Chapter 5 Section 3 Electron Configuration Ground State








- Slides: 13

Chapter 5 Section 3 Electron Configuration

Ground State Electron Configuration �The arrangement of electrons in an atom is called the atom’s electron configuration. �Electrons in atoms tend to assume the arrangement that gives the atom the lowest possible energy. �The stable, lowest-energy arrangement of the electrons is called the elements ground state electron

Ground State Electron Configuration �Three rules describe how electrons can be arranged in an atom’s orbitals ◦ The aufbau principle ◦ The Pauli exclusion principle ◦ Hund’s Rule

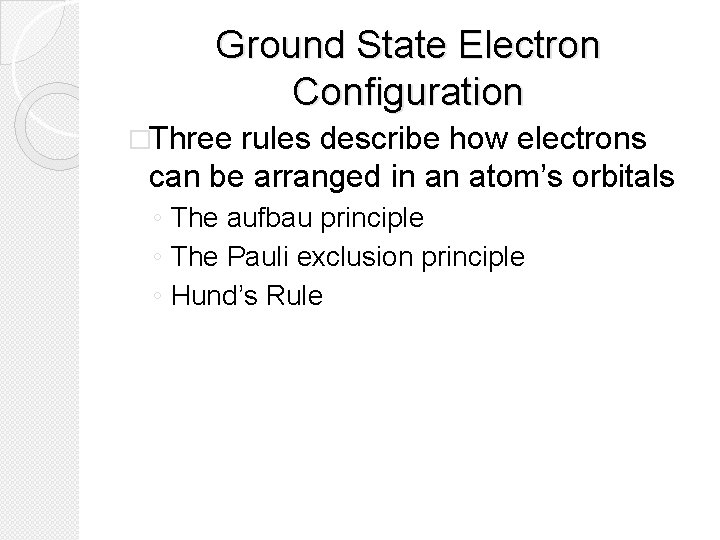
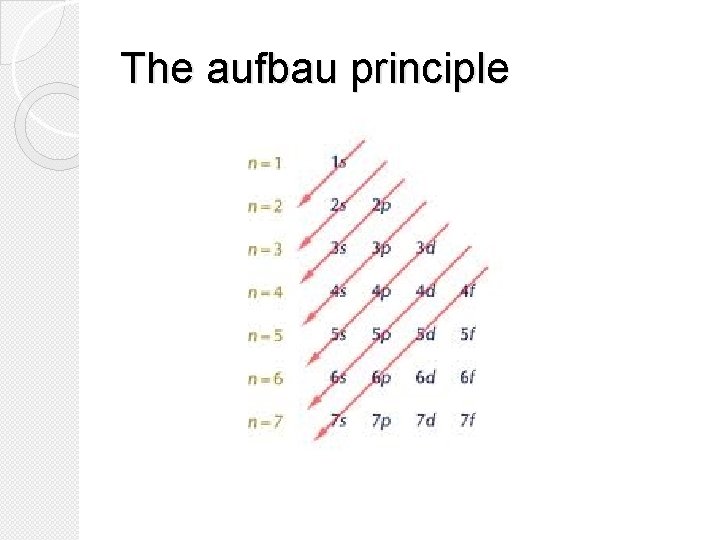
The aufbau principle �The aufbau principle states that each electron occupies the lowest energy orbital available. �You must fill electrons into orbitals from lowest energy to highest energy.

The aufbau principle

The aufbau principle

The Pauli Exclusion Principle �Electrons in an orbital can be represented by arrows in boxes. �Electrons have spin and can spin in one of two directions (like a top). �An arrow pointing up represents an electron spinning in one direction and an arrow pointing down represents an electron spinning in the other direction.

The Pauli Exclusion Principle �The Pauli Exclusion Principle states that a maximum of two electrons can occupy a single atomic orbital. �These two electrons must have opposite spins.

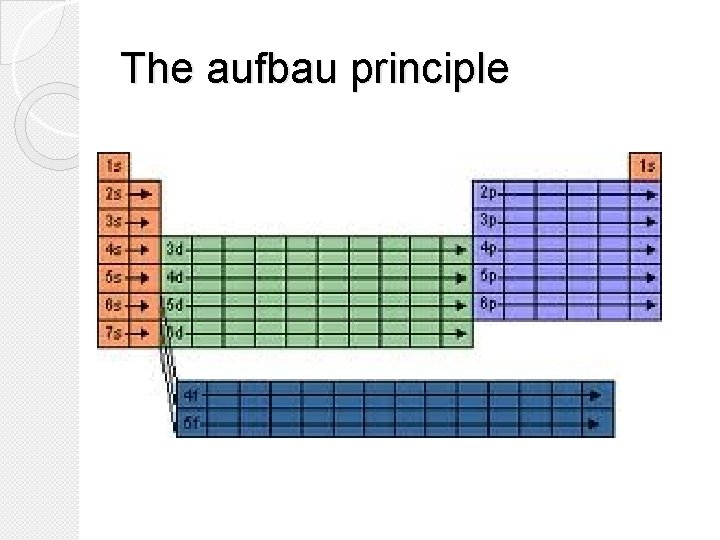
Hund’s Rule �Hund’s Rule states that single electrons with the same spin must occupy each equal-energy orbital before additional electrons with opposite spins can occupy the same orbital. �Simplified: We put one electron in each p, d, or f orbital before pairing them up.

Hund’s Rule

Electron Configurations �We will now practice writing electron configurations Li Na Cl As

Valence Electrons �Valence Electrons are defined as electrons in the atom’s outermost orbitals (highest principle quantum number). �These determine the elements chemical properties. �For the main group elements, the number of valence electrons is equal to the group number.

Electron Dot Diagrams �Electron dot diagrams are pictorial representations of the valence electrons in an element. �In writing an atom’s electron dot diagram, dots representing valence electrons are placed one at a time on the four sides of the symbol and then paired up until all are used. �An element can have a maximum of 8 electrons in it’s outer shell. This is the most stable form of an atom.