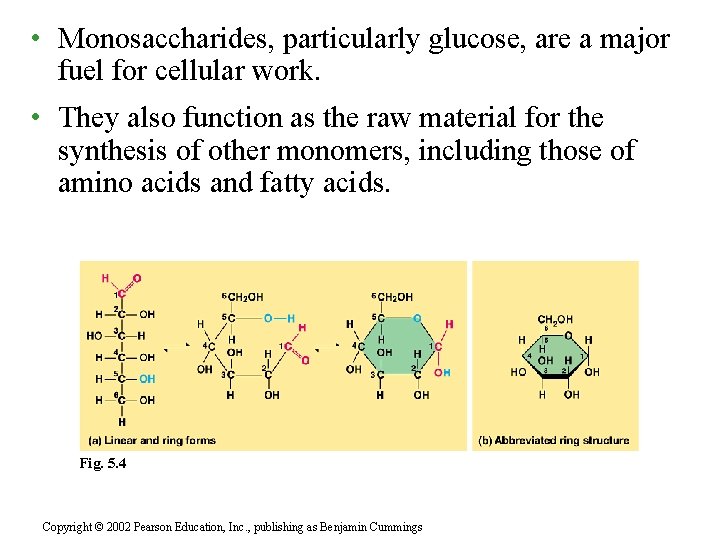
• Monosaccharides, particularly glucose, are a major fuel for cellular work. • They also function as the raw material for the synthesis of other monomers, including those of amino acids and fatty acids. Fig. 5. 4 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

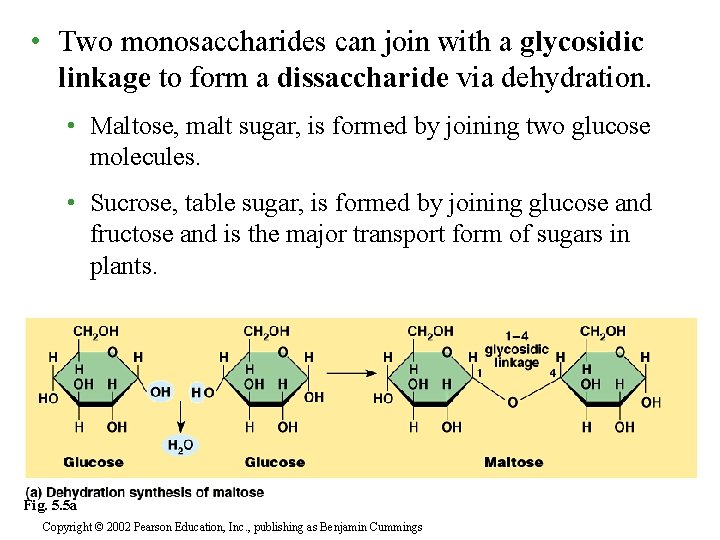
• Two monosaccharides can join with a glycosidic linkage to form a dissaccharide via dehydration. • Maltose, malt sugar, is formed by joining two glucose molecules. • Sucrose, table sugar, is formed by joining glucose and fructose and is the major transport form of sugars in plants. Fig. 5. 5 a Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

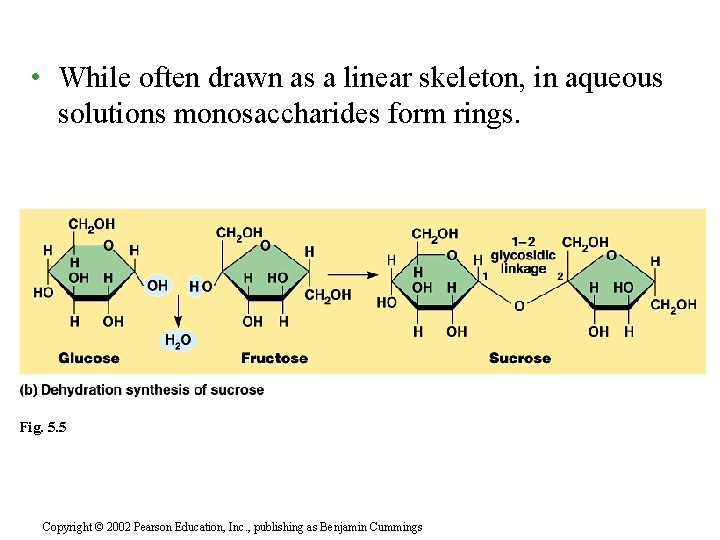
• While often drawn as a linear skeleton, in aqueous solutions monosaccharides form rings. Fig. 5. 5 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

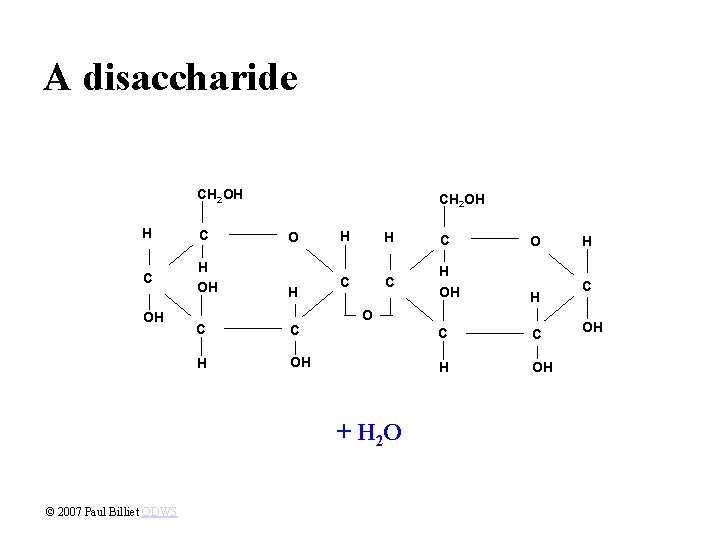
A disaccharide CH 2 OH H C C H OH OH O H H C O C C H OH H C C H OH O + H 2 O © 2007 Paul Billiet ODWS H C OH

List of Disaccharides • sucrose = glucose + fructose • lactose = glucose + galactose • maltose = glucose + glucose © 2007 Paul Billiet ODWS

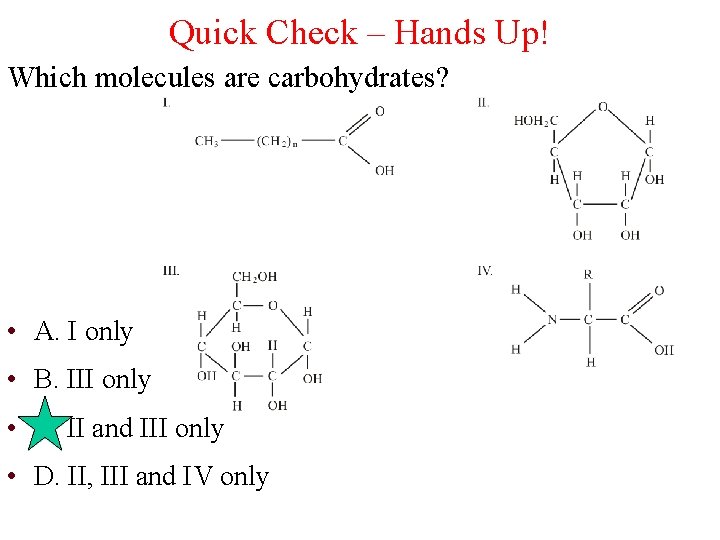
Quick Check – Hands Up! Which molecules are carbohydrates? • A. I only • B. III only • C. II and III only • D. II, III and IV only

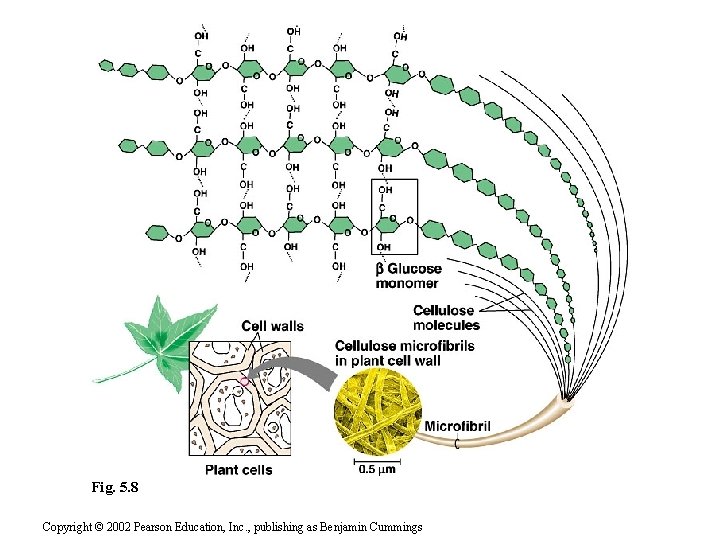
2. Polysaccharideshave storage and structural roles • Polysaccharides are polymers of hundreds to thousands of monosaccharides joined by glycosidic linkages. • One function of polysaccharides is as an energy storage macromolecule that is hydrolyzed as needed. • Other polysaccharides serve as building materials for the cell or whole organism. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

Polysaccharides • Macromolecules • Common ones based upon glucose Branched polysaccharides • Amylose & amylopectin (starches) are synthesised in plants. • Glycogen is synthesised in animals, more highly branched than starches = more compact Unbranched polysaccharides n Cellulose in plant cell walls © 2007 Paul Billiet ODWS

CARBOHYDRATE FUNCTIONS Sugars (mono and disaccharides) small molecules soluble in water: • Maintenance of osmotic balance (e. g. salts in blood plasma, plant cell turgidity); • transport of energy reserves (e. g. glucose in blood or sucrose in sap); • energy substrate (respiration and photosynthesis); • energy store (sugar cane); • flavouring (fruits); reward (nectar); • precursors (building blocks) of polysaccharides, nucleotides and © 2007 Paulamino Billiet ODWSacids

CARBOHYDRATE FUNCTIONS • Polysaccharides Large molecules insoluble in water: • Osmotically inactive carbohydrate storage, (seeds, roots, chloroplasts); • Structural (cellulose in plants) © 2007 Paul Billiet ODWS

Fig. 5. 8 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

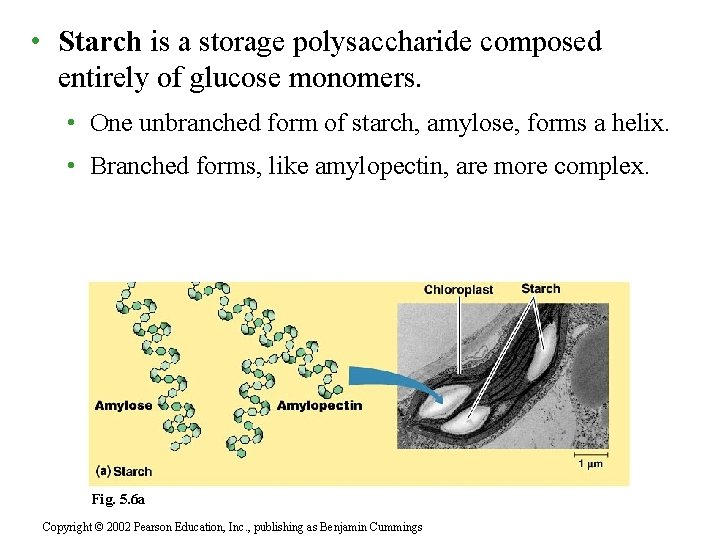
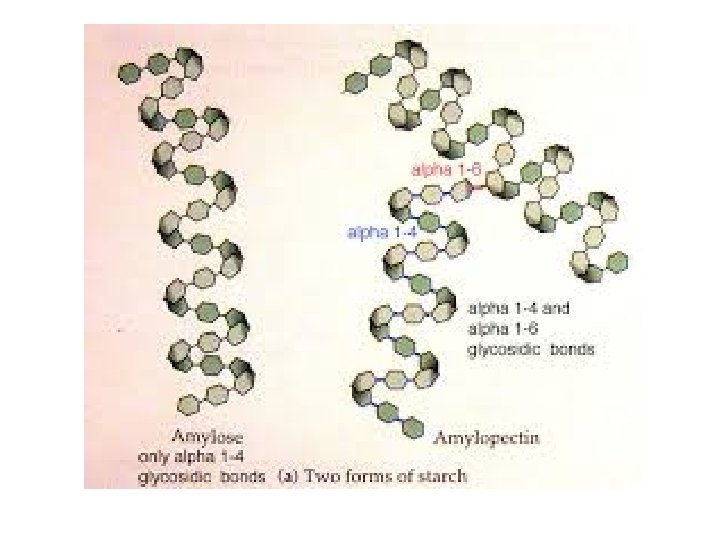
• Starch is a storage polysaccharide composed entirely of glucose monomers. • One unbranched form of starch, amylose, forms a helix. • Branched forms, like amylopectin, are more complex. Fig. 5. 6 a Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

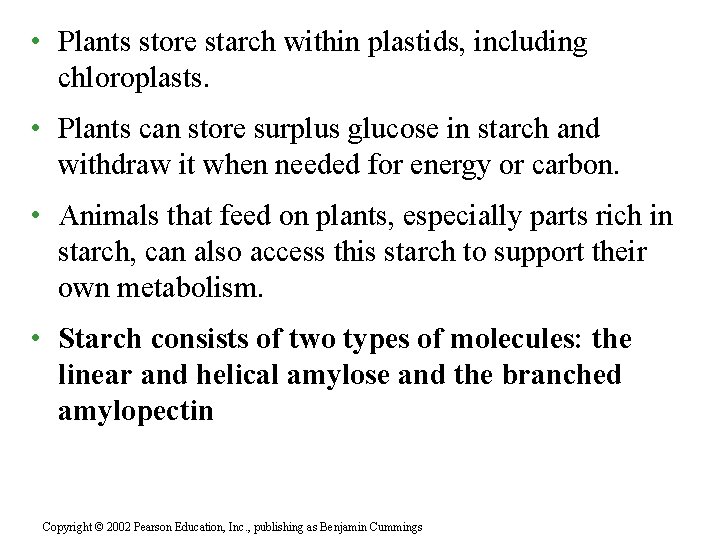
• Plants store starch within plastids, including chloroplasts. • Plants can store surplus glucose in starch and withdraw it when needed for energy or carbon. • Animals that feed on plants, especially parts rich in starch, can also access this starch to support their own metabolism. • Starch consists of two types of molecules: the linear and helical amylose and the branched amylopectin Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings


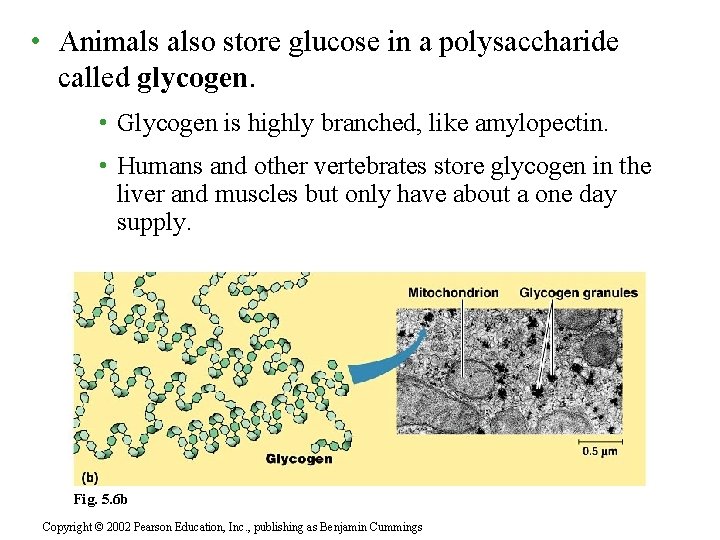
• Animals also store glucose in a polysaccharide called glycogen. • Glycogen is highly branched, like amylopectin. • Humans and other vertebrates store glycogen in the liver and muscles but only have about a one day supply. Insert Fig. 5. 6 b - glycogen Fig. 5. 6 b Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

• Structural polysaccharides form strong building materials. • Cellulose is a major component of the tough wall of plant cells. • Cellulose is also a polymer of glucose monomers, but using beta rings. Fig. 5. 7 c Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

• The enzymes that digest starch cannot hydrolyze the beta linkages in cellulose. • Cellulose in our food passes through the digestive tract and is eliminated in feces as “insoluble fiber”. • As it travels through the digestive tract, it abrades the intestinal walls and stimulates the secretion of mucus. • Some microbes can digest cellulose to its glucose monomers through the use of cellulase enzymes. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

• Another important structural polysaccharide is chitin, used in the exoskeletons of arthropods (including insects, spiders, and crustaceans). • Chitin is similar to cellulose, except that it contains a nitrogen-containing appendage on each glucose. • Pure chitin is leathery, but the addition of calcium carbonate hardens the chitin. • Chitin also forms the structural support for the cell walls of many fungi. Fig. 5. 9 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

Quick Check – Hands Up! Which molecule is a monosaccharide? • A. Fructose • B. Cellulose • C. Lipase • D. Lactose

Introduction • Lipids are an exception among macromolecules because they do not have polymers. • The unifying feature of lipids is that they all have little or no affinity for water. • This is because their structures are dominated by nonpolar covalent bonds. • Lipids are highly diverse in form and function. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

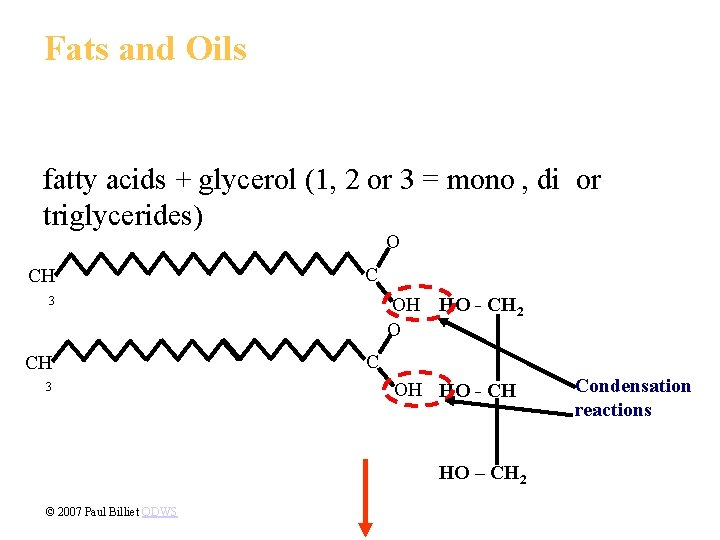
1. Fats store large amounts of energy • Although fats are not strictly polymers, they are large molecules assembled from smaller molecules by dehydration reactions. • A fat is constructed from two kinds of smaller molecules, glycerol and fatty acids. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

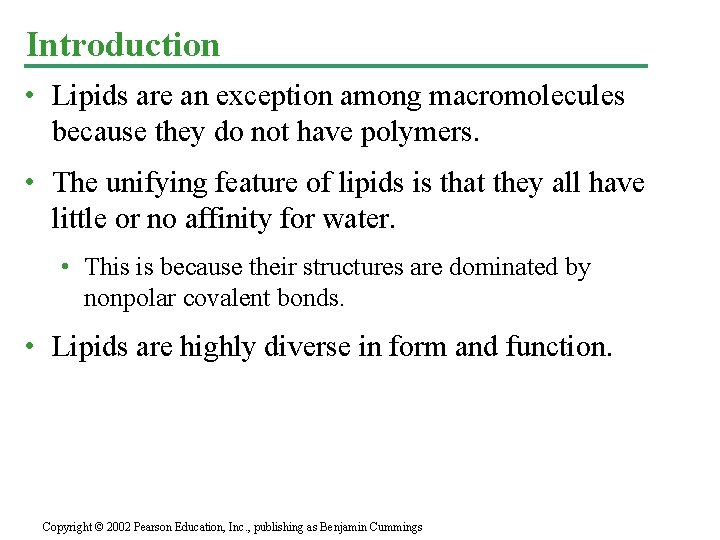
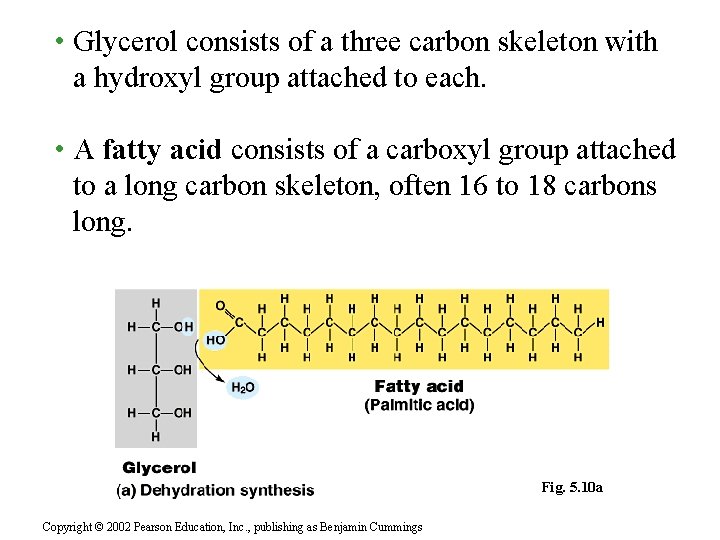
• Glycerol consists of a three carbon skeleton with a hydroxyl group attached to each. • A fatty acid consists of a carboxyl group attached to a long carbon skeleton, often 16 to 18 carbons long. Fig. 5. 10 a Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

Figure 3. 15 a

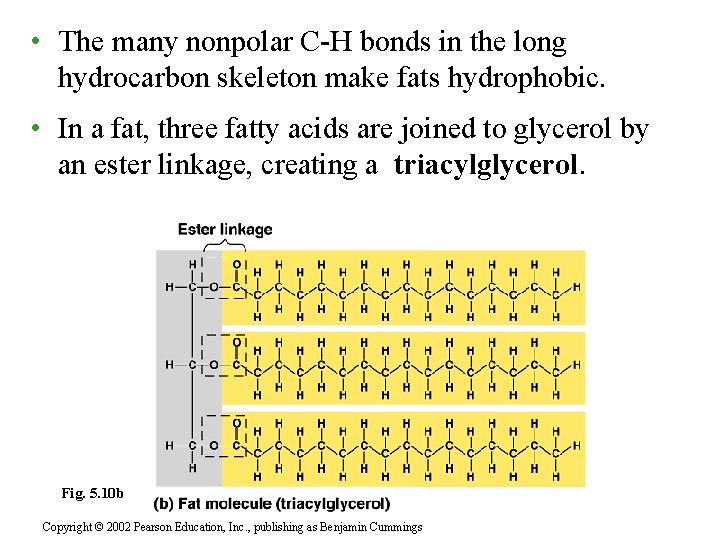
• The many nonpolar C-H bonds in the long hydrocarbon skeleton make fats hydrophobic. • In a fat, three fatty acids are joined to glycerol by an ester linkage, creating a triacylglycerol. Fig. 5. 10 b Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

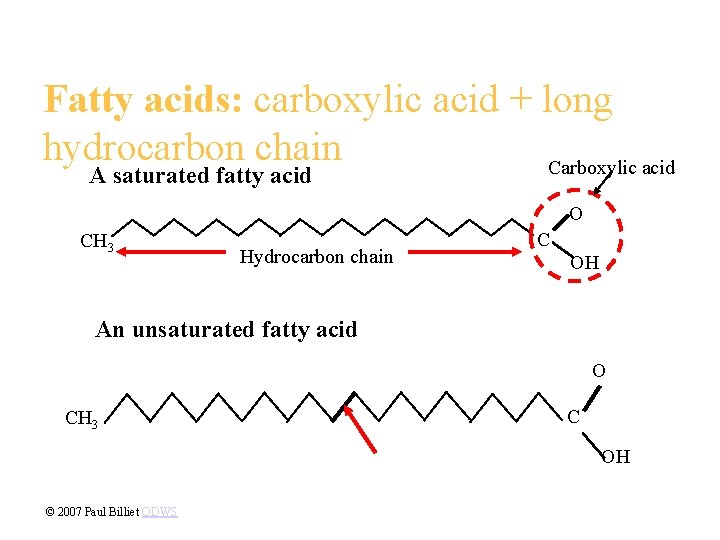
• The three fatty acids in a fat can be the same or different. • Fatty acids may vary in length (number of carbons) and in the number and locations of double bonds. • If there are no carbon-carbon double bonds, then the molecule is a saturated fatty acid - a hydrogen at every possible position. Fig. 5. 11 a Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

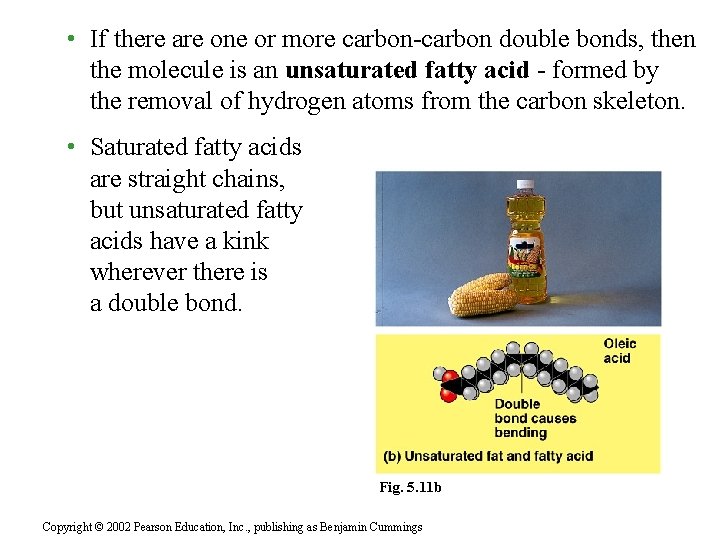
• If there are one or more carbon-carbon double bonds, then the molecule is an unsaturated fatty acid - formed by the removal of hydrogen atoms from the carbon skeleton. • Saturated fatty acids are straight chains, but unsaturated fatty acids have a kink wherever there is a double bond. Fig. 5. 11 b Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

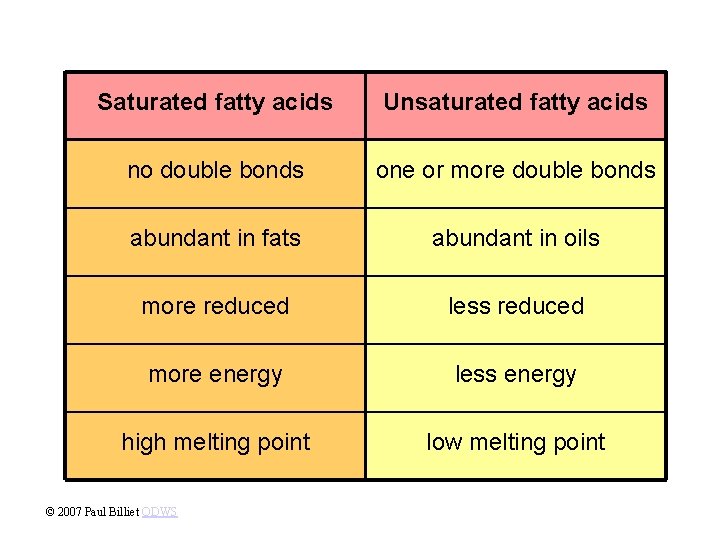
• Fats with saturated fatty acids are saturated fats. • Most animal fats are saturated. • Saturated fat are solid at room temperature. • A diet rich in saturated fats may contribute to cardiovascular disease (atherosclerosis) through plaque deposits. • Fats with unsaturated fatty acids are unsaturated fats. • Plant and fish fats, known as oils, are liquid are room temperature. • The kinks provided by the double bonds prevent the molecules from packing tightly together. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

• The major function of fats is energy storage. • A gram of fat stores more than twice as much energy as a gram of a polysaccharide. • Plants use starch for energy storage when mobility is not a concern but use oils when dispersal and packing is important, as in seeds. • Humans and other mammals store fats as long-term energy reserves in adipose cells. • Fat also functions to cushion vital organs. • A layer of fats can also function as insulation. • This subcutaneous layer is especially thick in whales, seals, and most other marine mammals. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

2. Phospholipids are major components of cell membranes • Phospholipids have two fatty acids attached to glycerol and a phosphate group at the third position. • The phosphate group carries a negative charge. • Additional smaller groups may be attached to the phosphate group. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

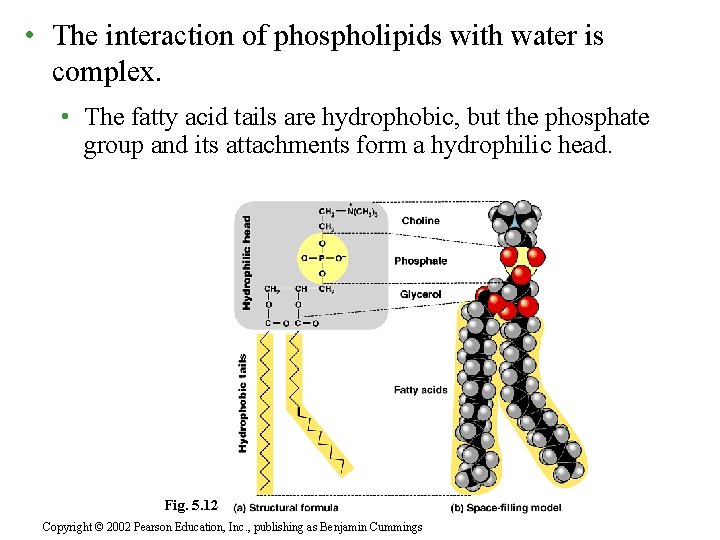
• The interaction of phospholipids with water is complex. • The fatty acid tails are hydrophobic, but the phosphate group and its attachments form a hydrophilic head. Fig. 5. 12 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

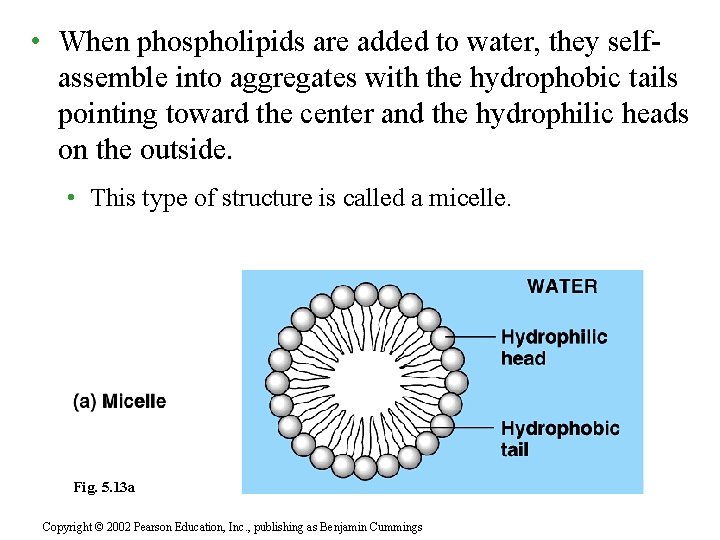
• When phospholipids are added to water, they selfassemble into aggregates with the hydrophobic tails pointing toward the center and the hydrophilic heads on the outside. • This type of structure is called a micelle. Fig. 5. 13 a Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

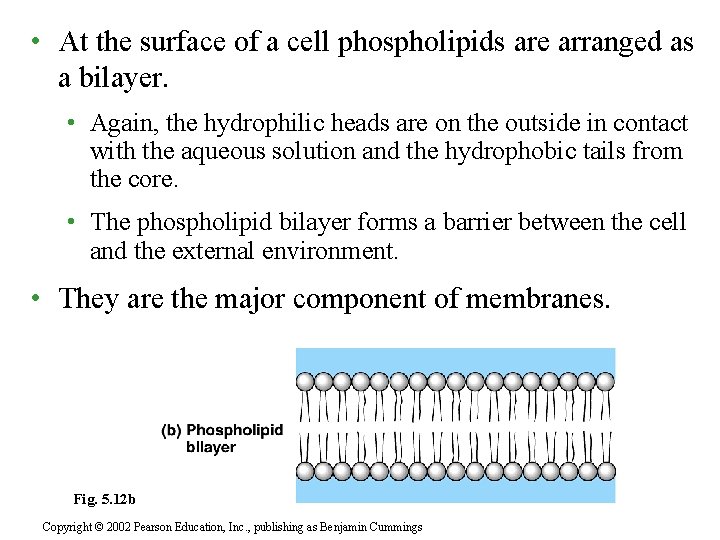
• At the surface of a cell phospholipids are arranged as a bilayer. • Again, the hydrophilic heads are on the outside in contact with the aqueous solution and the hydrophobic tails from the core. • The phospholipid bilayer forms a barrier between the cell and the external environment. • They are the major component of membranes. Fig. 5. 12 b Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

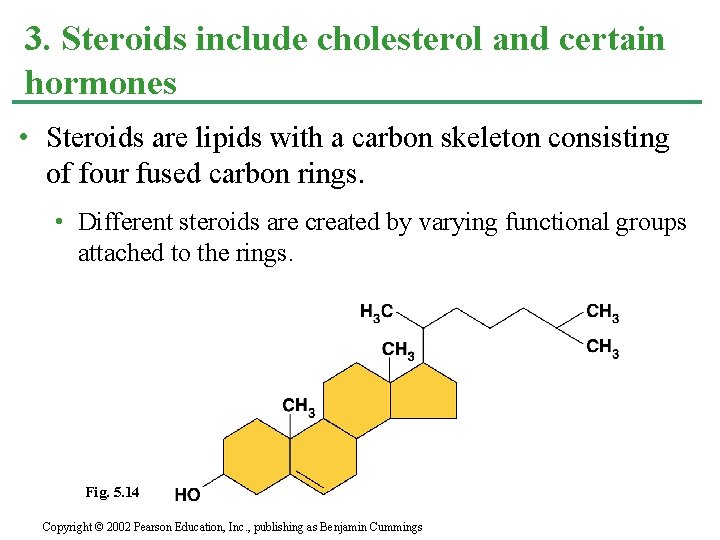
3. Steroids include cholesterol and certain hormones • Steroids are lipids with a carbon skeleton consisting of four fused carbon rings. • Different steroids are created by varying functional groups attached to the rings. Fig. 5. 14 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

• Cholesterol, an important steroid, is a component in animal cell membranes. • Cholesterol is also the precursor from which all other steroids are synthesized. • Many of these other steroids are hormones, including the vertebrate sex hormones. • While cholesterol is clearly an essential molecule, high levels of cholesterol in the blood may contribute to cardiovascular disease. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

LIPIDS C, H, O • More hydrogen (more reduced) than carbohydrates. • Insoluble in water, soluble in organic solvents (alcohols, acetone, chloroform etc) © 2007 Paul Billiet ODWS

Fatty acids: carboxylic acid + long hydrocarbon chain Carboxylic acid A saturated fatty acid O CH 3 Hydrocarbon chain C OH An unsaturated fatty acid O CH 3 C OH © 2007 Paul Billiet ODWS

Saturated fatty acids Unsaturated fatty acids no double bonds one or more double bonds abundant in fats abundant in oils more reduced less reduced more energy less energy high melting point low melting point © 2007 Paul Billiet ODWS

Fats and Oils fatty acids + glycerol (1, 2 or 3 = mono , di or triglycerides) O CH C 3 CH 3 OH HO - CH 2 O C OH HO - CH HO – CH 2 © 2007 Paul Billiet ODWS Condensation reactions

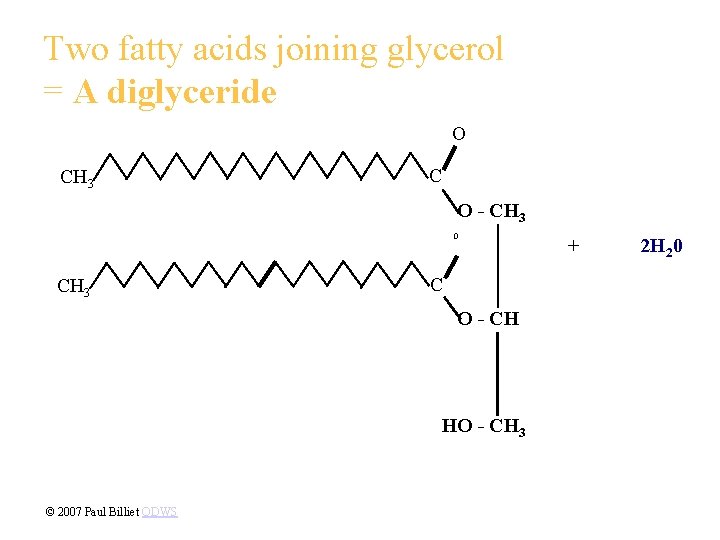
Two fatty acids joining glycerol = A diglyceride O CH 3 C O - CH 3 O CH 3 C O - CH HO - CH 3 © 2007 Paul Billiet ODWS + 2 H 20

Phospholipids • in lipoprotein membranes (plasma, nuclear, mitochondrial etc. ) © 2007 Paul Billiet ODWS

Other lipids Steroids: multiple ring structures (e. g. cholesterol) • Functions: cell membrane structure, digestion (help to emulsify fats), hormones (testosterone etc), vitamins (e. g. Vitamin D), poisons Waxes: long chain alcohol + fatty acids • Water proof coating to leaves, fur feathers, insect exoskeletons. • © 2007 Used by bees to construct their honey combs. Paul Billiet ODWS

LIPID FUNCTIONS IN GENERAL • STRUCTURAL: biological membranes (phospholipids, steroids, glycolipids), cushioning (fat deposits round the kidneys) • ELECTRICAL INSULATION: myelin sheath round axons • THERMAL INSULATION: subcutaneous fat deposits. • WATER PROOFING: waxes and oils • ENERGY STORE AND SUBSTRATE: very condensed form of energy (37 k. J g-1) used by animals and seeds. • HORMONES: steroids • VITAMINS: precursor to Vit D Paul Billiet ODWS • © 2007 BUOYANCY: oil droplets in plankton

Quick Check – Hands Up! • What is a role of lipids in animal cells? • A. As membrane receptor molecules • B. As enzymes • C. As energy storage • D. As components of the animal cell wall

Quick Check – Hands Up! • What is the maximum number of fatty acids that can be condensed with glycerol? • A. One • B. Two • C. Three • D. Four

Everyone Writes! Draw the structure of a fatty acid. Draw the structure of glycerol.