Chapter 5 Polymer structure Specific Instructional Objectives The
Chapter 5 Polymer structure
Specific Instructional Objectives The students should understand be able to explain the chemical and the properties of polymers in general, to distinguish different types of polymers and, to explain the crystalline polymers. Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 2
Contents 5. 1. Hydrocarbon molecules 5. 2. The chemistry of polymer molecules 5. 3. Molecular properties of polymer 5. 3. 1. Molecular weight 5. 3. 2. Molecular shape 5. 3. 3. Molecular structure 5. 3. 4. Molecular configurations 5. 4. Thermoplastics and thermosettings 5. 5. Copolymers 5. 6. Crystalline polymers Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 3
Polymers have been used for many centuries since human existed. Natural polymers: wood, rubber, cotton, wool, leather, and silk; proteins, enzymes, starches, and cellulose. Synthetic polymers most of plastics, rubbers and fiber materials. Polymer Poly means many Mer (a greek word “meros”, which means part) Polymer means many mers. Monomer refers to a stable molecule from which a polymer is synthesized Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 4
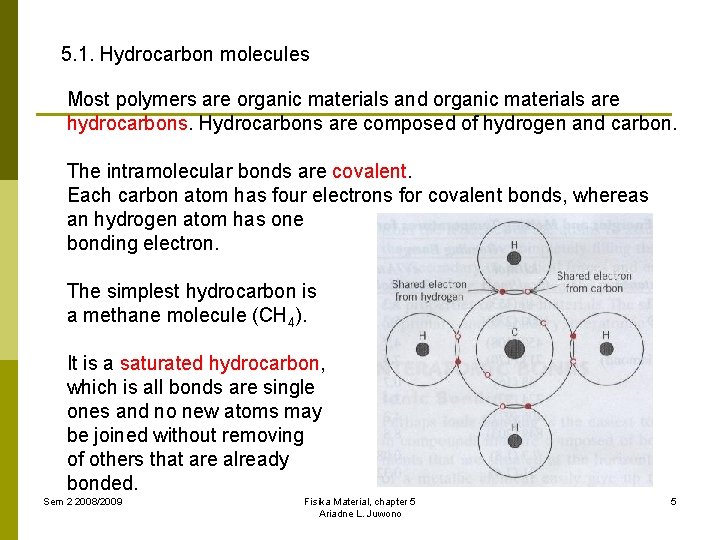
5. 1. Hydrocarbon molecules Most polymers are organic materials and organic materials are hydrocarbons. Hydrocarbons are composed of hydrogen and carbon. The intramolecular bonds are covalent. Each carbon atom has four electrons for covalent bonds, whereas an hydrogen atom has one bonding electron. The simplest hydrocarbon is a methane molecule (CH 4). It is a saturated hydrocarbon, which is all bonds are single ones and no new atoms may be joined without removing of others that are already bonded. Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 5
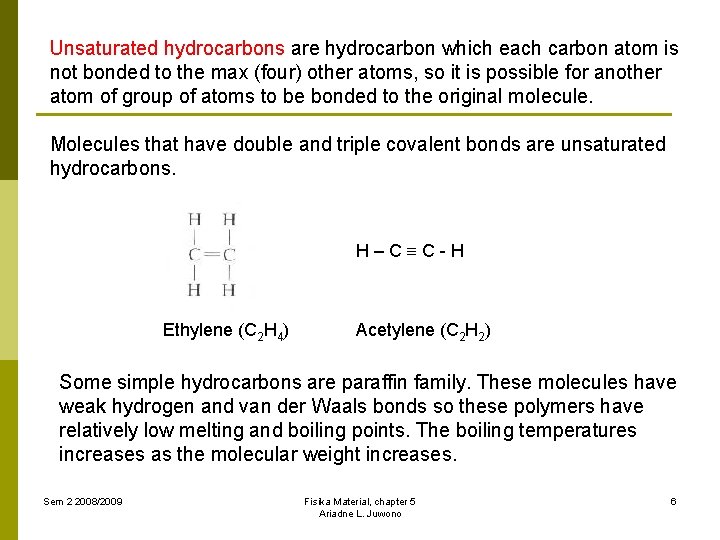
Unsaturated hydrocarbons are hydrocarbon which each carbon atom is not bonded to the max (four) other atoms, so it is possible for another atom of group of atoms to be bonded to the original molecule. Molecules that have double and triple covalent bonds are unsaturated hydrocarbons. H–C C-H Ethylene (C 2 H 4) Acetylene (C 2 H 2) Some simple hydrocarbons are paraffin family. These molecules have weak hydrogen and van der Waals bonds so these polymers have relatively low melting and boiling points. The boiling temperatures increases as the molecular weight increases. Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 6
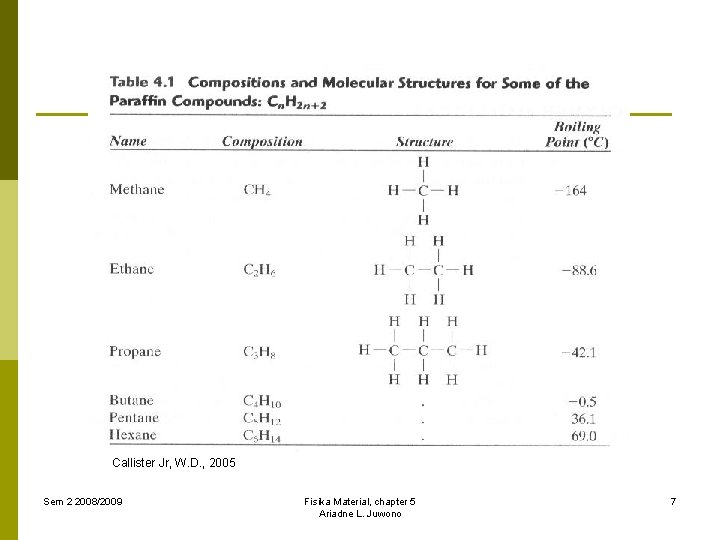
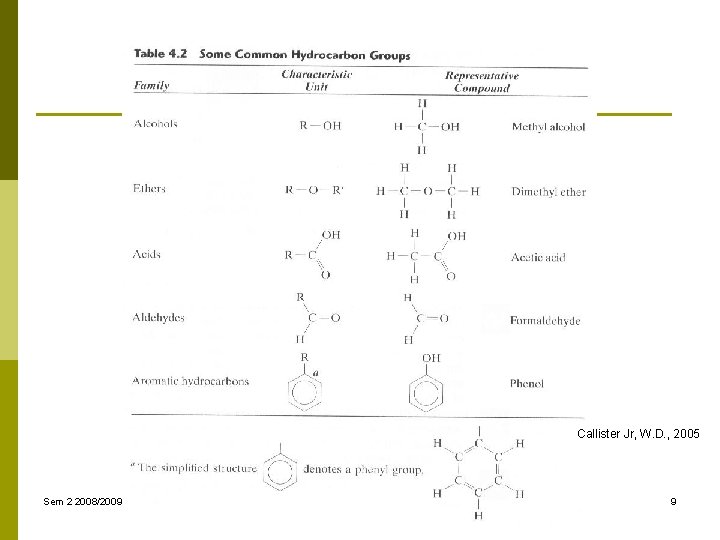
Callister Jr, W. D. , 2005 Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 7
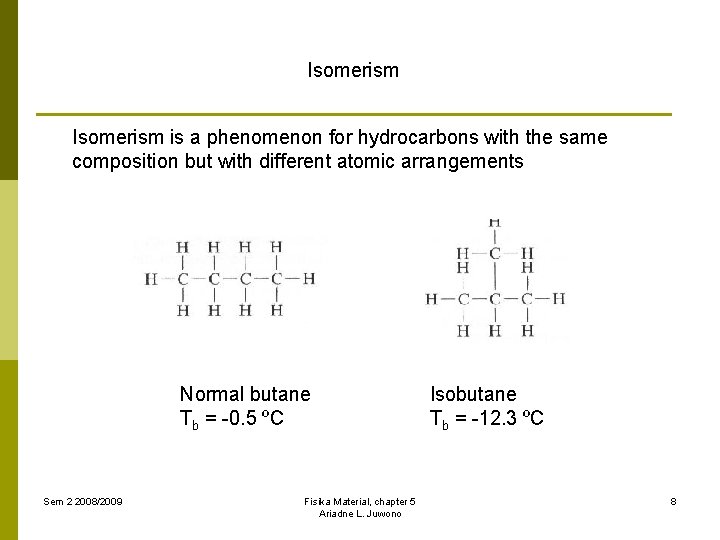
Isomerism is a phenomenon for hydrocarbons with the same composition but with different atomic arrangements Normal butane Tb = -0. 5 ºC Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono Isobutane Tb = -12. 3 ºC 8
Callister Jr, W. D. , 2005 Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 9
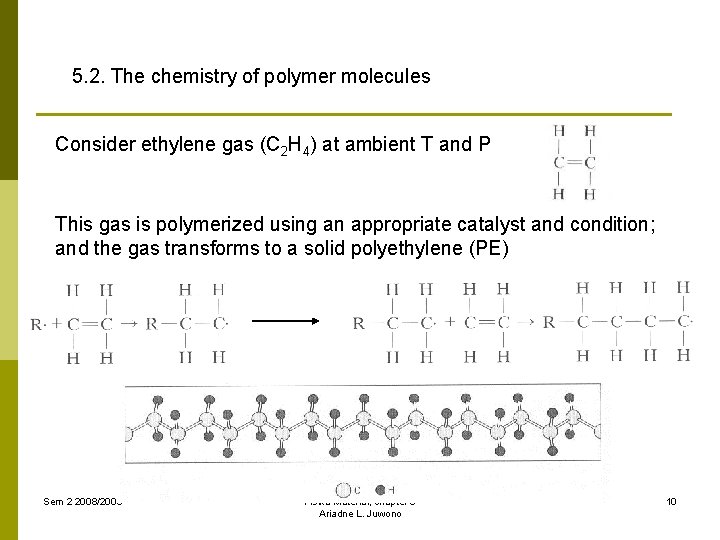
5. 2. The chemistry of polymer molecules Consider ethylene gas (C 2 H 4) at ambient T and P This gas is polymerized using an appropriate catalyst and condition; and the gas transforms to a solid polyethylene (PE) Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 10
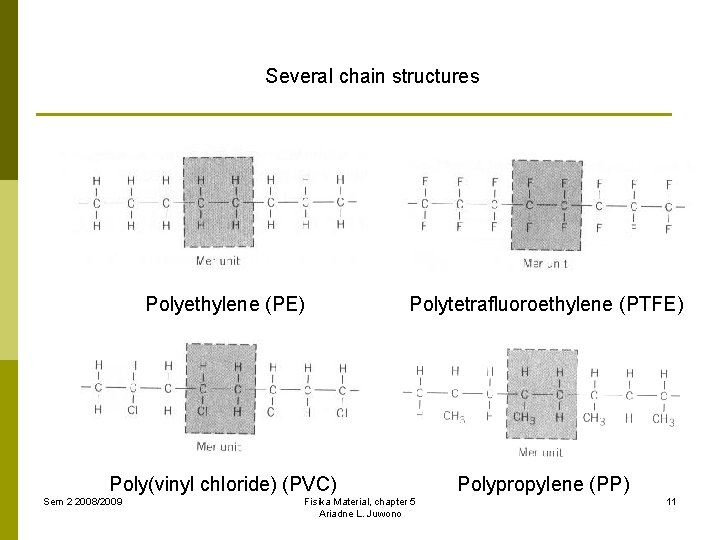
Several chain structures Polyethylene (PE) Polytetrafluoroethylene (PTFE) Poly(vinyl chloride) (PVC) Polypropylene (PP) Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 11
Some terms in polymers Homopolymer is a polymer which all the repeating units along a chain are of the same type. Copolymer is a polymer which the chains have two or more different mer units. Bifunctional monomer is a monomer which has two active bonds that may react with other monomers covalently to form [2] chain structures. Example : ethylene Trifunctional monomer is a monomer which has three active bonds to form [3] chain structures. Example : phenol-formaldehyde Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 12
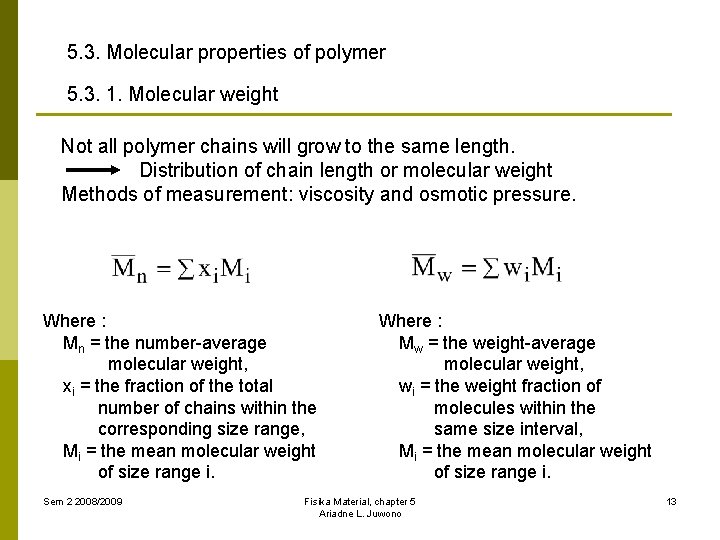
5. 3. Molecular properties of polymer 5. 3. 1. Molecular weight Not all polymer chains will grow to the same length. Distribution of chain length or molecular weight Methods of measurement: viscosity and osmotic pressure. Where : Mn = the number-average molecular weight, xi = the fraction of the total number of chains within the corresponding size range, Mi = the mean molecular weight of size range i. Sem 2 2008/2009 Where : Mw = the weight-average molecular weight, wi = the weight fraction of molecules within the same size interval, Mi = the mean molecular weight of size range i. Fisika Material, chapter 5 Ariadne L. Juwono 13
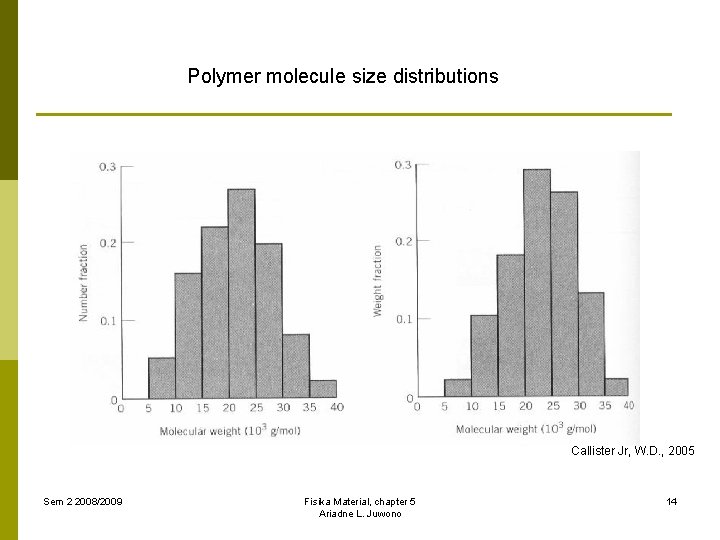
Polymer molecule size distributions Callister Jr, W. D. , 2005 Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 14

Degree of polymerization (n) Where: nn = number-average degrees nw = weight-average degrees of polymerization, Mn = number-average molecular Mw = weight-average molecular weights, m = the mer molecular weight (g) For a copolymer: Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 15
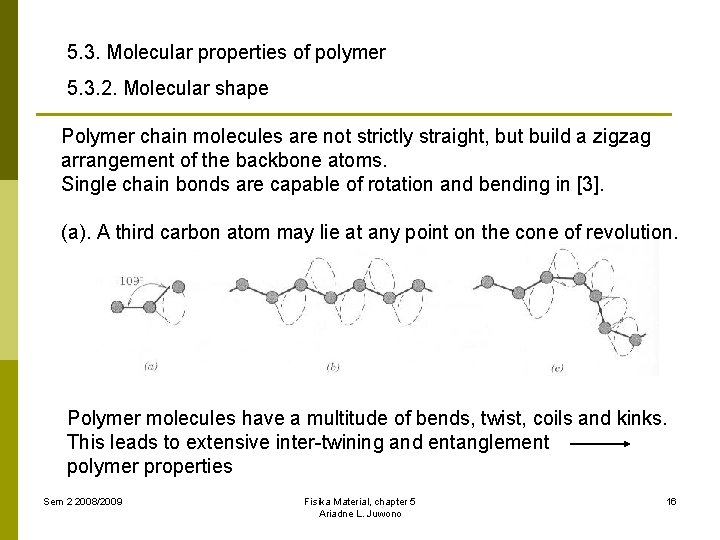
5. 3. Molecular properties of polymer 5. 3. 2. Molecular shape Polymer chain molecules are not strictly straight, but build a zigzag arrangement of the backbone atoms. Single chain bonds are capable of rotation and bending in [3]. (a). A third carbon atom may lie at any point on the cone of revolution. Polymer molecules have a multitude of bends, twist, coils and kinks. This leads to extensive inter-twining and entanglement polymer properties Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 16
5. 3. Molecular properties of polymer 5. 3. 3. Molecular structures There are 4 polymer structures are recognized, they are: 1. Linear polymers Mers are joined together end to end in single chains. (van der Waals and hydrogen bondings) Examples: PVC, PS, PMMC, nylon, fluorocarbons 2. Branched polymers Mers are connected in side-branch chains. 3. Crosslinked polymers Adjacent linear chains are joined one to another at various positions by covalent bonds. Example: rubbers (vulcanization process) 4. Network polymers Trifunctional mer units, that have three active covalent bonds, form [3] networks. The materials have special mechanical and thermal properties. Examples: epoxies and phenol-formaldehyde. Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 17
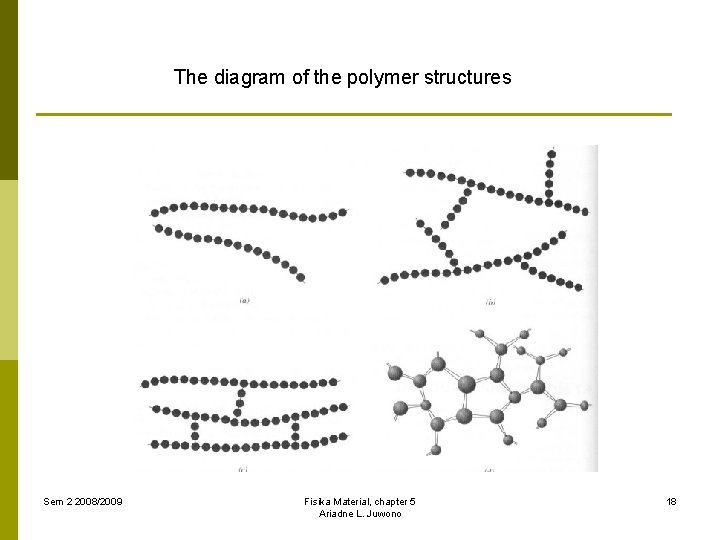
The diagram of the polymer structures Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 18
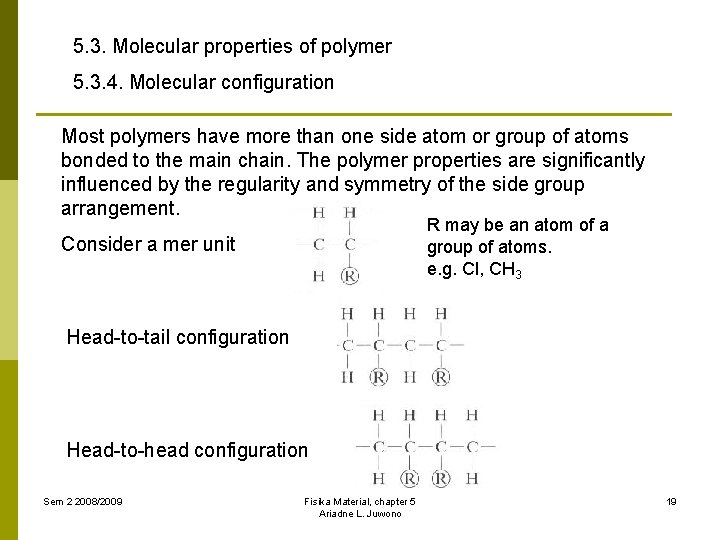
5. 3. Molecular properties of polymer 5. 3. 4. Molecular configuration Most polymers have more than one side atom or group of atoms bonded to the main chain. The polymer properties are significantly influenced by the regularity and symmetry of the side group arrangement. R may be an atom of a group of atoms. e. g. Cl, CH 3 Consider a mer unit Head-to-tail configuration Head-to-head configuration Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 19
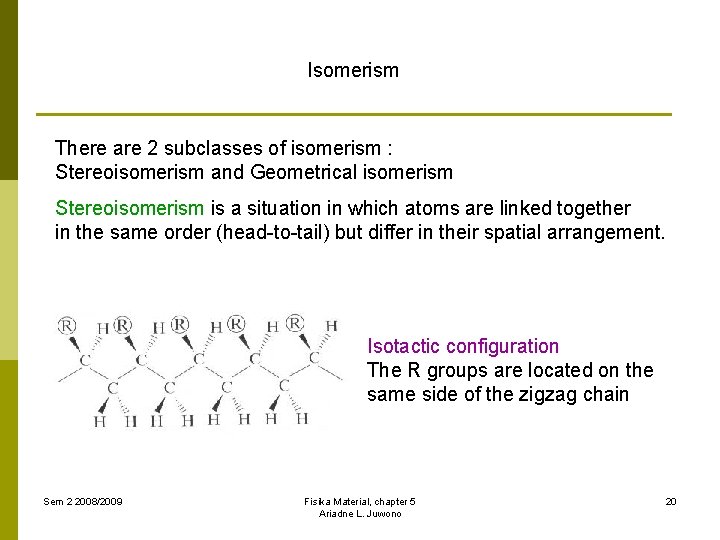
Isomerism There are 2 subclasses of isomerism : Stereoisomerism and Geometrical isomerism Stereoisomerism is a situation in which atoms are linked together in the same order (head-to-tail) but differ in their spatial arrangement. Isotactic configuration The R groups are located on the same side of the zigzag chain Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 20
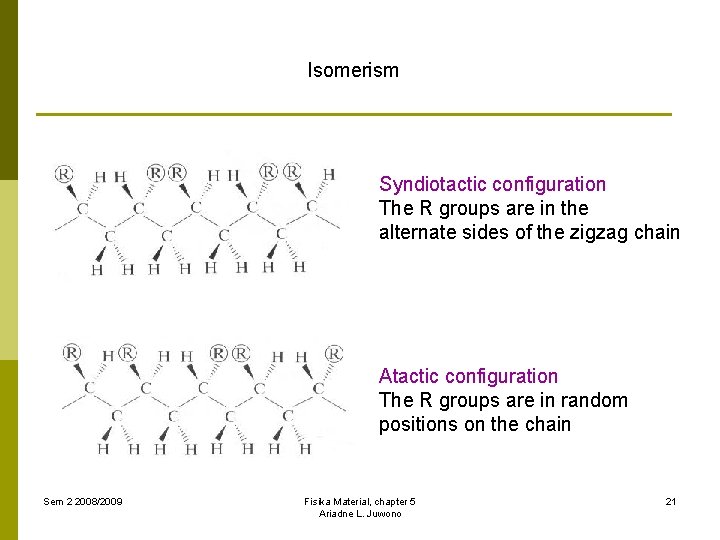
Isomerism Syndiotactic configuration The R groups are in the alternate sides of the zigzag chain Atactic configuration The R groups are in random positions on the chain Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 21
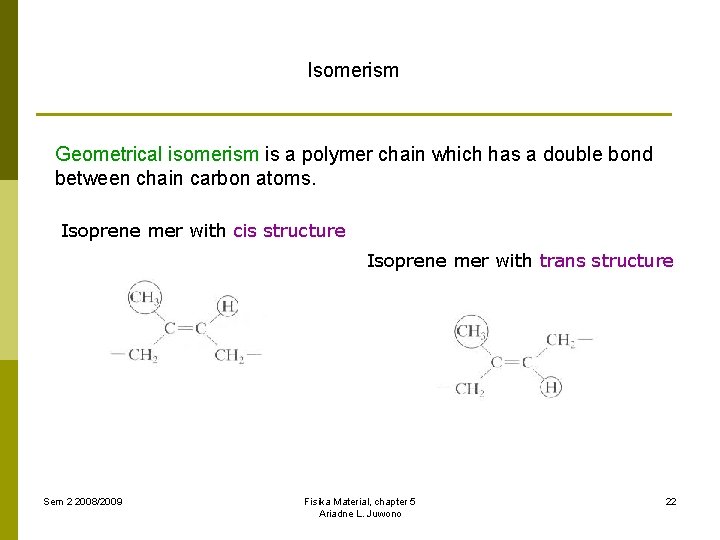
Isomerism Geometrical isomerism is a polymer chain which has a double bond between chain carbon atoms. Isoprene mer with cis structure Isoprene mer with trans structure Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 22
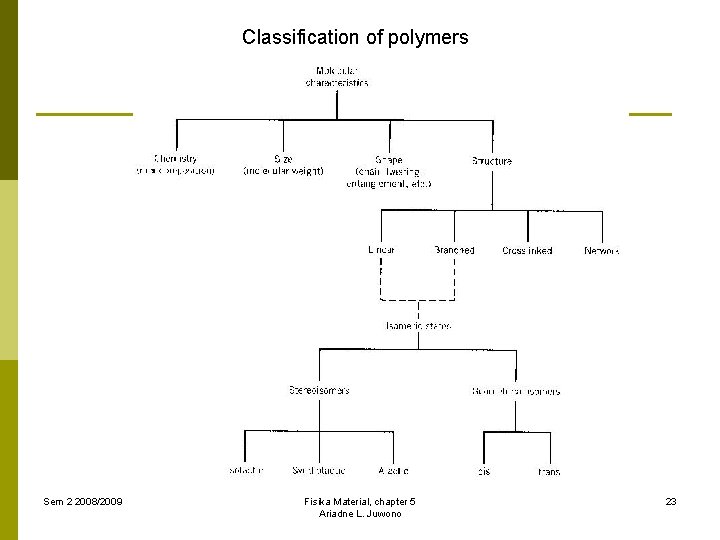
Classification of polymers Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 23
5. 4. Thermoplastic and thermosetting polymers Thermoplastic polymers are soften when heated (eventually liquefy) and harden when cooled. The process are totally reversible and may be repeated. Thermoplastics are relatively soft. Most of linear polymers and some branched structure polymers with flexible chains are thermoplastics. Thermosettings are permanently hard when heat is applied and do not soften upon subsequent heating. The process is irreversible. Thermosettings are relatively hard. Most cross-linked and network polymers are thermosettings and excessive temperatures cause severe polymer degradation. Example: rubbers, epoxies, phenolics and some polyester resins. Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 24
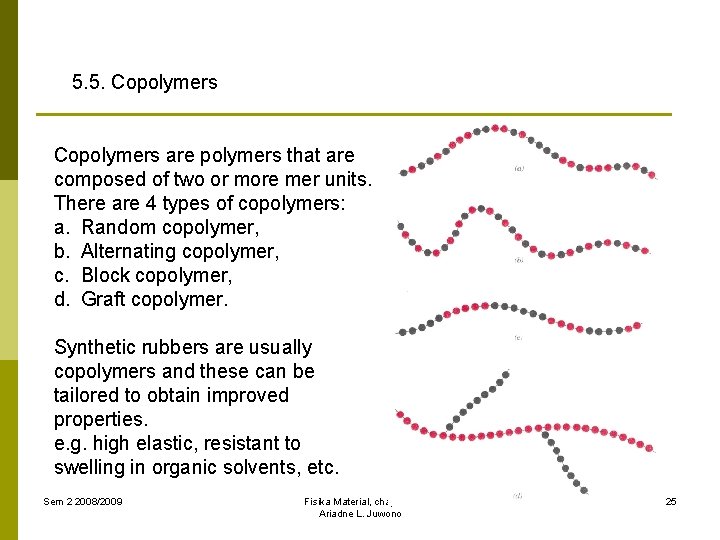
5. 5. Copolymers are polymers that are composed of two or more mer units. There are 4 types of copolymers: a. Random copolymer, b. Alternating copolymer, c. Block copolymer, d. Graft copolymer. Synthetic rubbers are usually copolymers and these can be tailored to obtain improved properties. e. g. high elastic, resistant to swelling in organic solvents, etc. Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 25
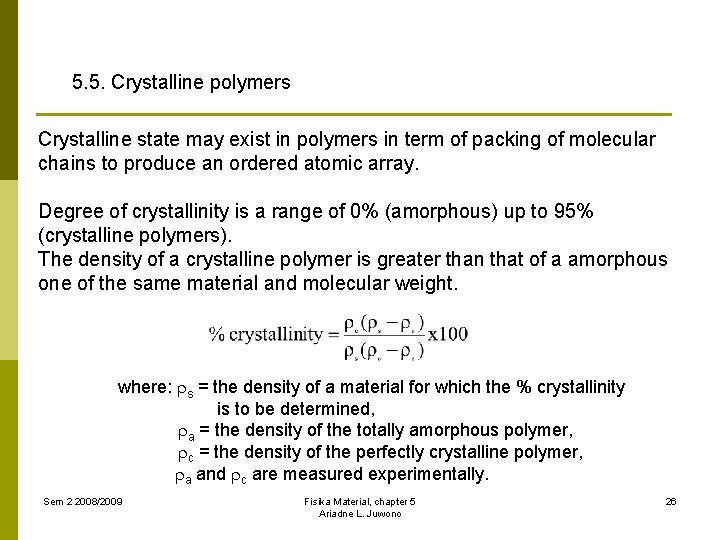
5. 5. Crystalline polymers Crystalline state may exist in polymers in term of packing of molecular chains to produce an ordered atomic array. Degree of crystallinity is a range of 0% (amorphous) up to 95% (crystalline polymers). The density of a crystalline polymer is greater than that of a amorphous one of the same material and molecular weight. where: s = the density of a material for which the % crystallinity is to be determined, a = the density of the totally amorphous polymer, c = the density of the perfectly crystalline polymer, a and c are measured experimentally. Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 26
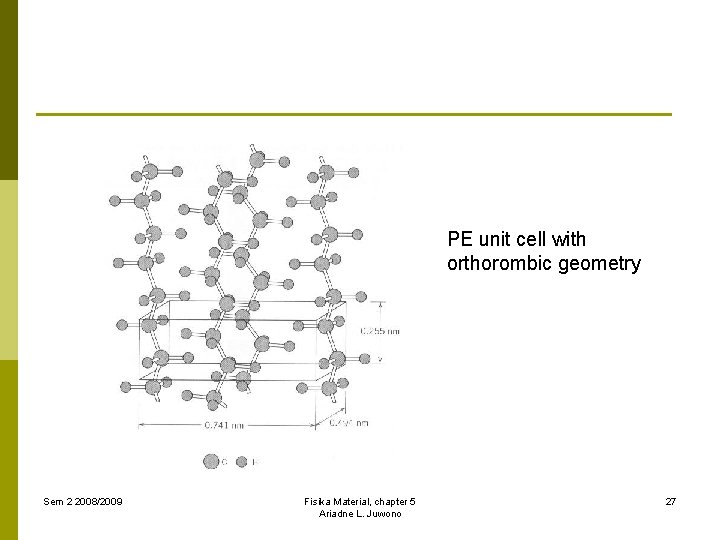
PE unit cell with orthorombic geometry Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 27
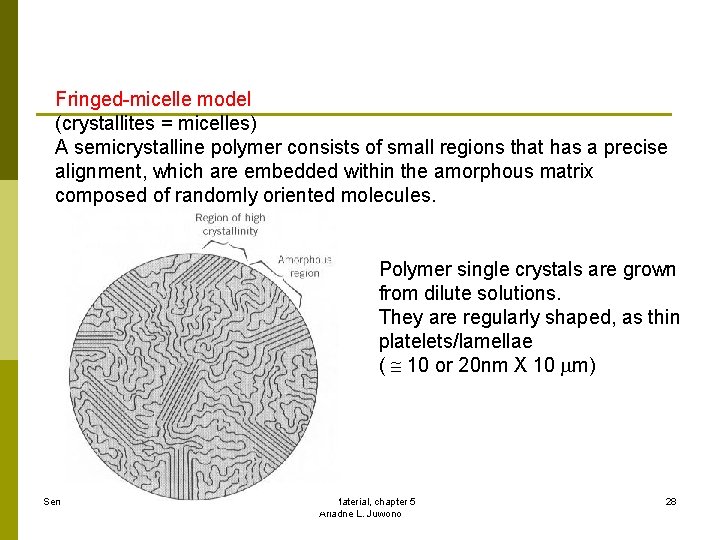
Fringed-micelle model (crystallites = micelles) A semicrystalline polymer consists of small regions that has a precise alignment, which are embedded within the amorphous matrix composed of randomly oriented molecules. Polymer single crystals are grown from dilute solutions. They are regularly shaped, as thin platelets/lamellae ( 10 or 20 nm X 10 m) Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 28
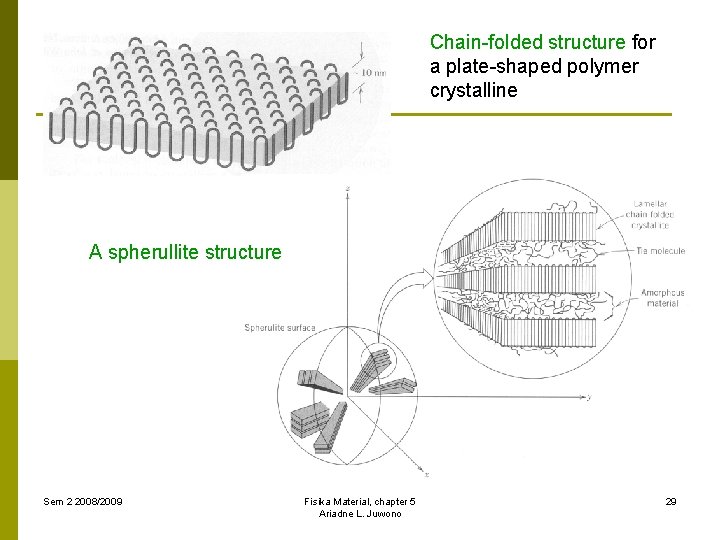
Chain-folded structure for a plate-shaped polymer crystalline A spherullite structure Sem 2 2008/2009 Fisika Material, chapter 5 Ariadne L. Juwono 29
- Slides: 29