Chapter 5 Section 6 Periodic Trends trend trend









- Slides: 30

Chapter 5 Section 6 Periodic Trends • trend: trend a predictable change in a particular direction. – allows for predictions about the chemical behavior of elements. – explained in terms of electron configurations. 1. Ionization energy (you will 2. Atomic radius (size of atom) know 3. Electronegativity reactivity from the lab!)

Octet Rule (8 is great!) • Elements typically will gain or lose electrons to have 8 valence electrons (a filled energy level) • 8 is desirable • Exceptions are hydrogen and helium which follow the DUET (2 electron) rule

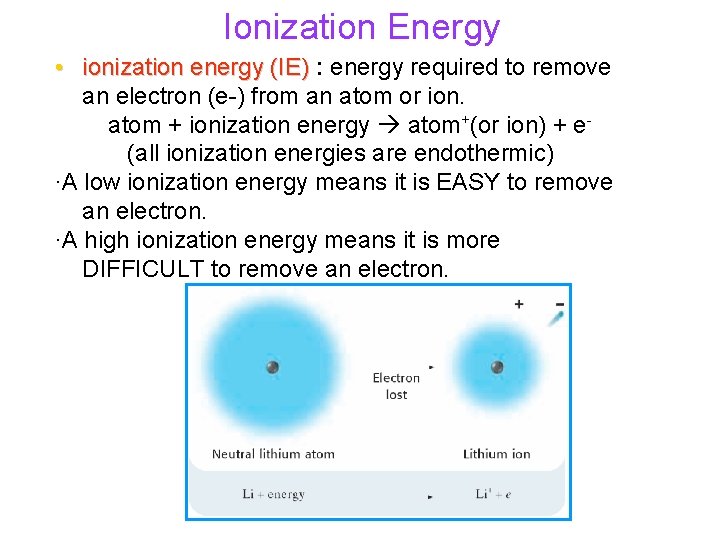
Ionization Energy • ionization energy (IE) : energy required to remove an electron (e-) from an atom or ion. atom + ionization energy atom+(or ion) + e(all ionization energies are endothermic) ∙A low ionization energy means it is EASY to remove an electron. ∙A high ionization energy means it is more DIFFICULT to remove an electron.

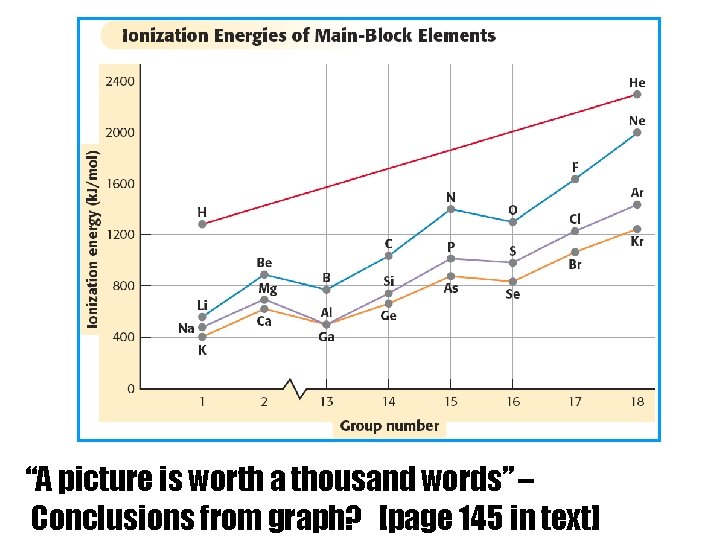
“A picture is worth a thousand words” – Conclusions from graph? [page 145 in text]

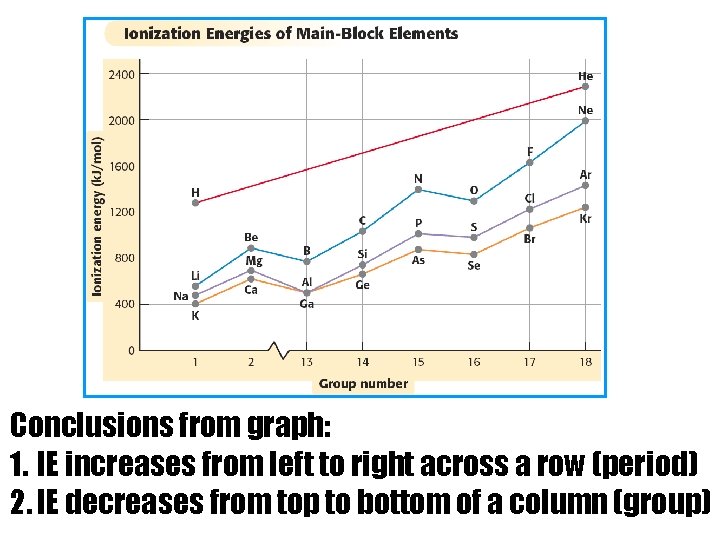
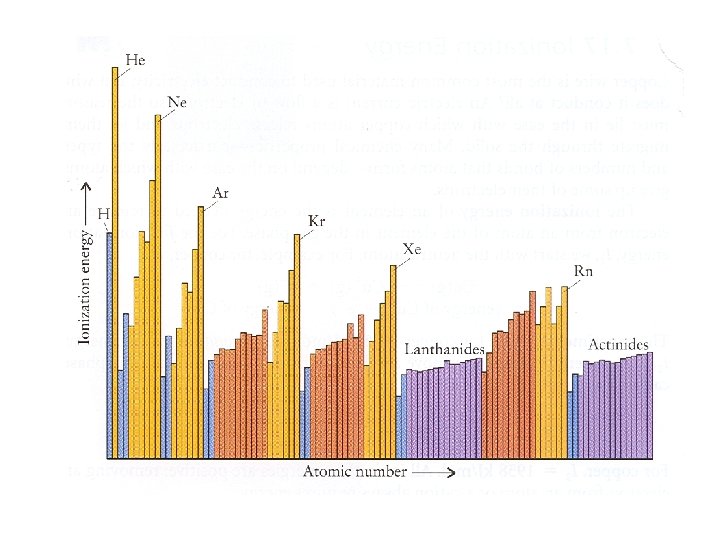
Conclusions from graph: 1. IE increases from left to right across a row (period) 2. IE decreases from top to bottom of a column (group)


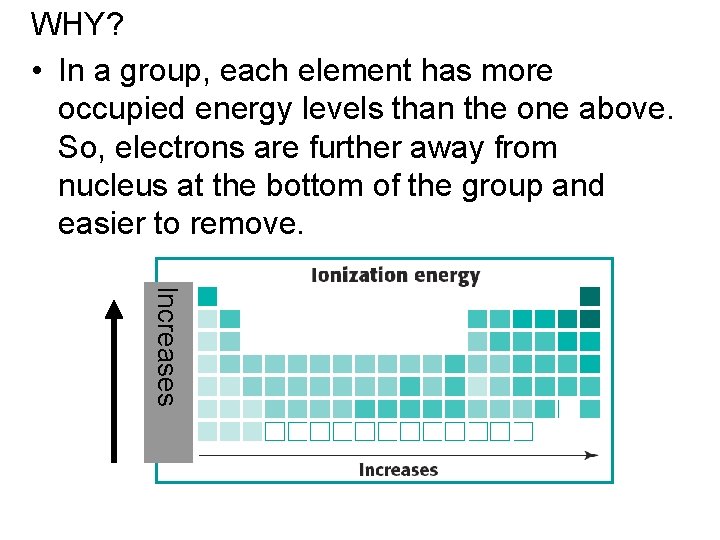
WHY? • In a group, each element has more occupied energy levels than the one above. So, electrons are further away from nucleus at the bottom of the group and easier to remove. Increases

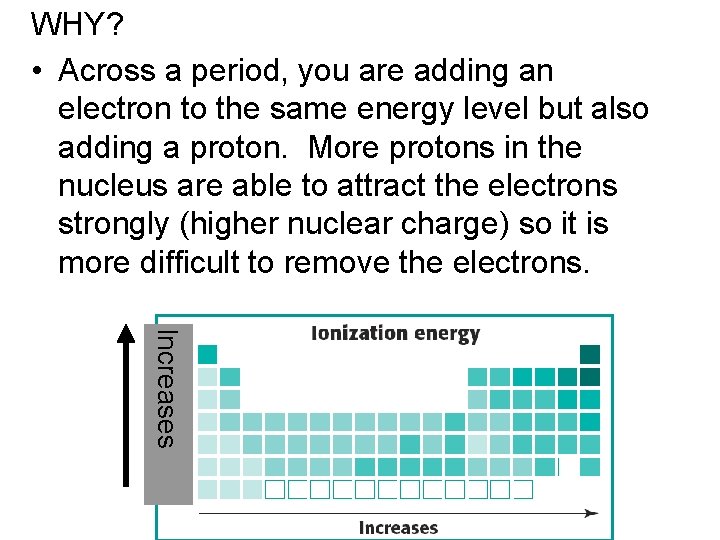
WHY? • Across a period, you are adding an electron to the same energy level but also adding a proton. More protons in the nucleus are able to attract the electrons strongly (higher nuclear charge) so it is more difficult to remove the electrons. Increases

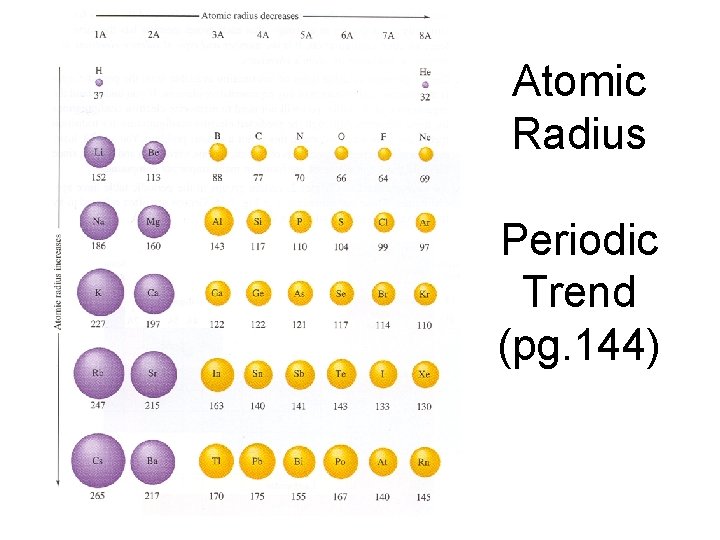
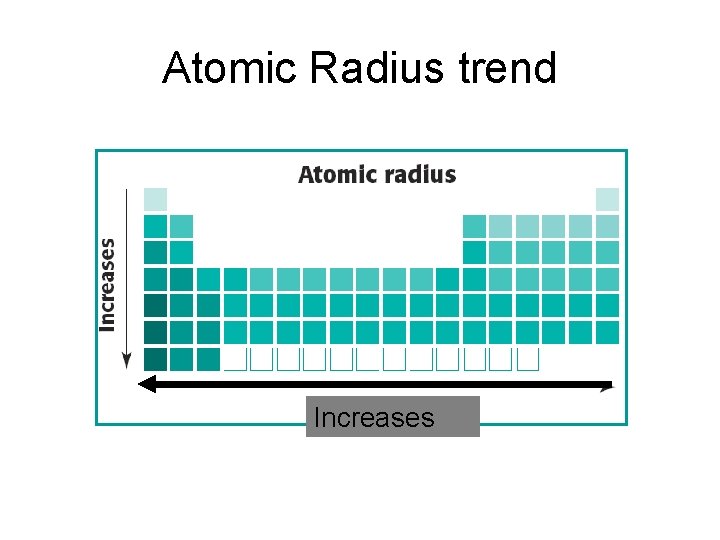
Atomic Radius- SIZE OF THE ATOM

Atomic Radius Periodic Trend (pg. 144)

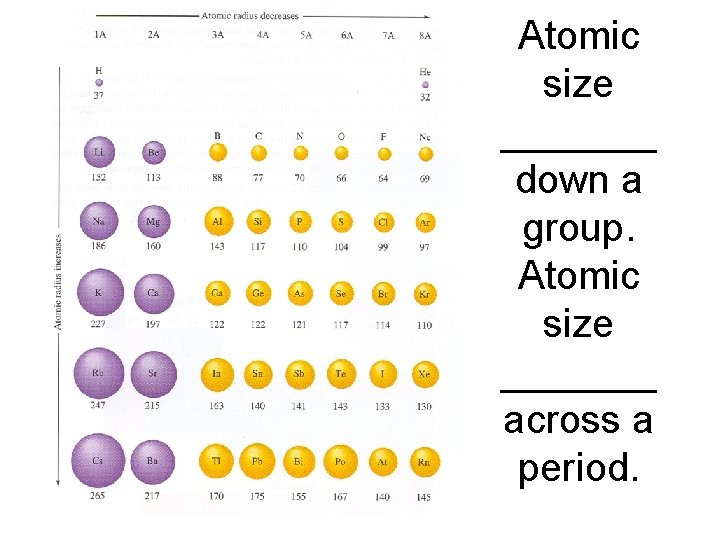
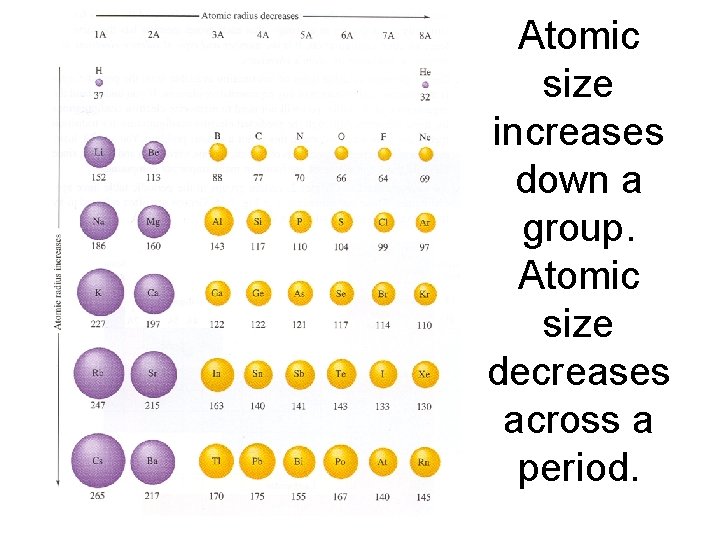
Atomic size _______ down a group. Atomic size _______ across a period.

Atomic size increases down a group. Atomic size decreases across a period.

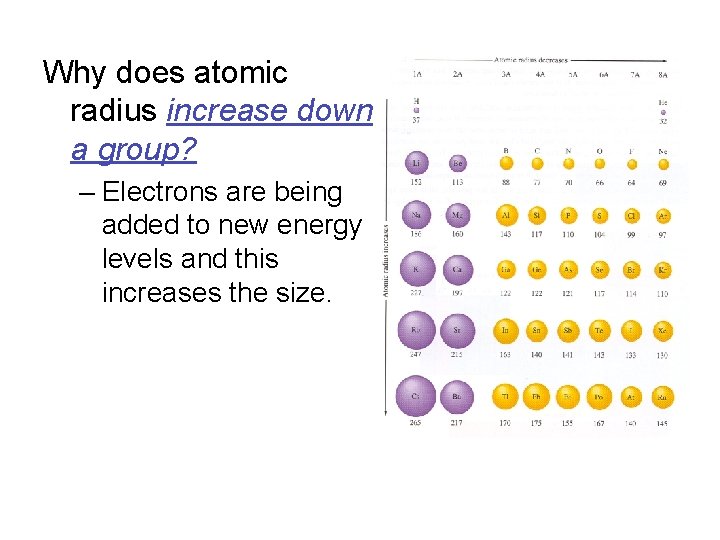
Why does atomic radius increase down a group? – Electrons are being added to new energy levels and this increases the size.

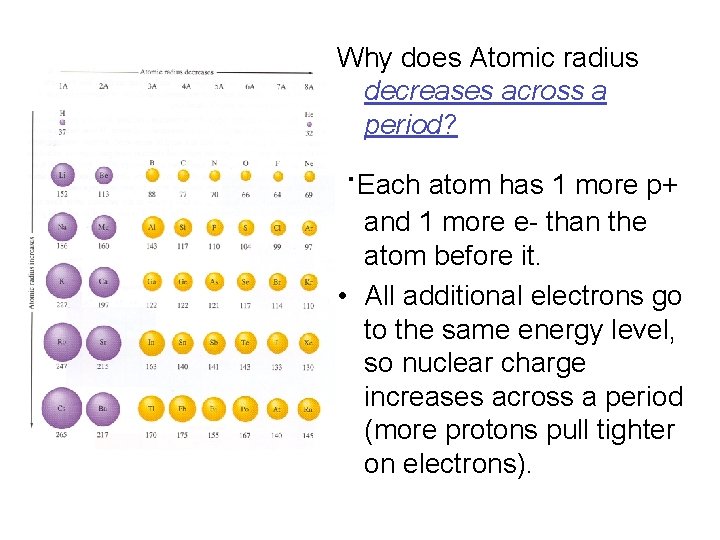
Why does Atomic radius decreases across a period? ∙Each atom has 1 more p+ and 1 more e- than the atom before it. • All additional electrons go to the same energy level, so nuclear charge increases across a period (more protons pull tighter on electrons).

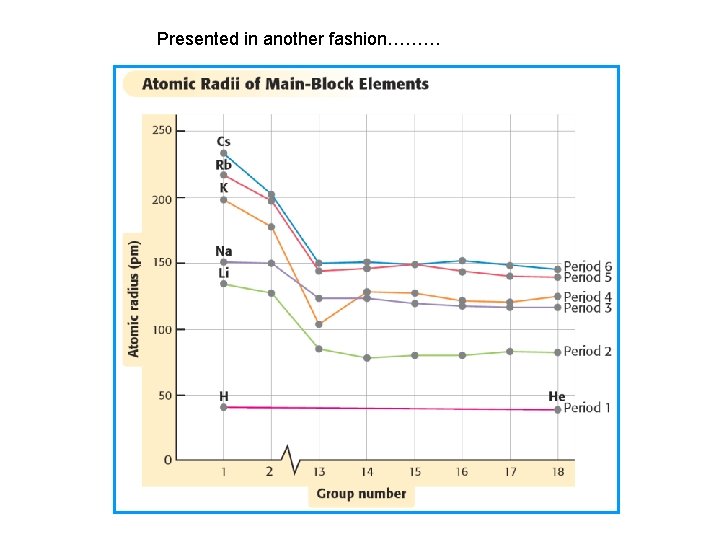
Presented in another fashion………

Atomic Radius trend Increases

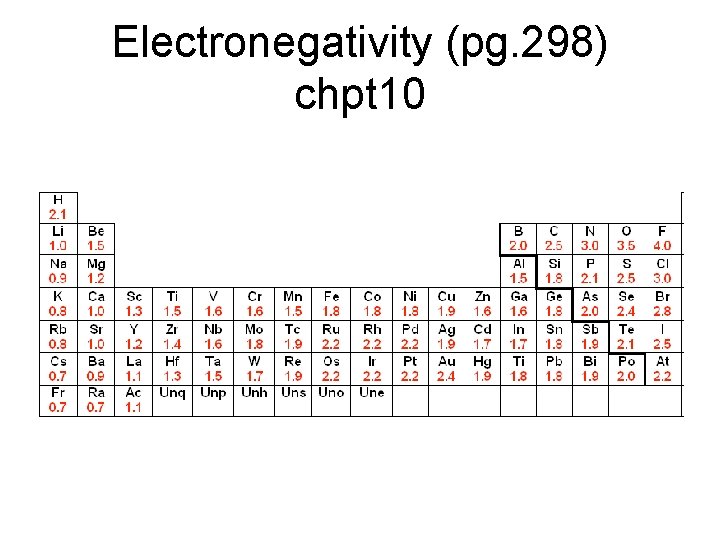
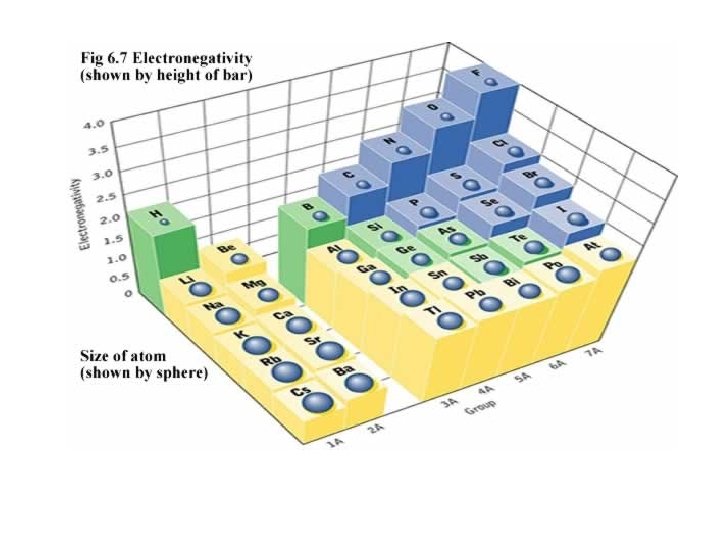
Electronegativity • Electronegativity: measures the ability of an atom in a compound to attract e-. – Not all atoms in a compound share e- equally. – Knowing attractions of bonding e- helps explain physical and chemical properties of compounds. • Linus Pauling created a scale of values (0 - 4) An atom with higher electronegativity (closer to 4) will pull on emore strongly.

Electronegativity (pg. 298) chpt 10

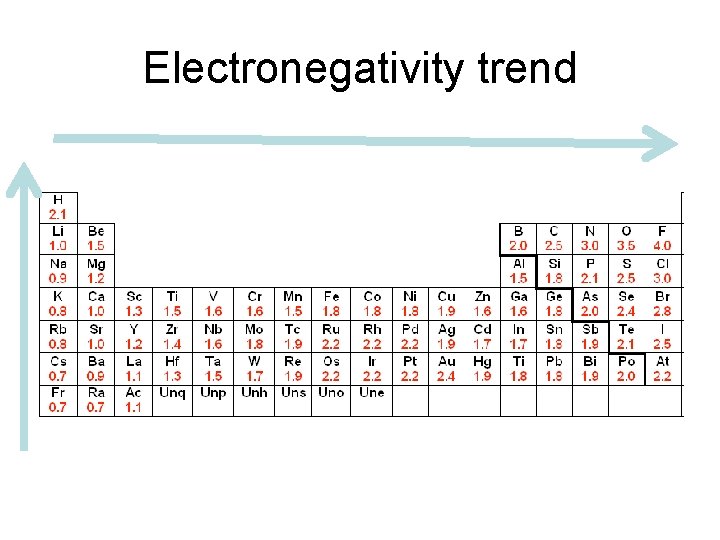
Electronegativity trend


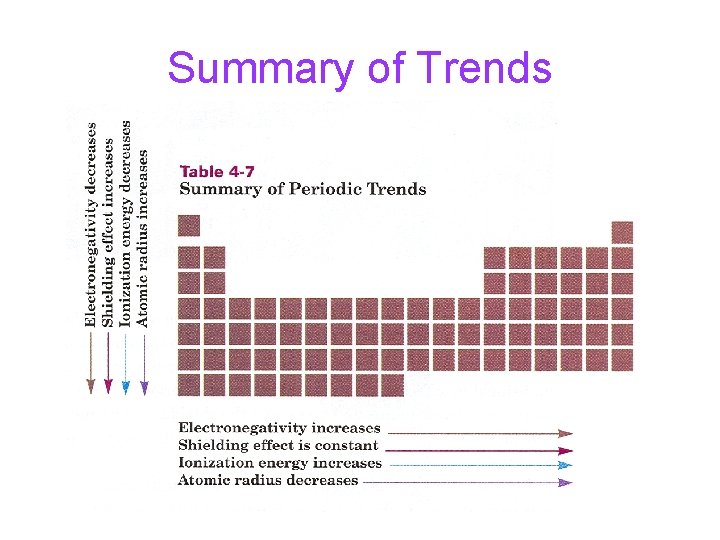
Summary of Trends

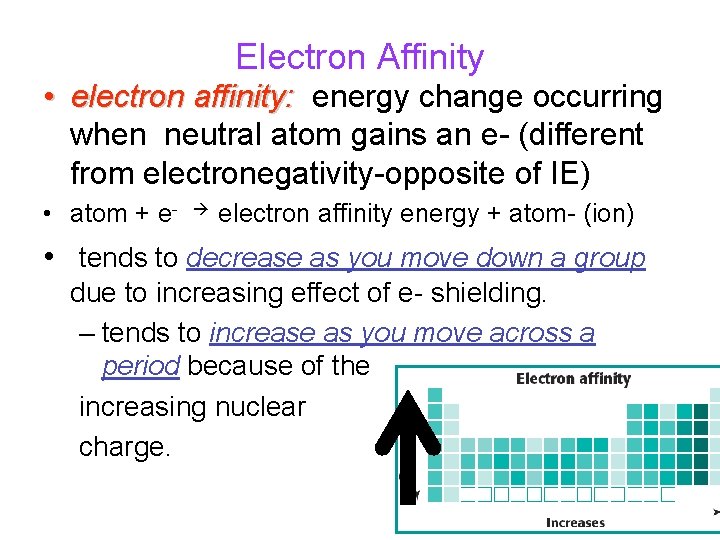
Electron Affinity • electron affinity: energy change occurring when neutral atom gains an e- (different from electronegativity-opposite of IE) • atom + e- electron affinity energy + atom- (ion) • tends to decrease as you move down a group due to increasing effect of e- shielding. – tends to increase as you move across a period because of the increasing nuclear charge.

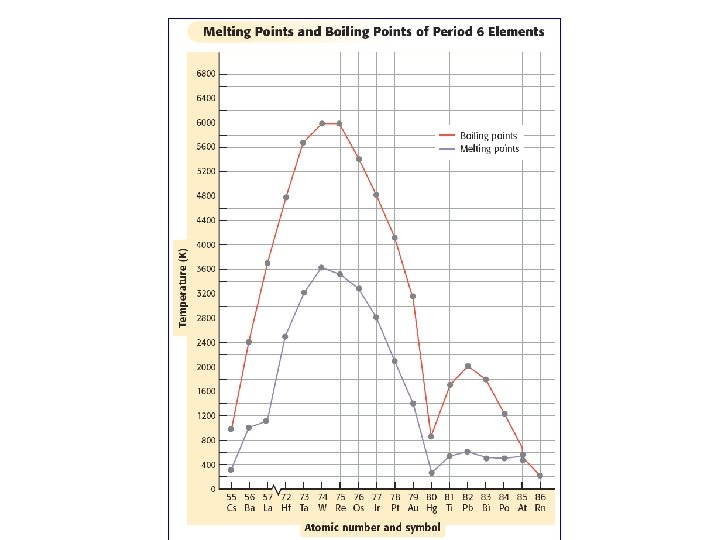
Trends in Melting & Boiling Pts. • melting & boiling points reach two different peaks as d and p orbitals fill. • across Period 6, the melting & boiling points initially increase. – As number of e- in each element increases, stronger bonds between atoms can form. More energy is needed for melting & boiling to occur. • Near middle of d-block, melting & boiling points peak. – As more e- added, pairs form within d orbitals. – Due to decrease in unpaired e-, bonds that atoms form with each other become weaker. – these elements have lower melting & boiling points.

• Moving past Hg, melting & boiling points again rise as e- are added to p orbital. – continue rising until they peak at the elements whose p orbitals are almost half filled. • Another decrease is seen as e- pair up to fill p orbitals. • Noble gases have no bonding forces between atoms. – melting & boiling points are unusually low.


electron shielding: shielding inner electrons reduce the attractive force between the nucleus (positive charge) and outermost e- due to e- in between.

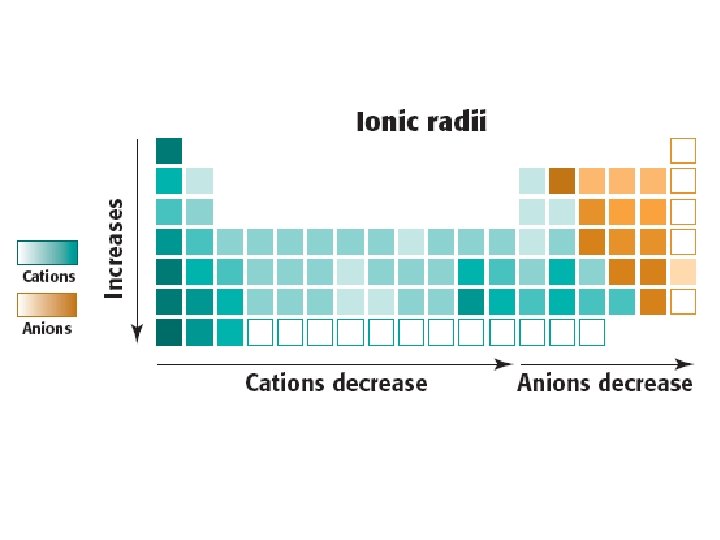
Ions • ions: ions charged atoms, become charged by gaining or losing electrons. – cations (metals) lose e-, form positive charge since p+ > e -. – anions (nonmetals) gain electrons, form negative charge since e- > p+ – results in change on the “pulling” power of nucleus toward e-, thus altering the size of ions vs. atoms. Cations are smaller than their atoms, while anions are larger than their atoms.


• atom with higher electronegativity will pull on e- more strongly. • F most strongly attracts shared e- in a compound. F = 4. 0 – Values for other elements were calculated in relation to this value. • values generally decrease moving down a group. – more p+ an atom has, the more strongly it attracts an e-. – e- shielding plays a role

• Electronegativity increases moving across a period. – Each atom has 1 more p+ and 1 more e- in the same pel as the atom before it. – E- shielding doesn’t change moving across period since no e- are added to inner levels. – Effective nuclear charge increases across period; e- strongly attracted resulting in increase in electronegativity. – increase across a period is more dramatic than the decrease going down a group.