Chapter 5 Section 3 MIXTURE NOTES A mixture






- Slides: 10

Chapter 5 – Section 3 MIXTURE NOTES

A mixture is a combination of two or more substances that are NOT chemically combined. Each substance in a mixture keeps its identity because no chemical change happens when a mixture is made. Mixtures can be separated by physical means paper chromatography (long strips of filter paper draw to separate different sized dissolved substance) decanting (pouring off a liquid at the top of an insoluble substance). PROPERTIES OF

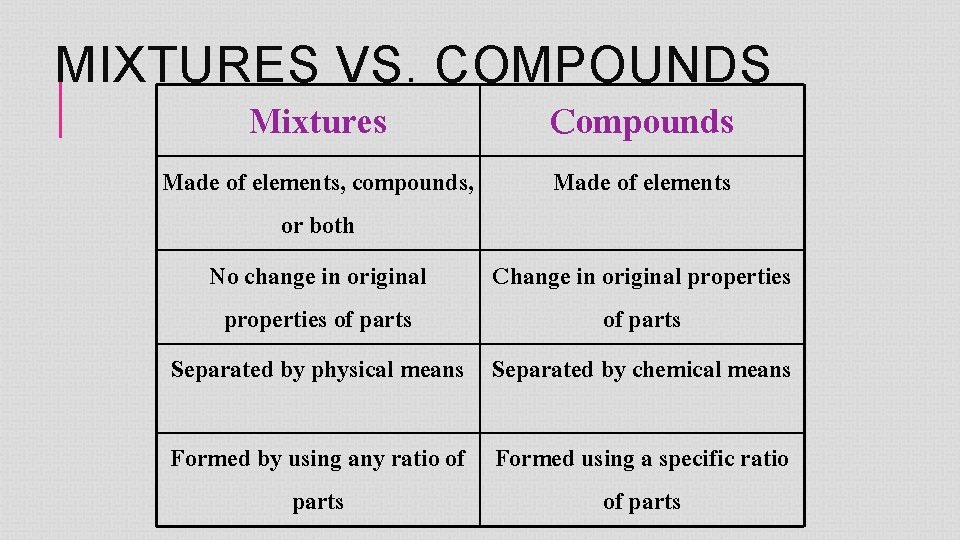
MIXTURES VS. COMPOUNDS Mixtures Compounds Made of elements, compounds, Made of elements or both No change in original Change in original properties of parts Separated by physical means Separated by chemical means Formed by using any ratio of Formed using a specific ratio parts of parts

Heterogeneous mixtures are mixtures in which you can visibly see the different substances that make it up. Homogeneous mixtures are so well mixed that you cannot differentiate (tell the difference between) the individual parts. A solution appears to be a single substance, but it is actually a homogeneous mixture composed of particles of two or more substances that are distributed evenly among each other. TYPES OF MIXTURES

In solutions, the solute is the substance that is dissolved (example: salt in salt water). The solvent is the substance in which the solute is dissolved (ex. water in salt water) Hint to remember which is which… solute is the smaller amount (and a shorter word), solvent is the larger amount (and a longer word) Adding a solute to a liquid solvent… raises the boiling point of the solvent (salt water boils at 102 C) lowers the freezing point of the solvent (salt water at saturation freezes @ -21. 1 C) PARTS OF A MIXTURE

Solutions may be liquids, gases, or solids. Gas in gas = the atmosphere Gas in liquid = (O 2 in ocean water) Liquid in liquid = antifreeze (alcohol in water) Solid in liquid = salt water (salt in water) liquid in solid = dental fillings Solid in solid = brass (zinc in copper) Alloys are solid solutions in which metals or nonmetals are dissolved in other metals (ex. bronze is an alloy of tin and copper) EXAMPLES OF SOLUTIONS

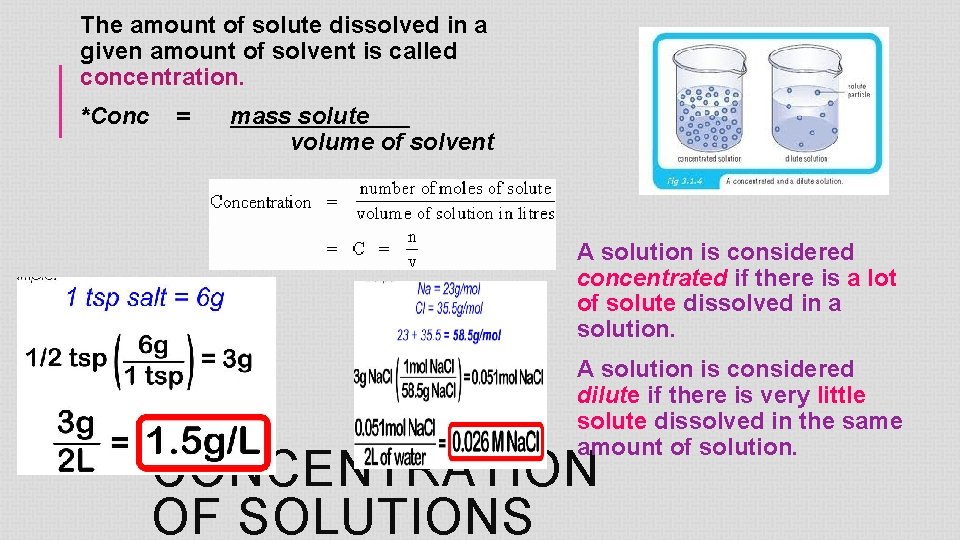
The amount of solute dissolved in a given amount of solvent is called concentration. *Conc = mass solute volume of solvent A solution is considered concentrated if there is a lot of solute dissolved in a solution. A solution is considered dilute if there is very little solute dissolved in the same amount of solution. CONCENTRATION OF SOLUTIONS

SOLUBILITY Solubility is the ability of one substance to dissolve in another at a certain temperature and pressure. A solute must be soluble, or able to dissolve, in a solvent to be a solution. Substances can also have varying degrees of solubility (high vs. low). If a substance is insoluble, or unable to dissolve, then it forms a mixture that is not a solution (ex. pepper in water).

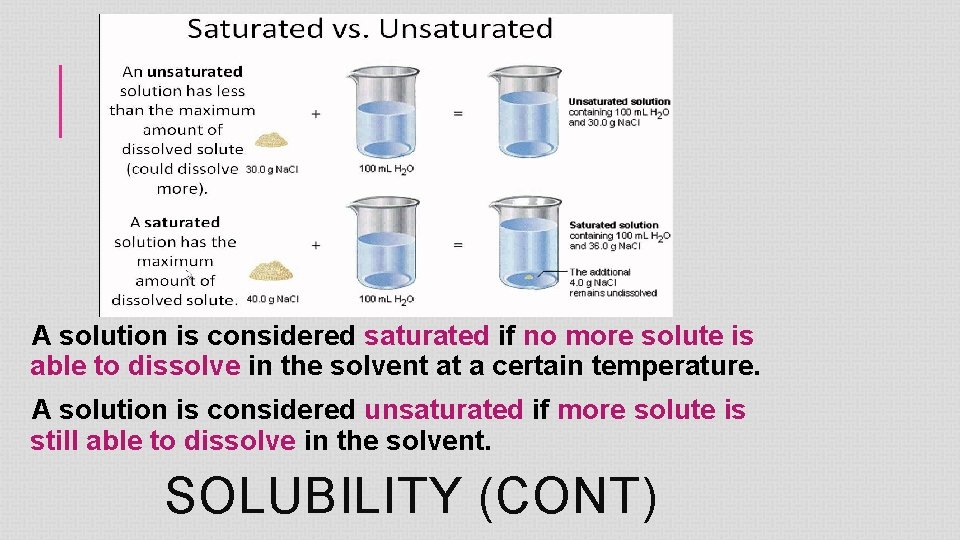
A solution is considered saturated if no more solute is able to dissolve in the solvent at a certain temperature. A solution is considered unsaturated if more solute is still able to dissolve in the solvent. SOLUBILITY (CONT)

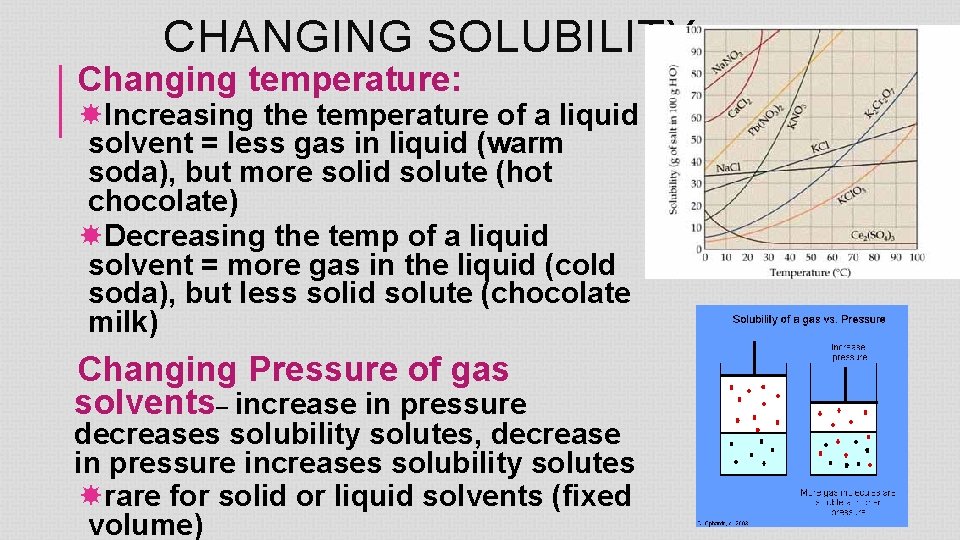
CHANGING SOLUBILITY Changing temperature: Increasing the temperature of a liquid solvent = less gas in liquid (warm soda), but more solid solute (hot chocolate) Decreasing the temp of a liquid solvent = more gas in the liquid (cold soda), but less solid solute (chocolate milk) Changing Pressure of gas solvents– increase in pressure decreases solubility solutes, decrease in pressure increases solubility solutes rare for solid or liquid solvents (fixed volume)