Chapter 5 The Structure and Function of Macromolecules







- Slides: 23

Chapter 5 The Structure and Function of Macromolecules Intro & Carbohydrates

Polymer Principles Classes of macromolecules include – Carbohydrates – Lipids – Proteins – Nucleic Acids Three of the four classes are polymers – Carbohydrates, Proteins, and Nucleic Acids

Polymer Principles, cont’d Polymer – A long molecule consisting of many similar or identical building blocks linked by covalent bonds Monomer – The small repeating units that serve as the building blocks of a polymer

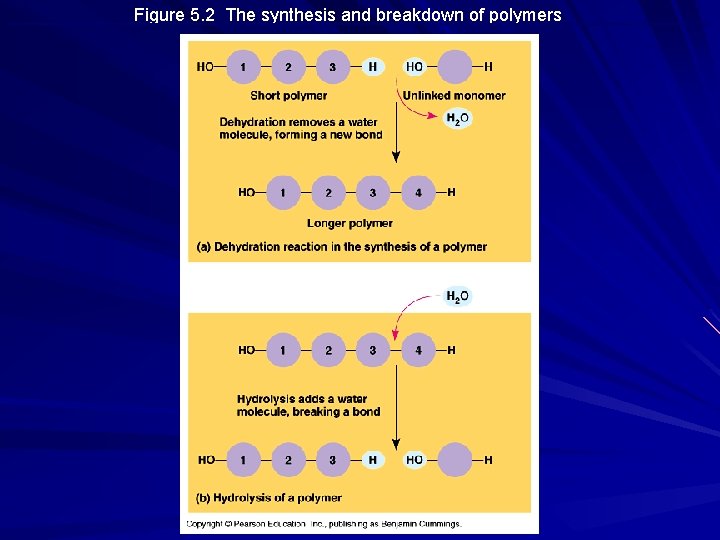
Polymer Principles, cont’d Condensation Reactions – Process by which monomers are covalently connected to each other – Typically are dehydration reactions since a water molecule is lost – A cell must expend energy to carry out dehydration reactions / Process requires enzymes – Note that an immense variety of polymers can be synthesized from a small set of monomers

Polymer Principles, cont’d Hydrolysis – process by which polymers are disassembled – Essentially the reverse of a dehydration rxn – Bonds between monomers are broken by the addition of water molecules across the bond – Ex: Digestion

Figure 5. 2 The synthesis and breakdown of polymers

Carbohydrates include both sugars and their polymers – Monosaccharides simple sugars Ex: glucose, fructose – Disaccharides two monosaccharides joined by condensation Ex: sucrose, maltose – Polysaccharides polymers of many monosaccharides Ex: cellulose, starch

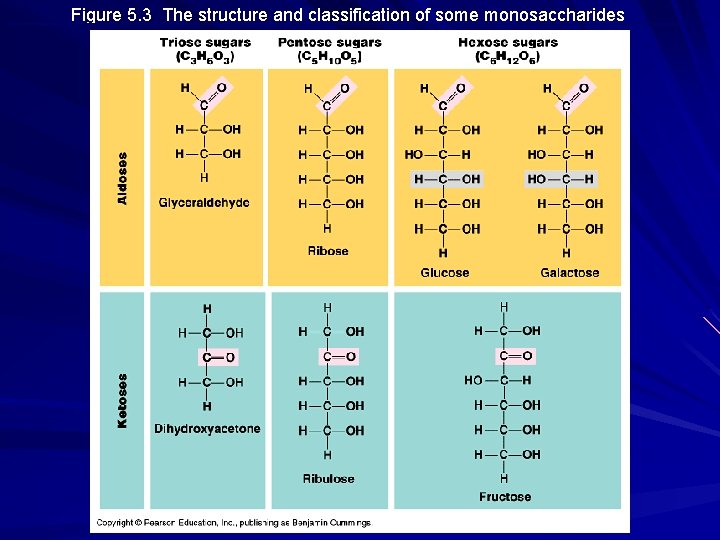
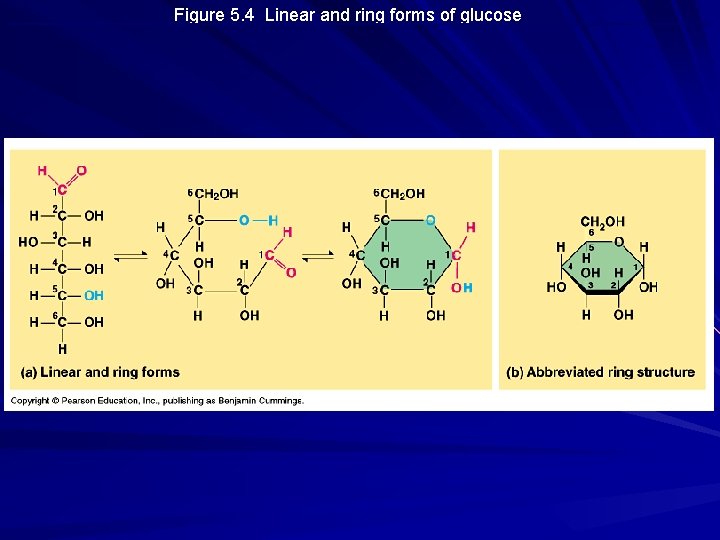
Monosaccharides Molecular formula = (CH 2 O)n Glucose (C 6 H 12 O 6) is the most common Contain a carbonyl carbon and multiple hydroxyls Classified as either aldose sugars or ketose sugars Also classified on the basis of length of carbon skeleton (triose, pentose, hexose, etc. )

Monosaccharides, cont’d Typically contain one or more asymmetric carbon atoms Importance to cells: – Major fuels for cellular work – Raw material for synthesis of other types of small organic molecules such as amino acids and fatty acids

Figure 5. 3 The structure and classification of some monosaccharides

Figure 5. 4 Linear and ring forms of glucose

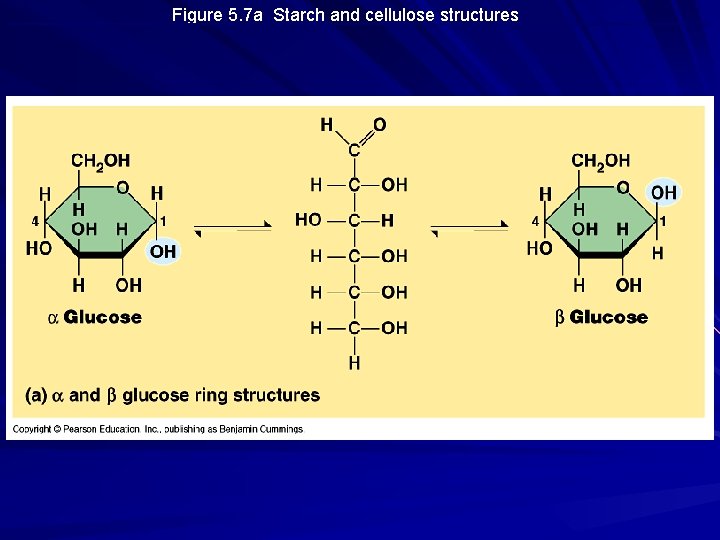
Figure 5. 7 a Starch and cellulose structures

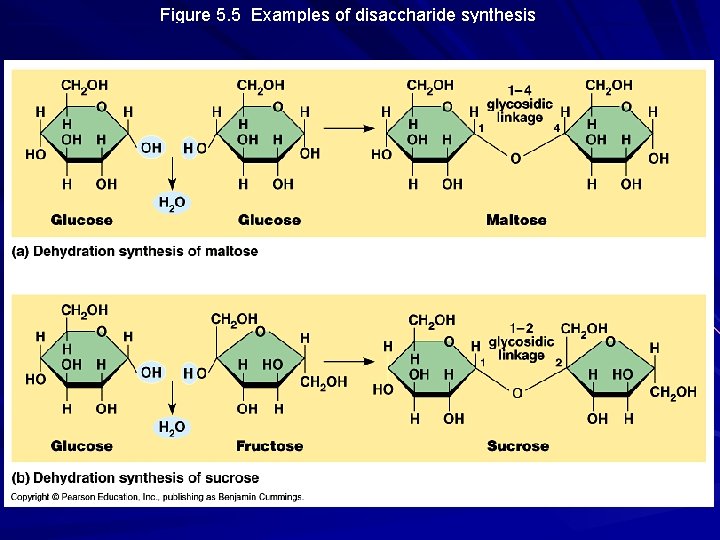
Disaccharides Two monosaccharides joined by a glycosidic linkage Ex: maltose = glucose + glucose Sucrose = glucose + fructose typical transport form of sugar in plants Lactose = glucose + galactose

Figure 5. 5 Examples of disaccharide synthesis

Polysaccharides - polymers with hundreds to thousands of monosaccharides joined by glycosidic linkages May be storage polysaccharides or structural polysaccharides Architecture and function determined by sugar monomers and position of glycosidic linkages

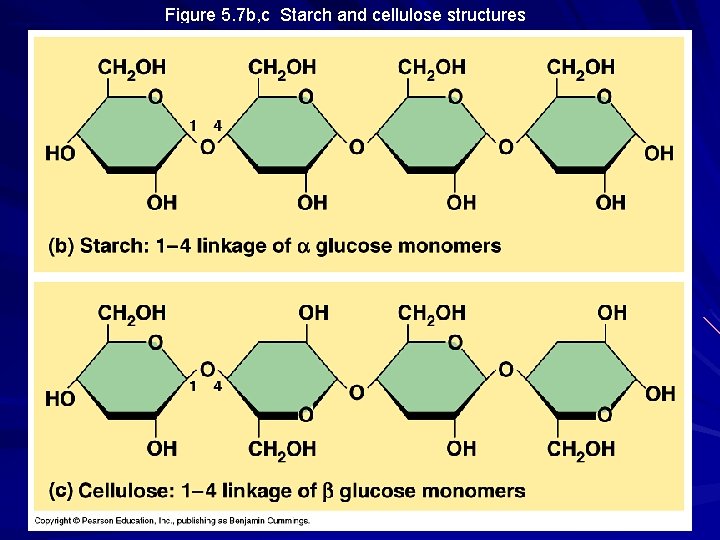
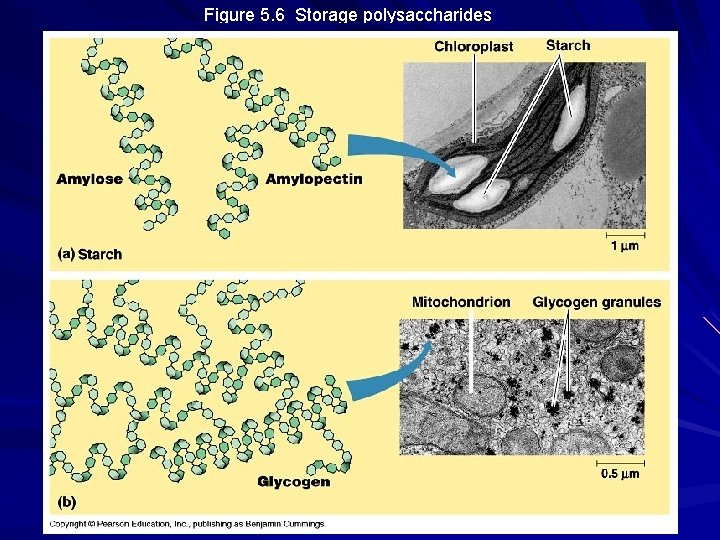
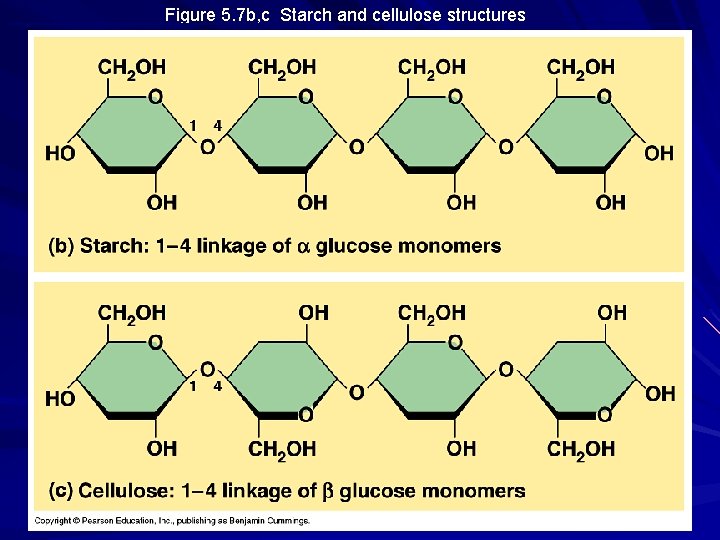
Storage Polysaccharides Starch – storage polysaccharide of plants – composed of series of glucose monomers with a 1 -4 glycosidic linkage – helical in structure – amylose = unbranched starch – amylopectin = branched polymer with 1 -6 linkages at branch points – stored within plastids

Storage Polysaccharides, cont’d Glycogen – storage polysaccharide of animals – composed of series of a-glucose monomers with a 1 -4 glycosidic linkage – highly branched structure with 1 -6 linkage at branchpoints – stored in liver and muscle cells

Figure 5. 7 b, c Starch and cellulose structures

Figure 5. 6 Storage polysaccharides

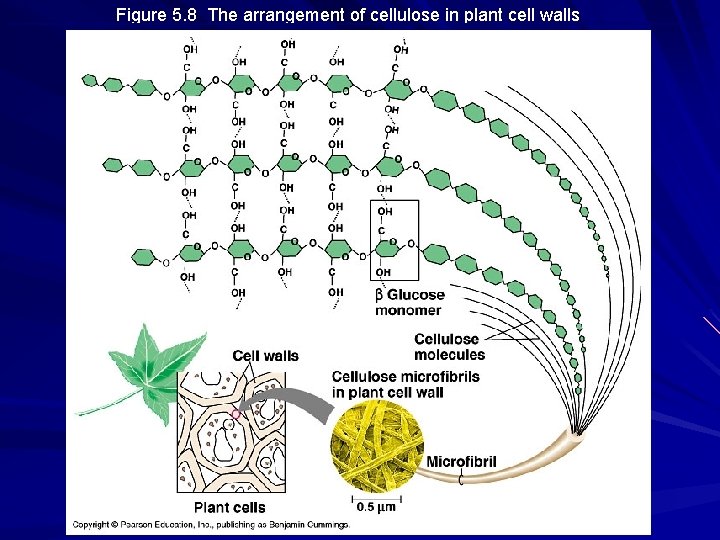
Structural Polysaccharides Cellulose – major component of tough wall that encloses plant cells – the most abundant organic compound on Earth (100 billion tons produced/year) – polymer with 1 -4 linkages of -glucose – straight, not branched, packing due to H-bonding – humans do not have the enzymes to digest cellulose / cow’s rumen contains bacteria that can digest cellulose

Figure 5. 7 b, c Starch and cellulose structures

Figure 5. 8 The arrangement of cellulose in plant cell walls

Structural Polysaccharides, cont’d Chitin – polymer used by arthropods to build their exoskeletons – also used by fungi to build their cell walls – similar to cellulose but the glucose monomer has a nitrogen containing appendage