Chapter 5 Patterns in Nature Minerals LECTURE OUTLINE



















- Slides: 47

Chapter 5 Patterns in Nature: Minerals LECTURE OUTLINE earth Portrait of a Planet Third Edition © 2008 W. W. Norton & Company, Inc. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

Patterns in Nature: Minerals Prepared by Ronald Parker Earlham College Department of Geosciences Richmond, Indiana

Minerals The “building blocks” of rocks, and hence, of Earth. < More than 4, 000 are known. < Dozens of new minerals are discovered annually. < Human interest in minerals spans millenia. < Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

Minerals < Developed societies depend on mineral resources. = Metals – Iron, copper, lead, zinc, nickel, aluminum, etc. = Non-metals – Gypsum, limestone, aggregate, clay. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

Minerals Economically important – Drive world economies. < Historically important – Dictated human history. < = Iron. = Copper. = Gold. = Diamonds. = Gems. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

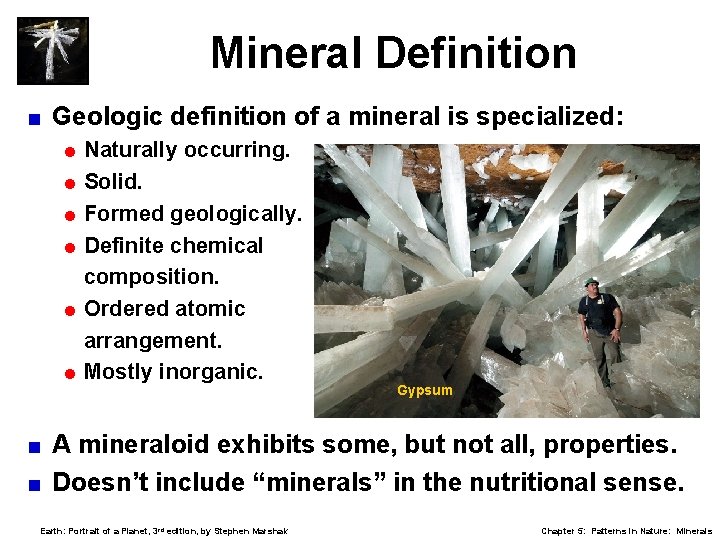
Mineral Definition < Geologic definition of a mineral is specialized: = Naturally occurring. = Solid. = Formed geologically. = Definite chemical composition. = Ordered atomic arrangement. = Mostly inorganic. Gypsum A mineraloid exhibits some, but not all, properties. < Doesn’t include “minerals” in the nutritional sense. < Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

Rocks are Earth materials made from minerals. < Most rocks have more than one kind of mineral. < = Example: Granite 4 Potassium feldspar. 4 Quartz. 4 Hornblende. < Some are monomineralic. = Limestone (Calcite). = Rock salt (Halite). = Glacial ice. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

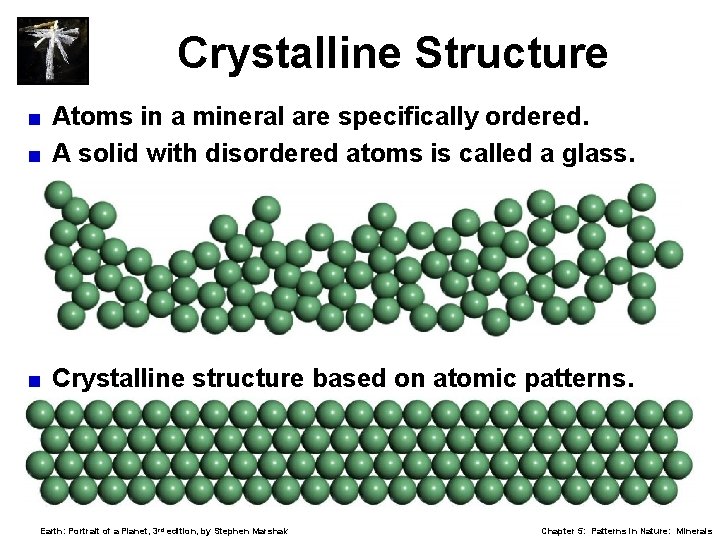
Crystalline Structure Atoms in a mineral are specifically ordered. < A solid with disordered atoms is called a glass. < < Crystalline structure based on atomic patterns. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

Crystals Rare minerals displaying flat external faces. < Crystal faces form best in open cavities. < Crystals are often prized mineral specimens. < Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

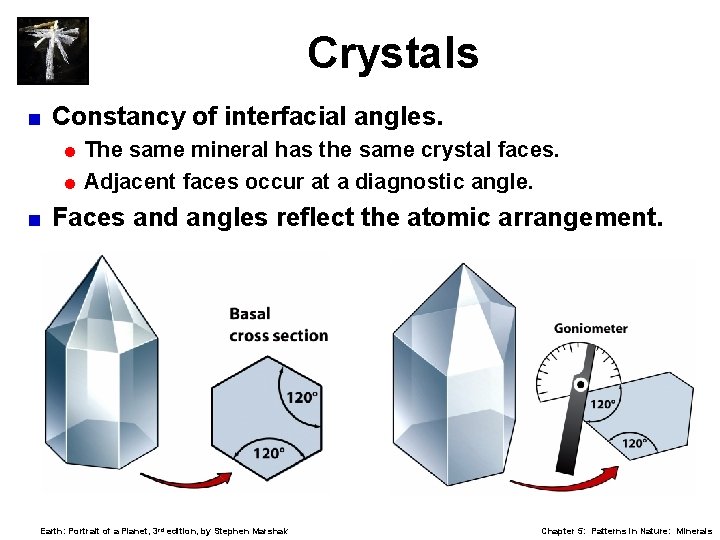
Crystals < Constancy of interfacial angles. = The same mineral has the same crystal faces. = Adjacent faces occur at a diagnostic angle. < Faces and angles reflect the atomic arrangement. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

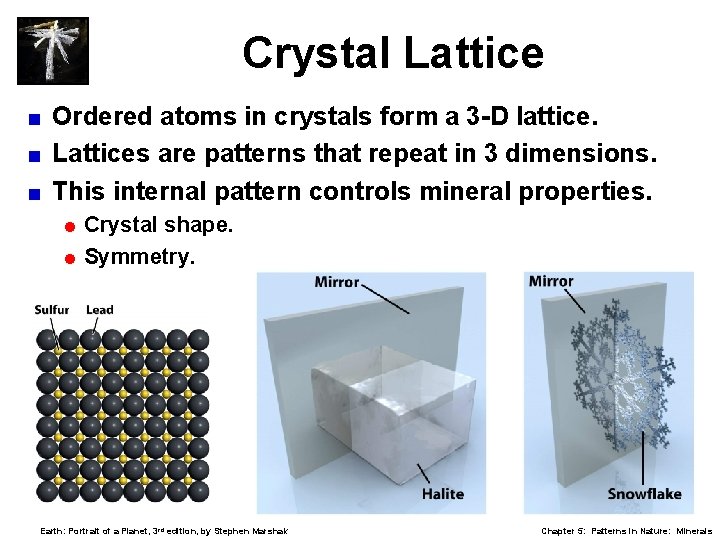
Crystal Lattice Ordered atoms in crystals form a 3 -D lattice. < Lattices are patterns that repeat in 3 dimensions. < This internal pattern controls mineral properties. < = Crystal shape. = Symmetry. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

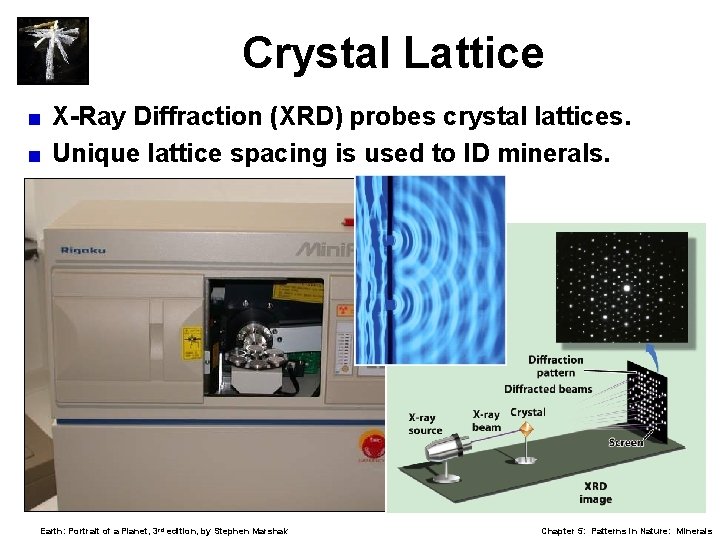
Crystal Lattice X-Ray Diffraction (XRD) probes crystal lattices. < Unique lattice spacing is used to ID minerals. < Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

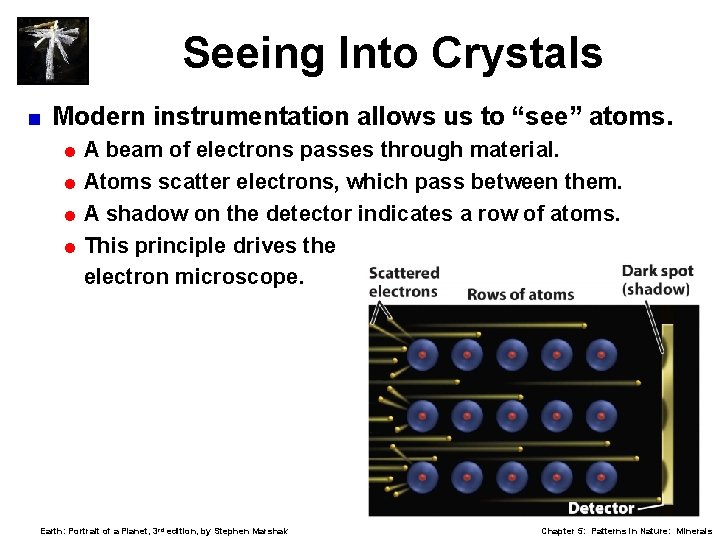
Seeing Into Crystals < Modern instrumentation allows us to “see” atoms. =A beam of electrons passes through material. = Atoms scatter electrons, which pass between them. = A shadow on the detector indicates a row of atoms. = This principle drives the electron microscope. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

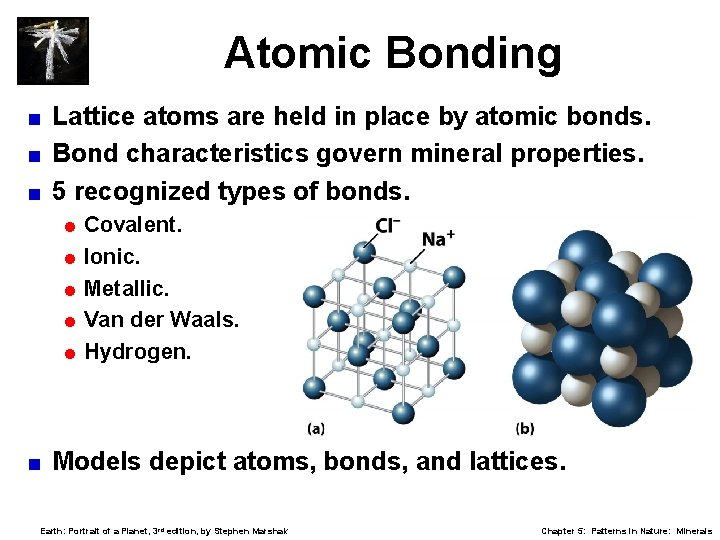
Atomic Bonding Lattice atoms are held in place by atomic bonds. < Bond characteristics govern mineral properties. < 5 recognized types of bonds. < = Covalent. = Ionic. = Metallic. = Van der Waals. = Hydrogen. < Models depict atoms, bonds, and lattices. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

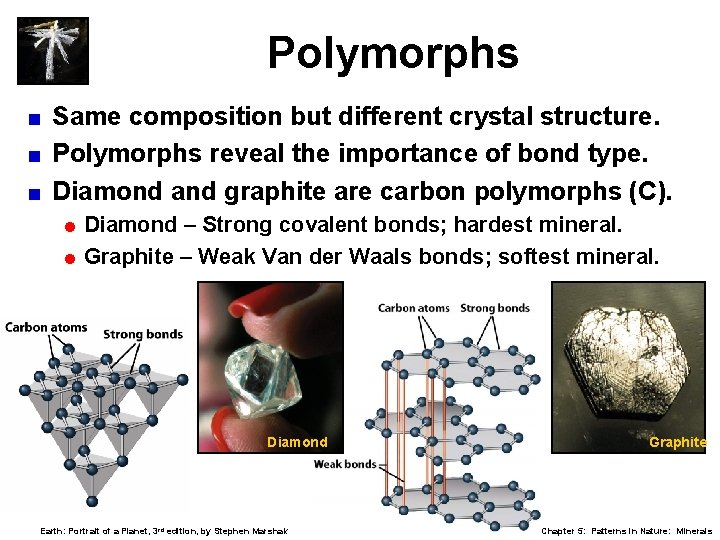
Polymorphs Same composition but different crystal structure. < Polymorphs reveal the importance of bond type. < Diamond and graphite are carbon polymorphs (C). < = Diamond – Strong covalent bonds; hardest mineral. = Graphite – Weak Van der Waals bonds; softest mineral. Diamond Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Graphite Chapter 5: Patterns in Nature: Minerals

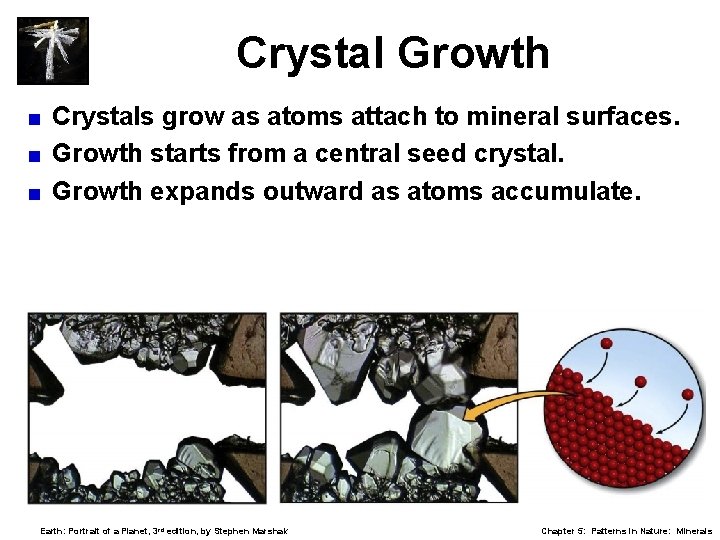
Crystal Growth Crystals grow as atoms attach to mineral surfaces. < Growth starts from a central seed crystal. < Growth expands outward as atoms accumulate. < Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

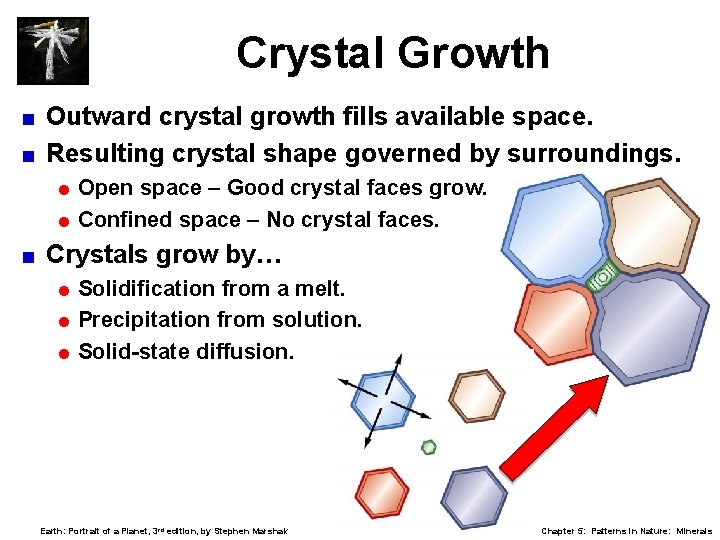
Crystal Growth Outward crystal growth fills available space. < Resulting crystal shape governed by surroundings. < = Open space – Good crystal faces grow. = Confined space – No crystal faces. < Crystals grow by… = Solidification from a melt. = Precipitation from solution. = Solid-state diffusion. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

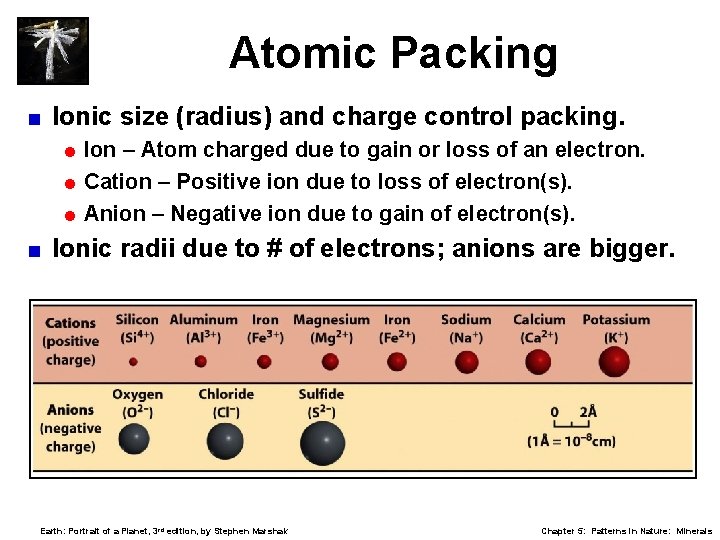
Atomic Packing < Ionic size (radius) and charge control packing. = Ion – Atom charged due to gain or loss of an electron. = Cation – Positive ion due to loss of electron(s). = Anion – Negative ion due to gain of electron(s). < Ionic radii due to # of electrons; anions are bigger. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

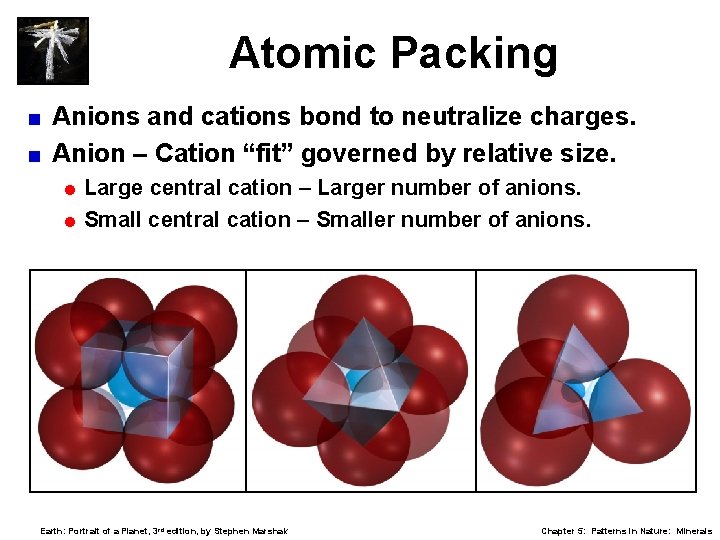
Atomic Packing Anions and cations bond to neutralize charges. < Anion – Cation “fit” governed by relative size. < = Large central cation – Larger number of anions. = Small central cation – Smaller number of anions. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

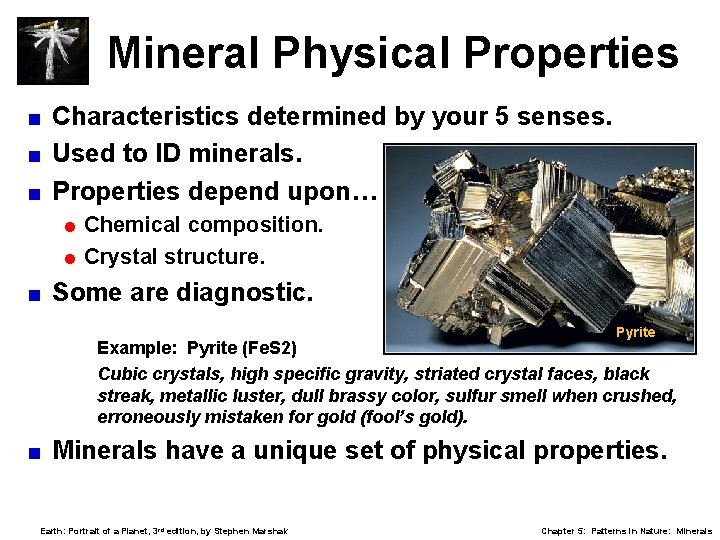
Mineral Physical Properties Characteristics determined by your 5 senses. < Used to ID minerals. < Properties depend upon… < = Chemical composition. = Crystal structure. < Some are diagnostic. Pyrite Example: Pyrite (Fe. S 2) Cubic crystals, high specific gravity, striated crystal faces, black streak, metallic luster, dull brassy color, sulfur smell when crushed, erroneously mistaken for gold (fool’s gold). < Minerals have a unique set of physical properties. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

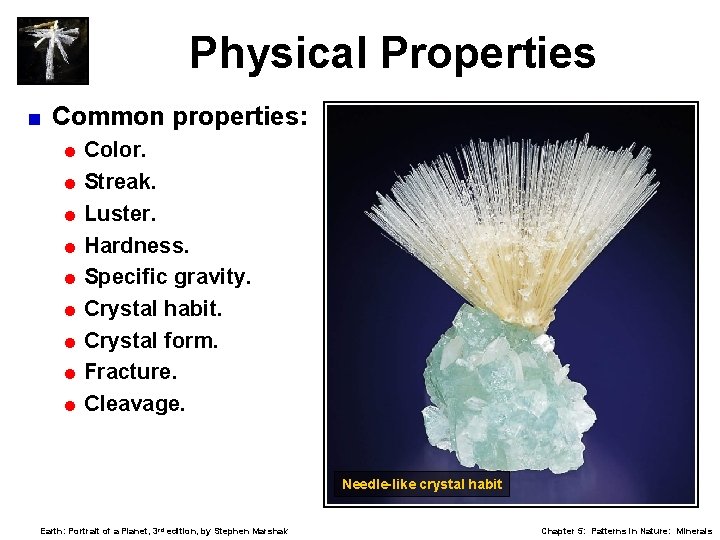
Physical Properties < Common properties: = Color. = Streak. = Luster. = Hardness. = Specific gravity. = Crystal habit. = Crystal form. = Fracture. = Cleavage. Needle-like crystal habit Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

Physical Properties < Less common physical properties: = Taste. = Smell. = Feel. = Elasticity. = Magnetism. = Effervescence. = Diaphaneity. = Piezoelectricity. Magnetite crystals on a large magnet. = Pyroelectricity. = Refractive index. = Malleability. = Ductility. Calcite effervesces with acid = Sectility. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

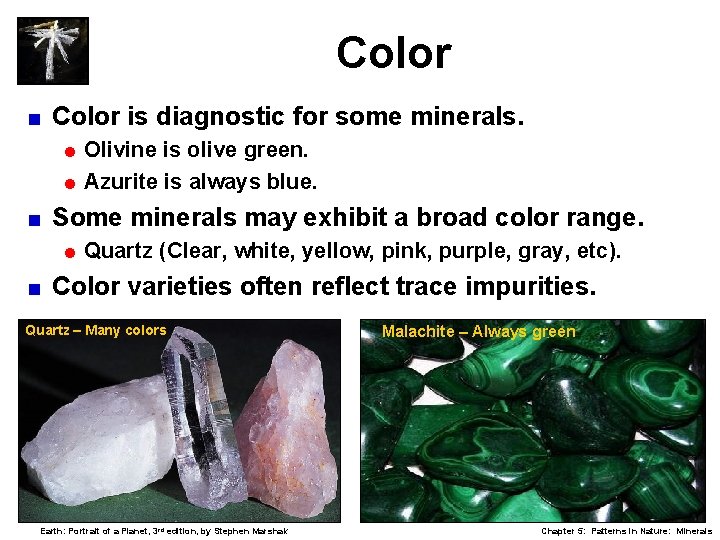
Color < Color is diagnostic for some minerals. = Olivine is olive green. = Azurite is always blue. < Some minerals may exhibit a broad color range. = Quartz < (Clear, white, yellow, pink, purple, gray, etc). Color varieties often reflect trace impurities. Quartz – Many colors Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Malachite – Always green Chapter 5: Patterns in Nature: Minerals

Streak Color of a mineral crushed on unglazed porcelain. < Streak is often a useful diagnostic property. < = Congruent streak – Streak color same as mineral. 4 Magnetite – Black mineral; black streak. = Incongruent streak – Streak color different than mineral. 4 Chromite – Black mineral; greenish-brown streak. Hematite – Red-brown streak Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

Luster The way a mineral scatters light. < Two subdivisions. < = Metallic – Looks like a metal. = Nonmetallic. 4 Vitreous (glassy). 4 Satiny. 4 Silky. 4 Resinous. 4 Pearly. 4 Earthy (dull). 4 Adamantine (brilliant). Quartz – Vitreous luster Satin spar Gypsum – Satiny luster Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

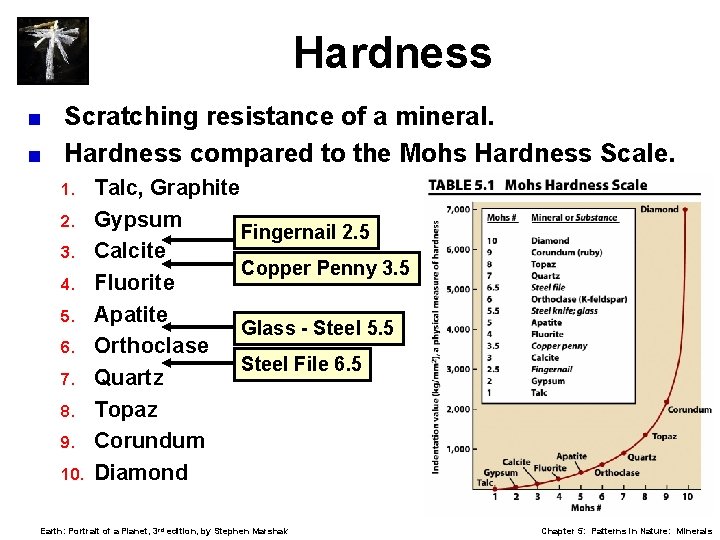
Hardness < < Scratching resistance of a mineral. Hardness compared to the Mohs Hardness Scale. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Talc, Graphite Gypsum Fingernail 2. 5 Calcite Copper Penny 3. 5 Fluorite Apatite Glass - Steel 5. 5 Orthoclase Steel File 6. 5 Quartz Topaz Corundum Diamond Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

Specific Gravity Related to density (mass per volume). < Mineral weight over weight of equal water volume. < Specific gravity is “heft”– How heavy it feels. < = Pyrite – Heavy (SG 5. 0) = Feldspar – Light (SG 2. 6) < Pyrite “feels” heavier that feldspar. Potassium Feldspar Pyrite Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

Crystal Habit Crystal habit is the ideal shape of crystal faces. < Ideal growth requires ideal conditions. < Many terms are used to describe habit. < Cubes Dodecahedra Octahedra Compound Forms Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Blades Rhombohedra Hexagonal Prisms Tetragonal Prisms Chapter 5: Patterns in Nature: Minerals

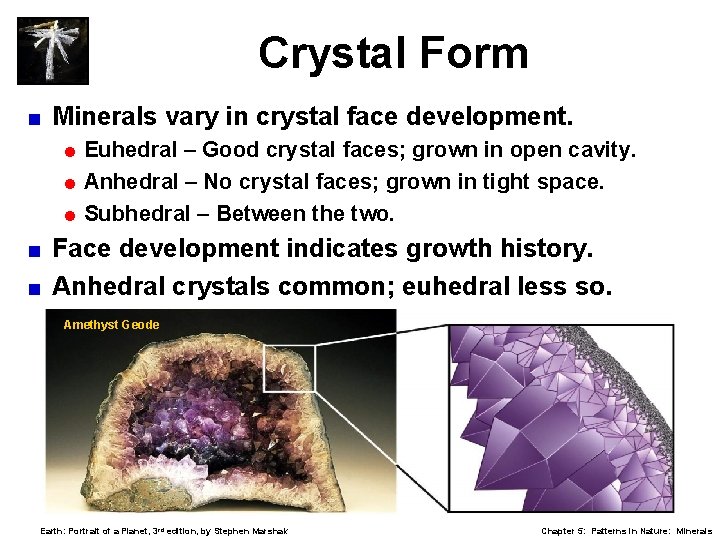
Crystal Form < Minerals vary in crystal face development. = Euhedral – Good crystal faces; grown in open cavity. = Anhedral – No crystal faces; grown in tight space. = Subhedral – Between the two. Face development indicates growth history. < Anhedral crystals common; euhedral less so. < Amethyst Geode Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

Fracture Some minerals lack planes of weakness. < Due to equal molecular bonds in all directions. < These minerals don’t have cleavage; they fracture. < = Example: Quartz displays conchoidal fracture. 4 Shaped like the inside of a clam shell. 4 Breaks along smooth curved surfaces. 4 Produces extremely sharp edges. Obsidian Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

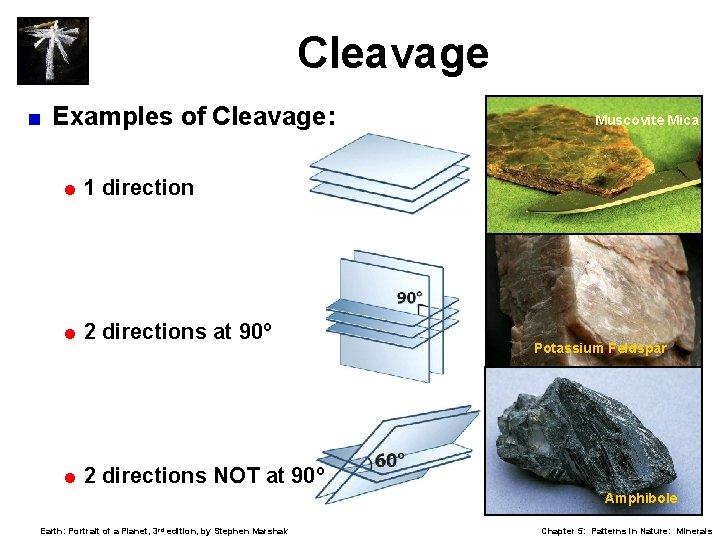
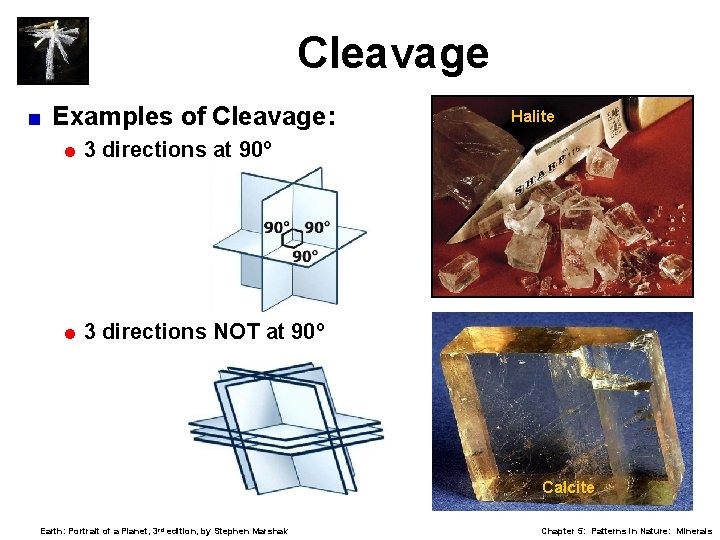
Cleavage Tendency to break along planes of weakness. < Cleavage produces flat, shiny surfaces. < Described by number of planes and their angles. < Sometimes mistaken for crystal habit. < = Cleavage is through-going; often forms parallel “steps. ” = Crystal habit is only on external surfaces. < 1, 2, 3, 4, and 6 cleavages possible. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

Cleavage < Examples of Cleavage: =1 direction =2 directions at 90º =2 directions NOT at 90º Muscovite Mica Potassium Feldspar Amphibole Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

Cleavage < Examples of Cleavage: =3 directions at 90º =3 directions NOT at 90º Halite Calcite Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

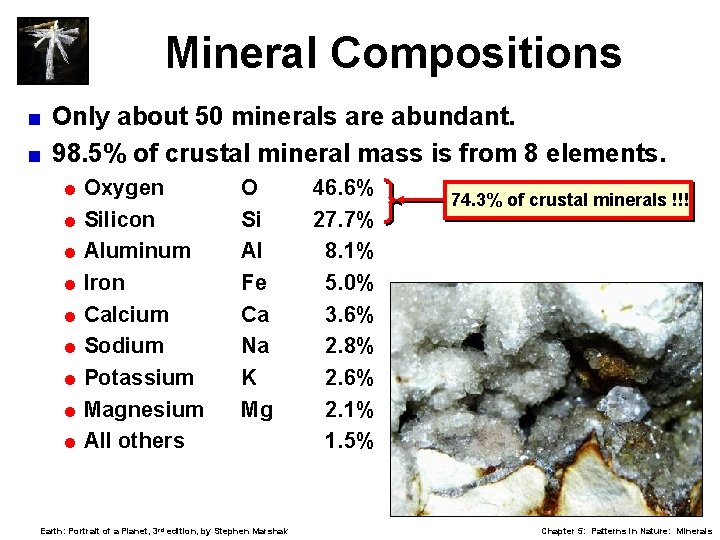
Mineral Compositions Only about 50 minerals are abundant. < 98. 5% of crustal mineral mass is from 8 elements. < = Oxygen = Silicon = Aluminum = Iron = Calcium = Sodium = Potassium = Magnesium = All O Si Al Fe Ca Na K Mg others Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak 46. 6% 27. 7% 8. 1% 5. 0% 3. 6% 2. 8% 2. 6% 2. 1% 1. 5% 74. 3% of crustal minerals !!! Chapter 5: Patterns in Nature: Minerals

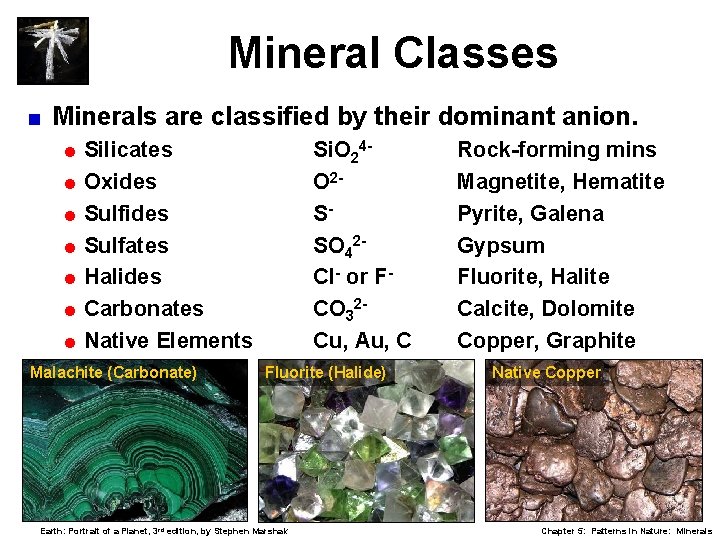
Mineral Classes < Minerals are classified by their dominant anion. = Silicates Si. O 24 O 2 SSO 42 Cl- or FCO 32 Cu, Au, C = Oxides = Sulfates = Halides = Carbonates = Native Elements Malachite (Carbonate) Fluorite (Halide) Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Rock-forming mins Magnetite, Hematite Pyrite, Galena Gypsum Fluorite, Halite Calcite, Dolomite Copper, Graphite Native Copper Chapter 5: Patterns in Nature: Minerals

Silicate Minerals Silicates are know as the rock-forming minerals. < They dominate the Earth’s crust. < = Oxygen and silicon… 4 Make up 94. 7 % of crustal volume, and. . . 474. 3 % of crustal mass. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

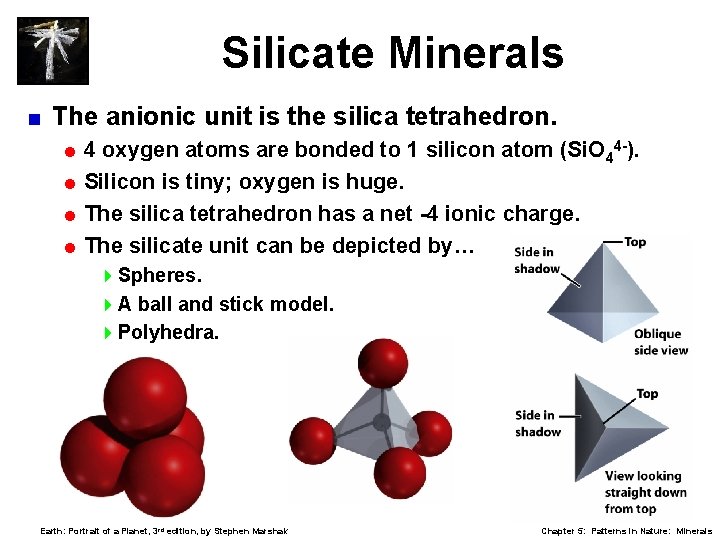
Silicate Minerals < The anionic unit is the silica tetrahedron. =4 oxygen atoms are bonded to 1 silicon atom (Si. O 44 -). = Silicon is tiny; oxygen is huge. = The silica tetrahedron has a net -4 ionic charge. = The silicate unit can be depicted by… 4 Spheres. 4 A ball and stick model. 4 Polyhedra. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

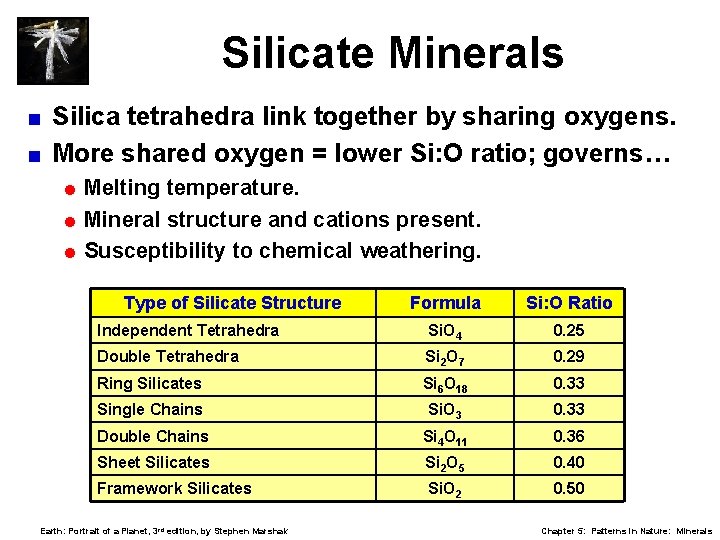
Silicate Minerals Silica tetrahedra link together by sharing oxygens. < More shared oxygen = lower Si: O ratio; governs… < = Melting temperature. = Mineral structure and cations present. = Susceptibility to chemical weathering. Type of Silicate Structure Formula Si: O Ratio Independent Tetrahedra Si. O 4 0. 25 Double Tetrahedra Si 2 O 7 0. 29 Ring Silicates Si 6 O 18 0. 33 Single Chains Si. O 3 0. 33 Double Chains Si 4 O 11 0. 36 Sheet Silicates Si 2 O 5 0. 40 Framework Silicates Si. O 2 0. 50 Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

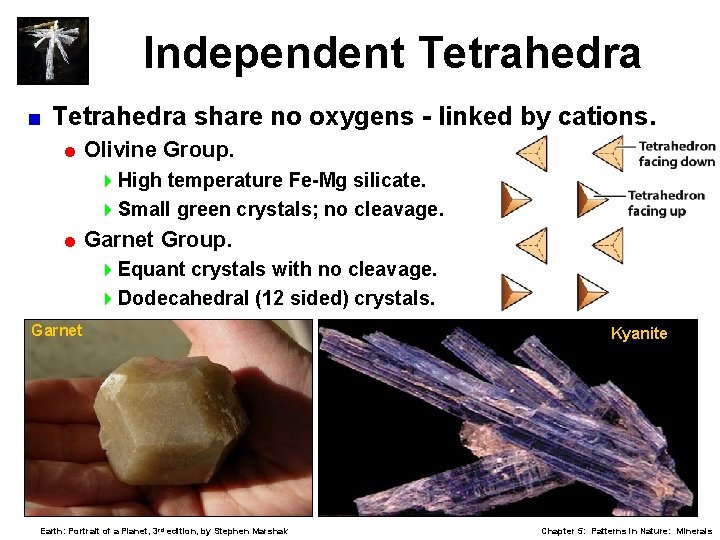
Independent Tetrahedra < Tetrahedra share no oxygens - linked by cations. = Olivine Group. 4 High temperature Fe-Mg silicate. 4 Small green crystals; no cleavage. = Garnet Group. 4 Equant crystals with no cleavage. 4 Dodecahedral (12 sided) crystals. Garnet Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Kyanite Chapter 5: Patterns in Nature: Minerals

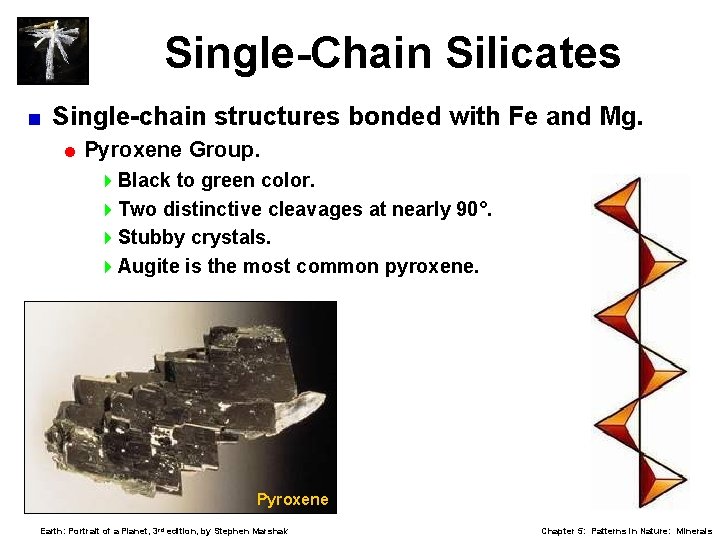
Single-Chain Silicates < Single-chain structures bonded with Fe and Mg. = Pyroxene Group. 4 Black to green color. 4 Two distinctive cleavages at nearly 90°. 4 Stubby crystals. 4 Augite is the most common pyroxene. Pyroxene Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

Double-Chain Silicates Double chain of silica tetrahedra bonded together. < Contain a variety of cations. < = Amphibole Group - Two perfect cleavages; elongate crystals. Hornblende Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

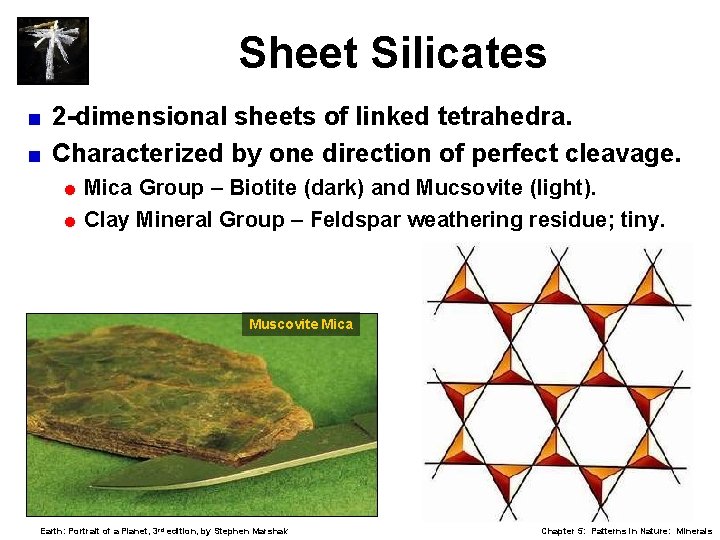
Sheet Silicates 2 -dimensional sheets of linked tetrahedra. < Characterized by one direction of perfect cleavage. < = Mica Group – Biotite (dark) and Mucsovite (light). = Clay Mineral Group – Feldspar weathering residue; tiny. Muscovite Mica Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

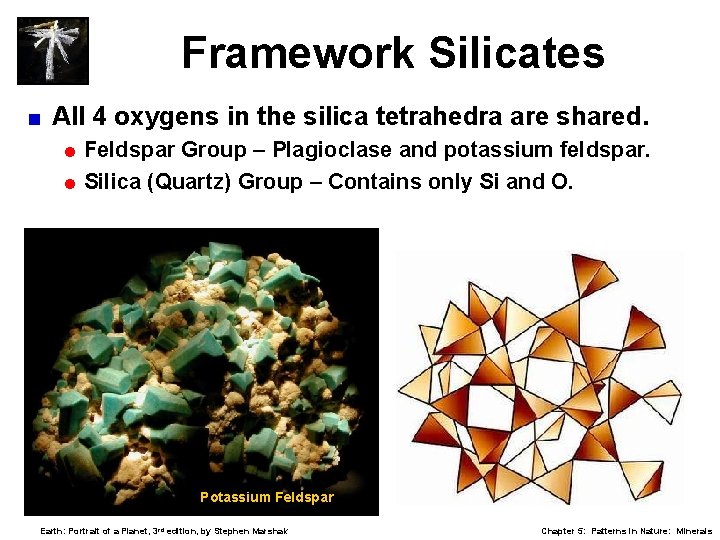
Framework Silicates < All 4 oxygens in the silica tetrahedra are shared. = Feldspar Group – Plagioclase and potassium feldspar. = Silica (Quartz) Group – Contains only Si and O. Potassium Feldspar Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

Gems < Minerals with special value. = Rarity. = Beauty. 4 Color. 4 Interaction with light. a. Dispersion. a. High refractive index. Aquamarine Beryl Watermelon Tourmaline Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

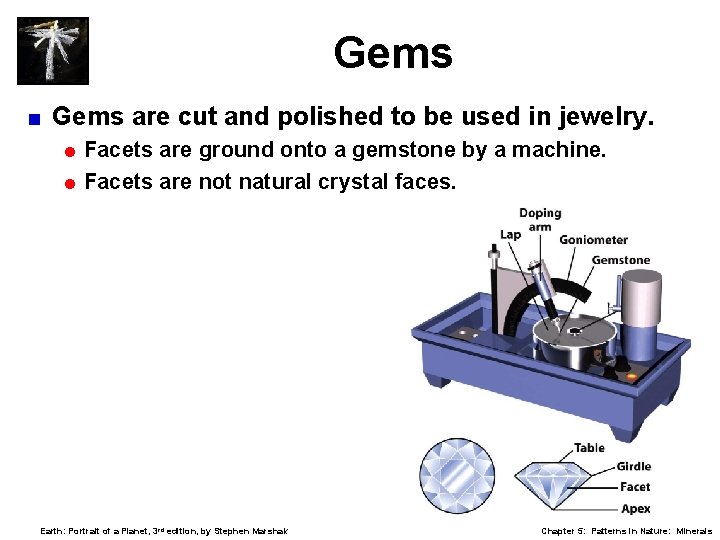
Gems < Gems are cut and polished to be used in jewelry. = Facets are ground onto a gemstone by a machine. = Facets are not natural crystal faces. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

Diamonds < Diamonds originate under extremely high pressure. =~ 150 km deep – in the upper mantle. = Pure carbon is compressed into the diamond structure. Rifting causes deep mantle rock to move upward. < Diamonds are found in kimberlite pipes. < Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals

This concludes the Chapter 5 Patterns in Nature: Minerals LECTURE OUTLINE earth Portrait of a Planet Third Edition © 2008 W. W. Norton & Company, Inc. Earth: Portrait of a Planet, 3 rd edition, by Stephen Marshak Chapter 5: Patterns in Nature: Minerals