Chapter 5 Section 1 pg 176 Atoms Bonding

Chapter 5 Section 1 – pg 176 Atoms, Bonding, and the Periodic Table
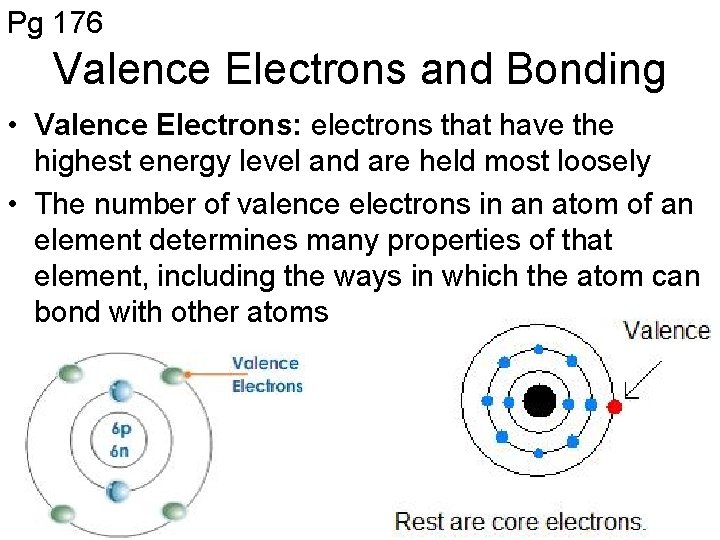
Pg 176 Valence Electrons and Bonding • Valence Electrons: electrons that have the highest energy level and are held most loosely • The number of valence electrons in an atom of an element determines many properties of that element, including the ways in which the atom can bond with other atoms
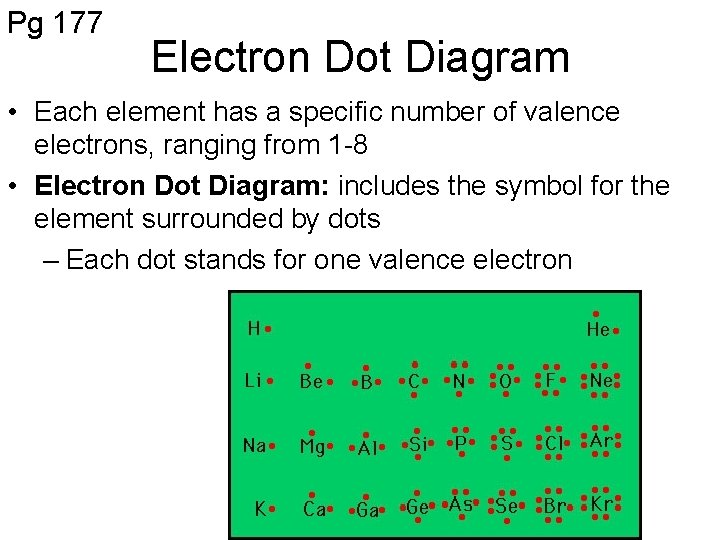
Pg 177 Electron Dot Diagram • Each element has a specific number of valence electrons, ranging from 1 -8 • Electron Dot Diagram: includes the symbol for the element surrounded by dots – Each dot stands for one valence electron
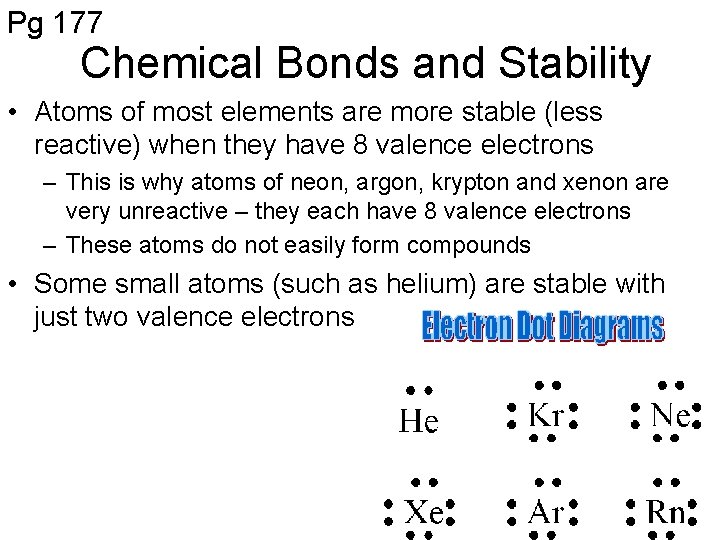
Pg 177 Chemical Bonds and Stability • Atoms of most elements are more stable (less reactive) when they have 8 valence electrons – This is why atoms of neon, argon, krypton and xenon are very unreactive – they each have 8 valence electrons – These atoms do not easily form compounds • Some small atoms (such as helium) are stable with just two valence electrons
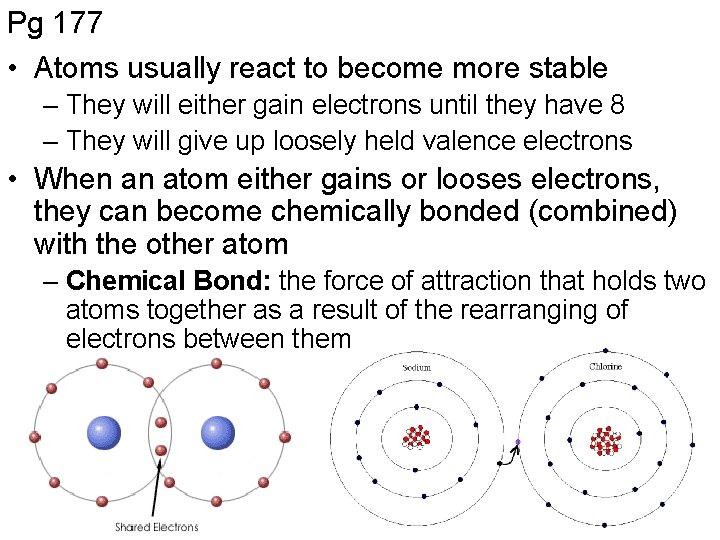
Pg 177 • Atoms usually react to become more stable – They will either gain electrons until they have 8 – They will give up loosely held valence electrons • When an atom either gains or looses electrons, they can become chemically bonded (combined) with the other atom – Chemical Bond: the force of attraction that holds two atoms together as a result of the rearranging of electrons between them
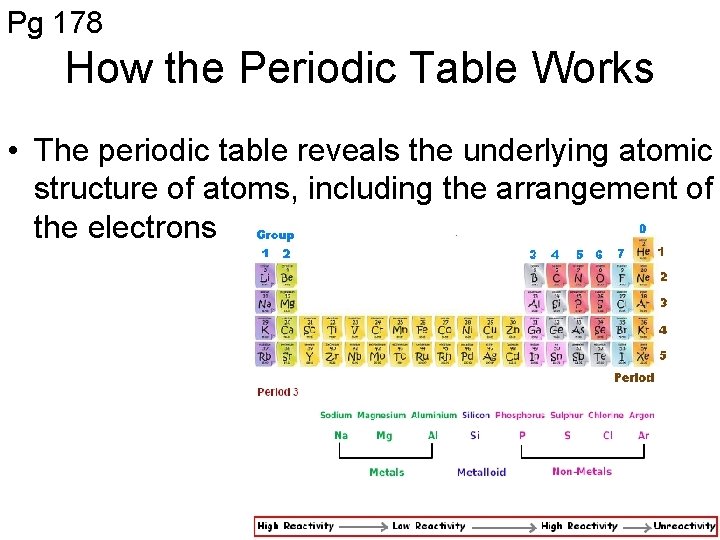
Pg 178 How the Periodic Table Works • The periodic table reveals the underlying atomic structure of atoms, including the arrangement of the electrons
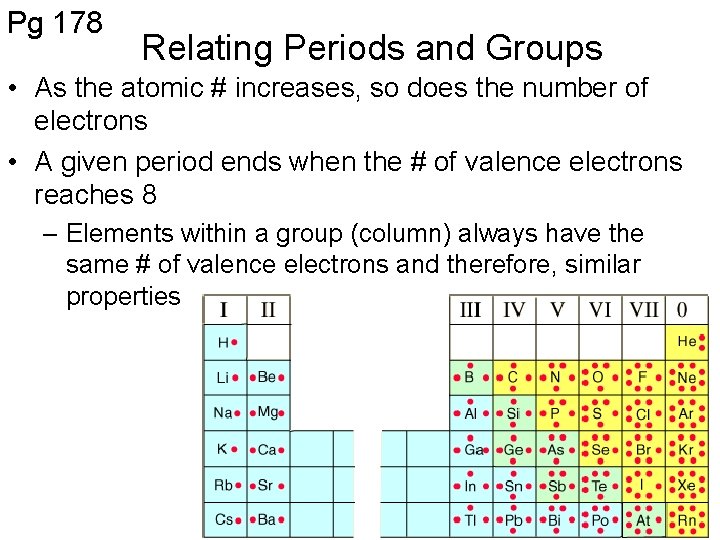
Pg 178 Relating Periods and Groups • As the atomic # increases, so does the number of electrons • A given period ends when the # of valence electrons reaches 8 – Elements within a group (column) always have the same # of valence electrons and therefore, similar properties
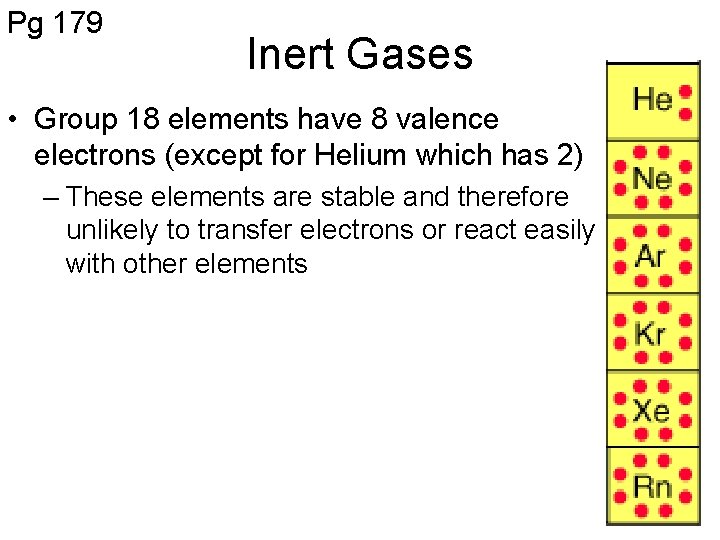
Pg 179 Inert Gases • Group 18 elements have 8 valence electrons (except for Helium which has 2) – These elements are stable and therefore unlikely to transfer electrons or react easily with other elements
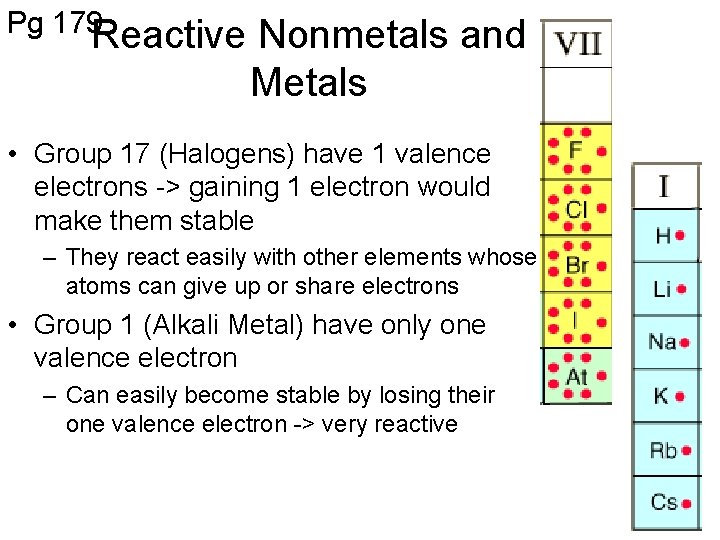
Pg 179 Reactive Nonmetals and Metals • Group 17 (Halogens) have 1 valence electrons -> gaining 1 electron would make them stable – They react easily with other elements whose atoms can give up or share electrons • Group 1 (Alkali Metal) have only one valence electron – Can easily become stable by losing their one valence electron -> very reactive
Pg 180 Other Metals • Groups 2 – 12 (metals) have 1 -3 valence electrons – React by losing these valence electrons, esp. when combined with oxygen or a halogen • Reactivity of metals decreases from left to right • In Groups 1 & 2, reactivity increases from top to bottom
Pg 181 Other Nonmetals • All of the nonmetals have four or more valence electrons and become stable when they have a set of 8 – Combine with metals by gaining electrons – Combine with other nonmetals by sharing electrons
Pg 182 Semimetals • Have 3 -6 valence electrons • Can either lose or share electrons when combined with other elements

Pg 182 Hydrogen • Has 1 valence electron • NOT a metal

Chapter 5 Section 1 Homework – pg 182

1 A. What are valence electrons?

1 B. What role do valence electrons play in the formation of compounds from elements?
1 C. Do oxygen atoms become more stable or less stable when oxygen forms compounds? Explain

2 A. Summarize how the periodic table is organized, and tell why this organization is useful.

2 B. Why do the properties of elements change in a regular way across a period?

2 C. Explain the reactivity of the inert gases in terms of valence electrons.
- Slides: 20