Chapter 5 part 2 The Structure and Function




- Slides: 49

Chapter 5 – part 2 The Structure and Function of Large Biological Molecules

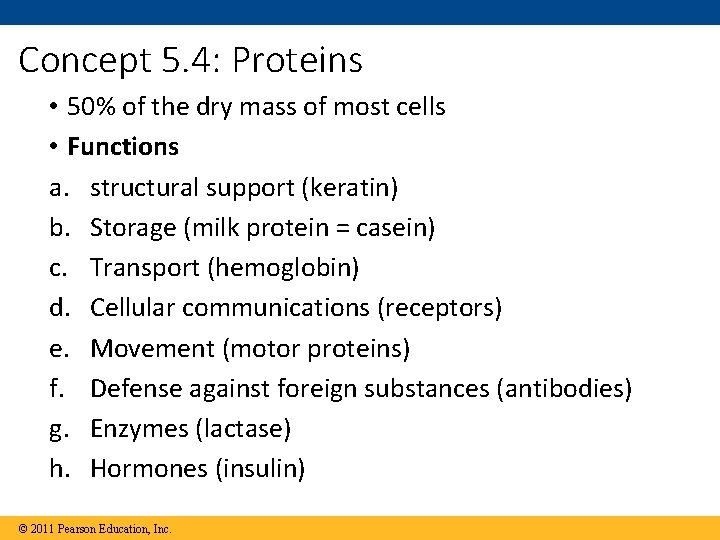
Concept 5. 4: Proteins • 50% of the dry mass of most cells • Functions a. structural support (keratin) b. Storage (milk protein = casein) c. Transport (hemoglobin) d. Cellular communications (receptors) e. Movement (motor proteins) f. Defense against foreign substances (antibodies) g. Enzymes (lactase) h. Hormones (insulin) © 2011 Pearson Education, Inc.

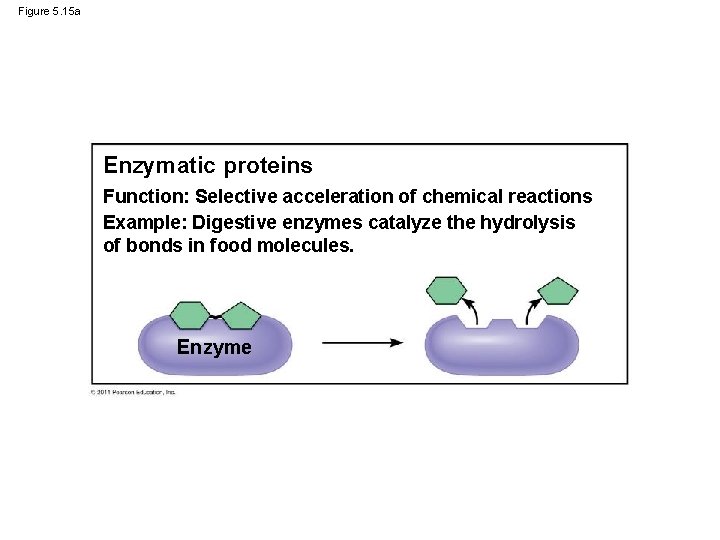
Figure 5. 15 a Enzymatic proteins Function: Selective acceleration of chemical reactions Example: Digestive enzymes catalyze the hydrolysis of bonds in food molecules. Enzyme

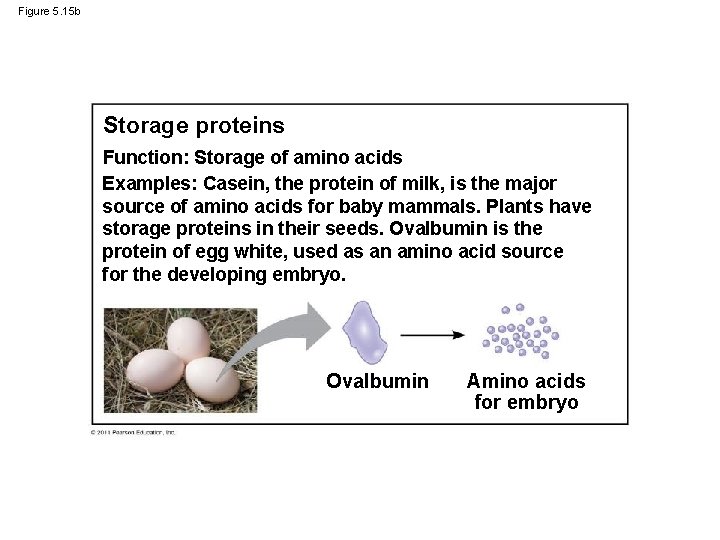
Figure 5. 15 b Storage proteins Function: Storage of amino acids Examples: Casein, the protein of milk, is the major source of amino acids for baby mammals. Plants have storage proteins in their seeds. Ovalbumin is the protein of egg white, used as an amino acid source for the developing embryo. Ovalbumin Amino acids for embryo

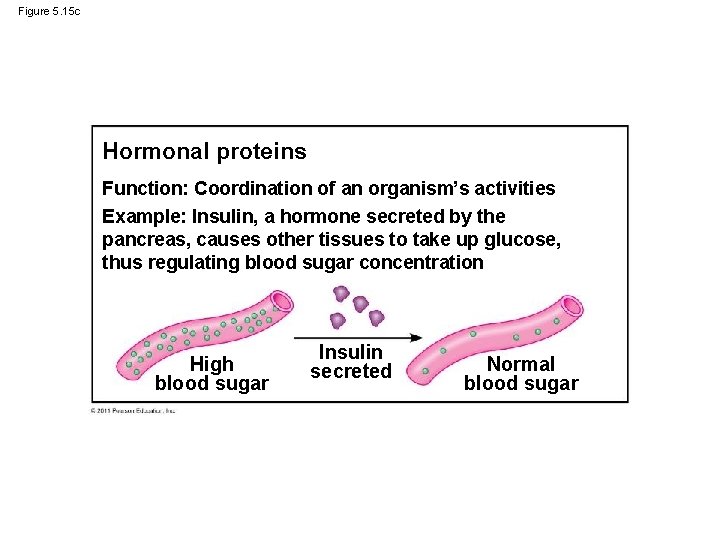
Figure 5. 15 c Hormonal proteins Function: Coordination of an organism’s activities Example: Insulin, a hormone secreted by the pancreas, causes other tissues to take up glucose, thus regulating blood sugar concentration High blood sugar Insulin secreted Normal blood sugar

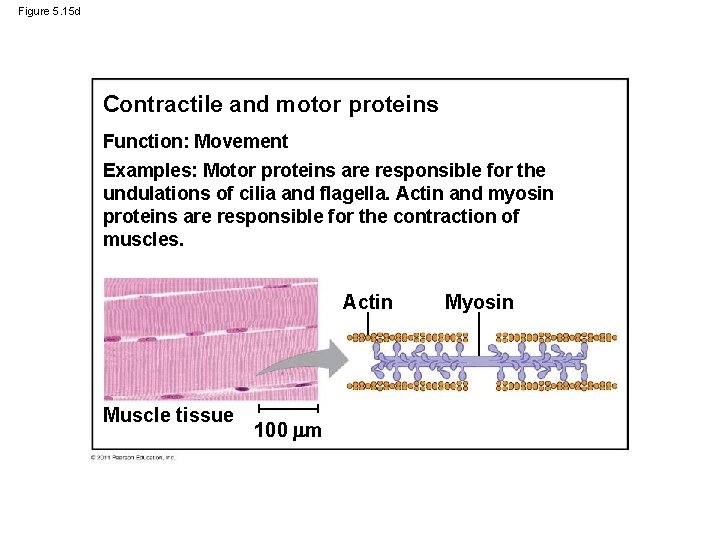
Figure 5. 15 d Contractile and motor proteins Function: Movement Examples: Motor proteins are responsible for the undulations of cilia and flagella. Actin and myosin proteins are responsible for the contraction of muscles. Actin Muscle tissue 100 m Myosin

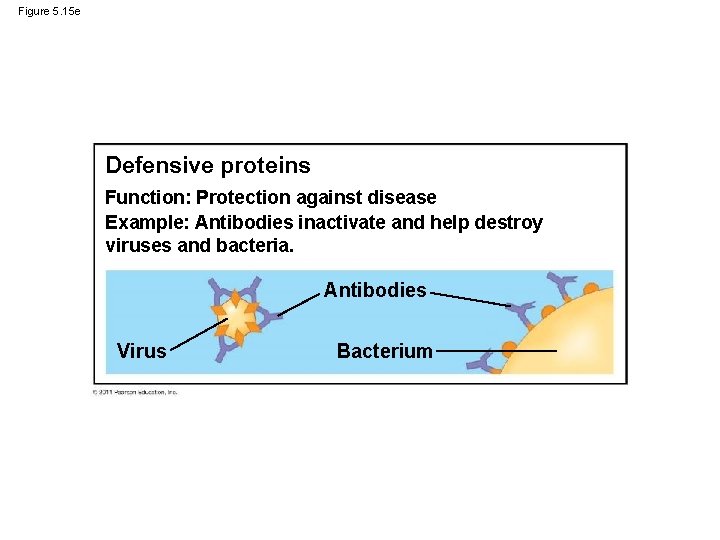
Figure 5. 15 e Defensive proteins Function: Protection against disease Example: Antibodies inactivate and help destroy viruses and bacteria. Antibodies Virus Bacterium

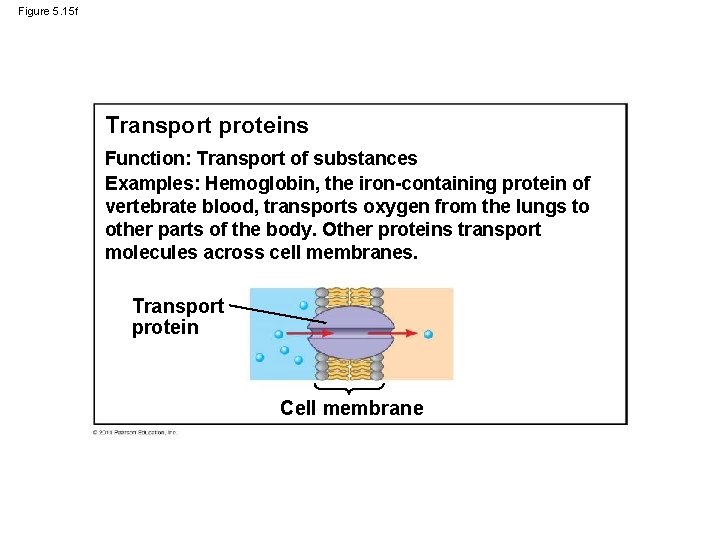
Figure 5. 15 f Transport proteins Function: Transport of substances Examples: Hemoglobin, the iron-containing protein of vertebrate blood, transports oxygen from the lungs to other parts of the body. Other proteins transport molecules across cell membranes. Transport protein Cell membrane

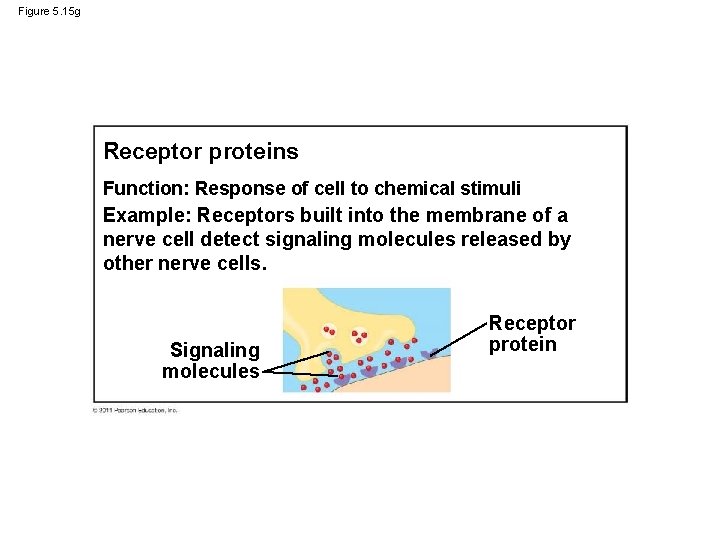
Figure 5. 15 g Receptor proteins Function: Response of cell to chemical stimuli Example: Receptors built into the membrane of a nerve cell detect signaling molecules released by other nerve cells. Signaling molecules Receptor protein

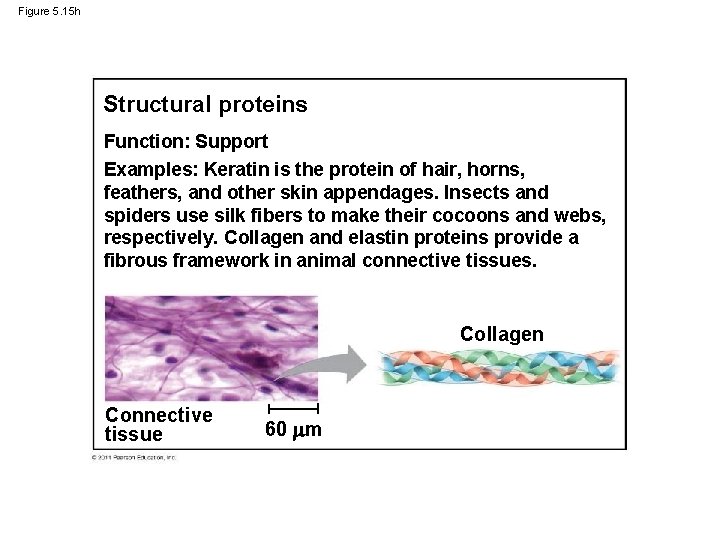
Figure 5. 15 h Structural proteins Function: Support Examples: Keratin is the protein of hair, horns, feathers, and other skin appendages. Insects and spiders use silk fibers to make their cocoons and webs, respectively. Collagen and elastin proteins provide a fibrous framework in animal connective tissues. Collagen Connective tissue 60 m

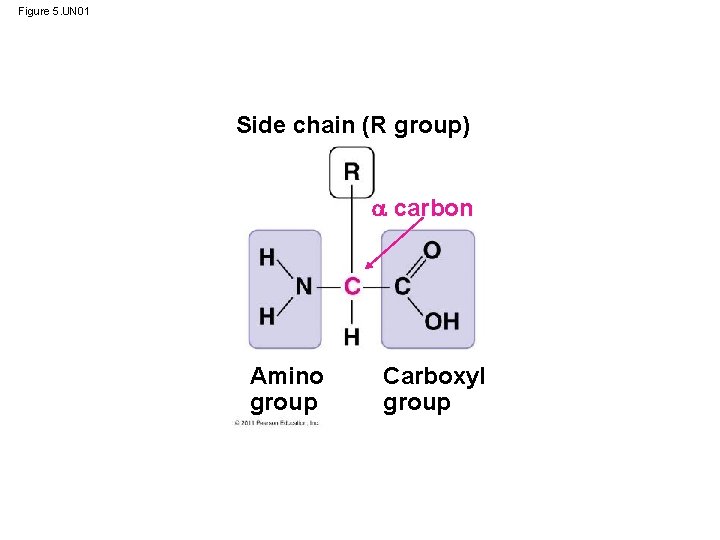
Amino Acid Monomers • carboxyl and amino groups • differ in their properties due to differing side chains, called R groups © 2011 Pearson Education, Inc.

Figure 5. UN 01 Side chain (R group) carbon Amino group Carboxyl group

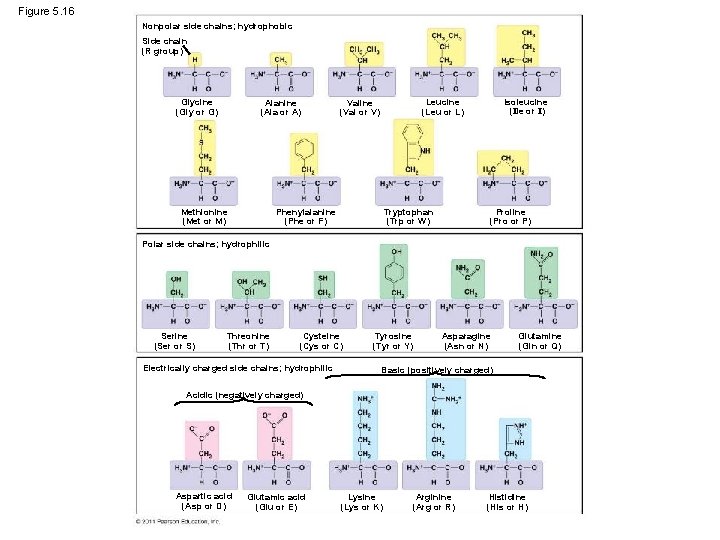
Figure 5. 16 Nonpolar side chains; hydrophobic Side chain (R group) Glycine (Gly or G) Alanine (Ala or A) Methionine (Met or M) Isoleucine (Ile or I) Leucine (Leu or L) Valine (Val or V) Phenylalanine (Phe or F) Tryptophan (Trp or W) Proline (Pro or P) Polar side chains; hydrophilic Serine (Ser or S) Threonine (Thr or T) Cysteine (Cys or C) Electrically charged side chains; hydrophilic Tyrosine (Tyr or Y) Asparagine (Asn or N) Glutamine (Gln or Q) Basic (positively charged) Acidic (negatively charged) Aspartic acid (Asp or D) Glutamic acid (Glu or E) Lysine (Lys or K) Arginine (Arg or R) Histidine (His or H)

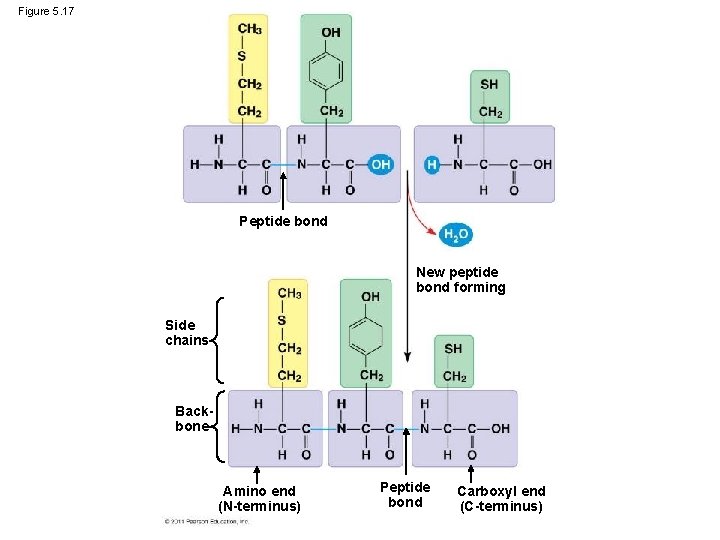
Figure 5. 17 Peptide bond New peptide bond forming Side chains Backbone Amino end (N-terminus) Peptide bond Carboxyl end (C-terminus)

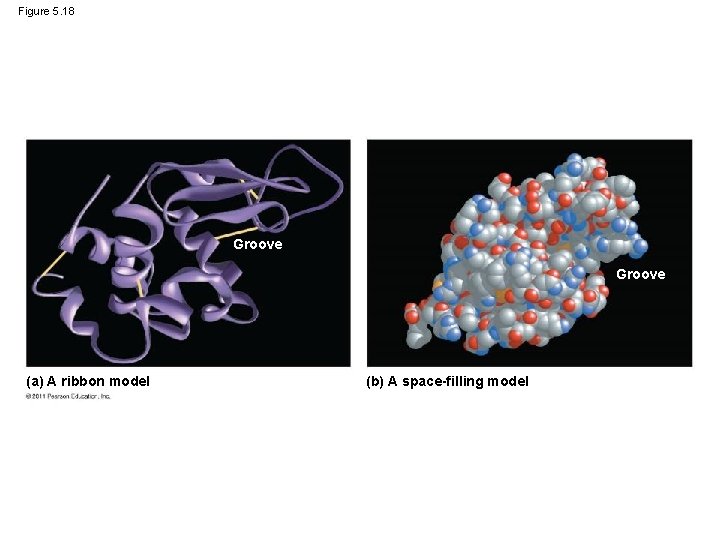
Figure 5. 18 Groove (a) A ribbon model (b) A space-filling model

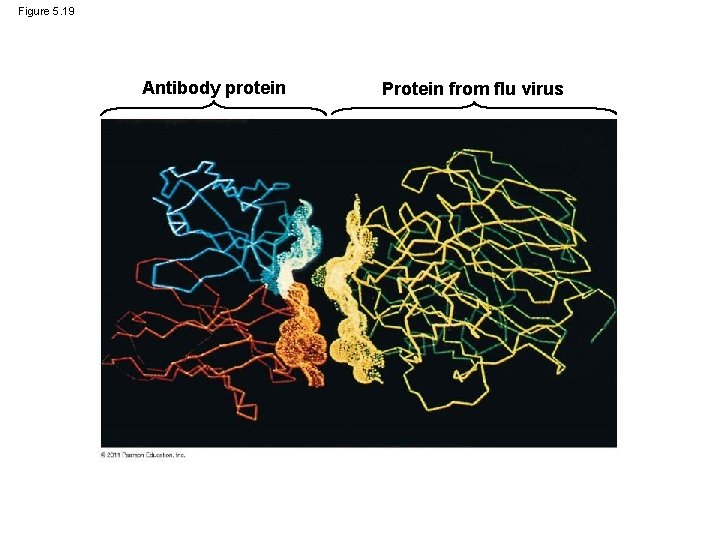
Figure 5. 19 Antibody protein Protein from flu virus

Four Levels of Protein Structure • • primary structure Secondary structure Tertiary Quaternary Animation: Protein Structure Introduction © 2011 Pearson Education, Inc.

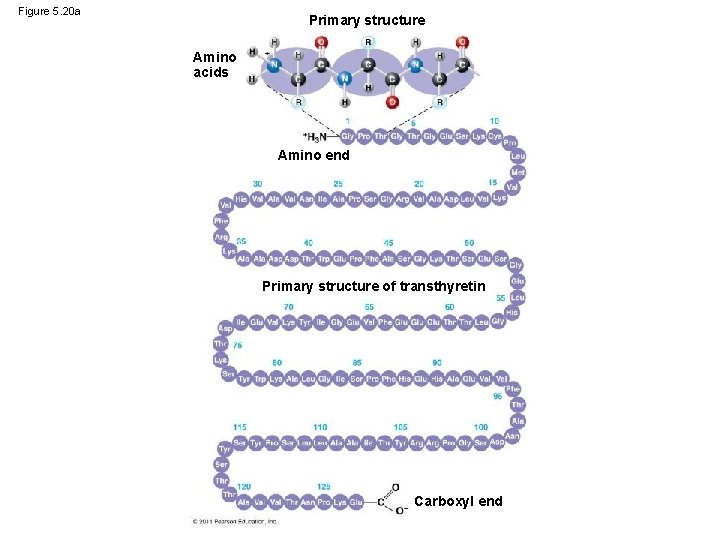
Figure 5. 20 a Primary structure Amino acids Amino end Primary structure of transthyretin Carboxyl end

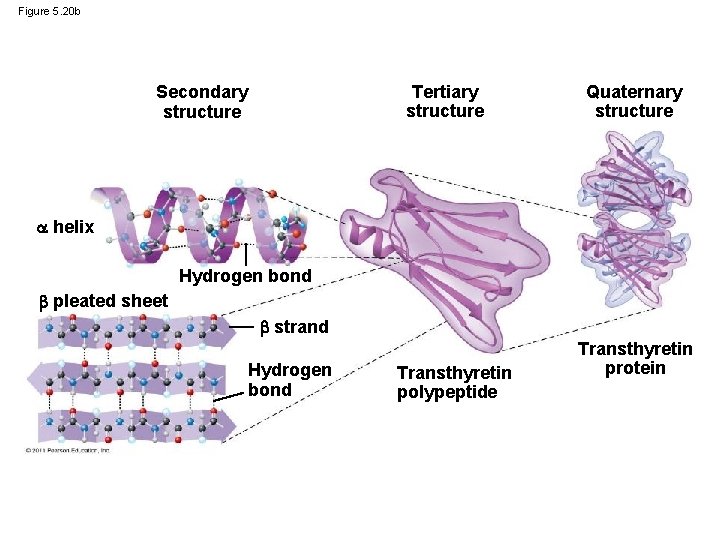
Figure 5. 20 b Tertiary structure Secondary structure Quaternary structure helix Hydrogen bond pleated sheet strand Hydrogen bond Transthyretin polypeptide Transthyretin protein

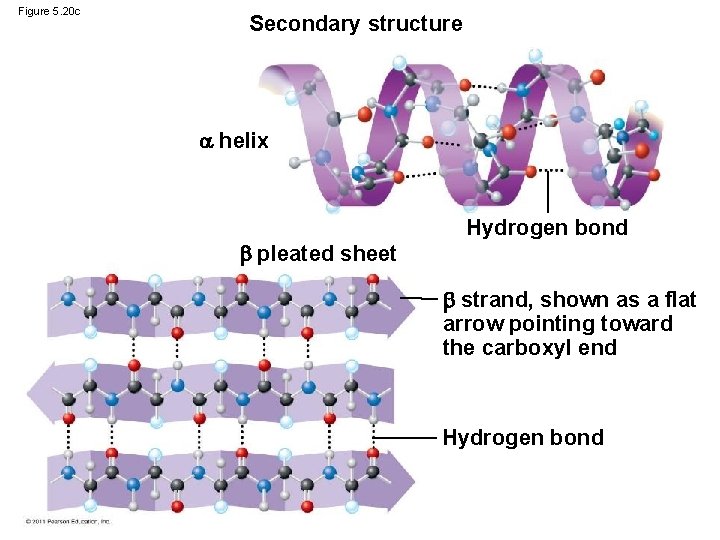
Figure 5. 20 c Secondary structure helix pleated sheet Hydrogen bond strand, shown as a flat arrow pointing toward the carboxyl end Hydrogen bond

Figure 5. 20 d

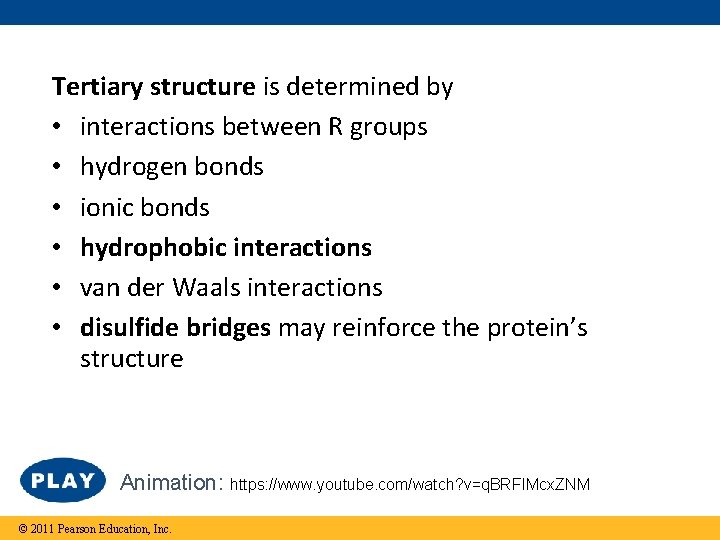
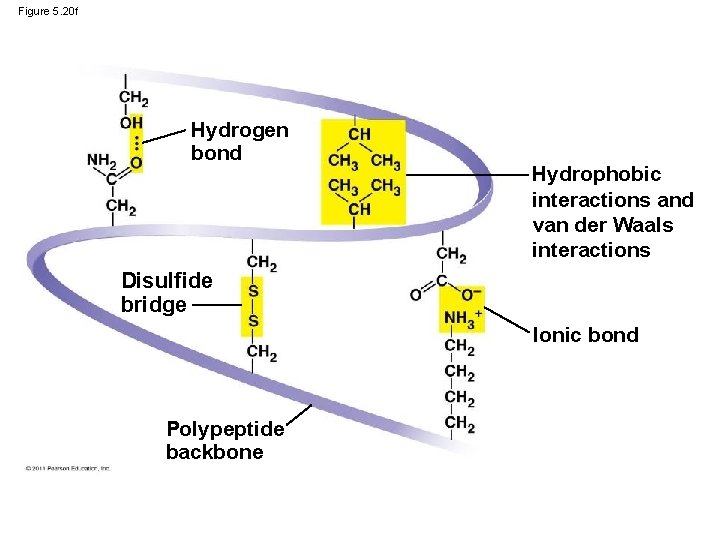
Tertiary structure is determined by • interactions between R groups • hydrogen bonds • ionic bonds • hydrophobic interactions • van der Waals interactions • disulfide bridges may reinforce the protein’s structure Animation: https: //www. youtube. com/watch? v=q. BRFIMcx. ZNM © 2011 Pearson Education, Inc.

Figure 5. 20 f Hydrogen bond Hydrophobic interactions and van der Waals interactions Disulfide bridge Ionic bond Polypeptide backbone

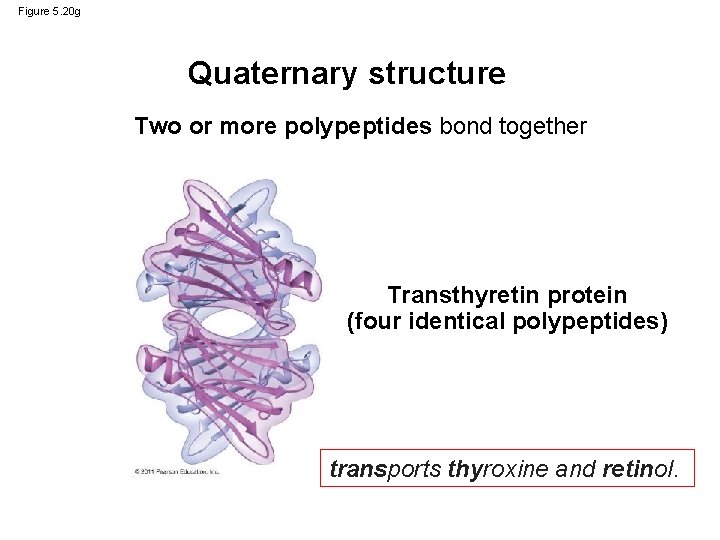
Figure 5. 20 g Quaternary structure Two or more polypeptides bond together Transthyretin protein (four identical polypeptides) transports thyroxine and retinol.

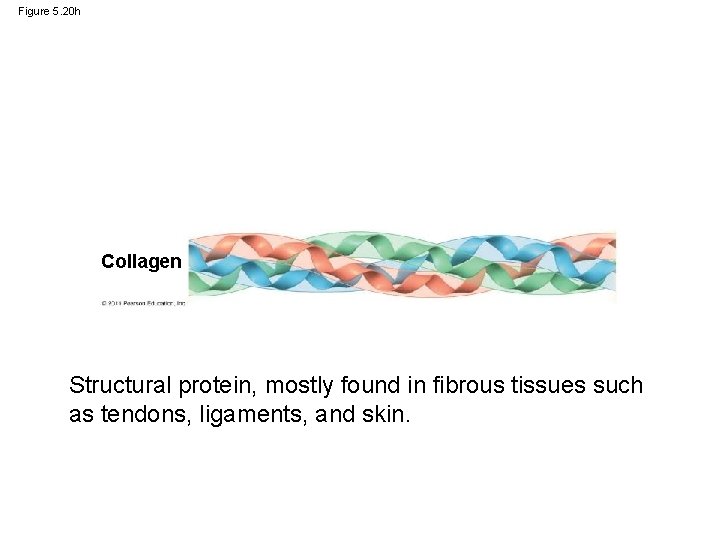
Figure 5. 20 h Collagen Structural protein, mostly found in fibrous tissues such as tendons, ligaments, and skin.

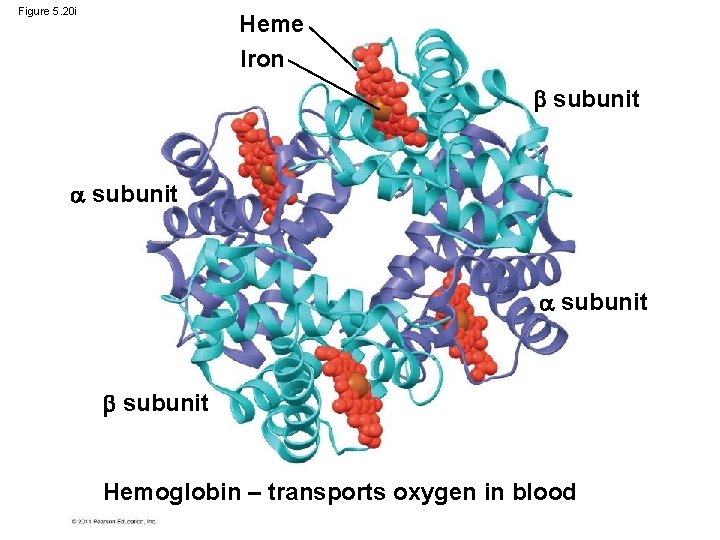
Figure 5. 20 i Heme Iron subunit Hemoglobin – transports oxygen in blood

Figure 5. 20 j Red blood cells

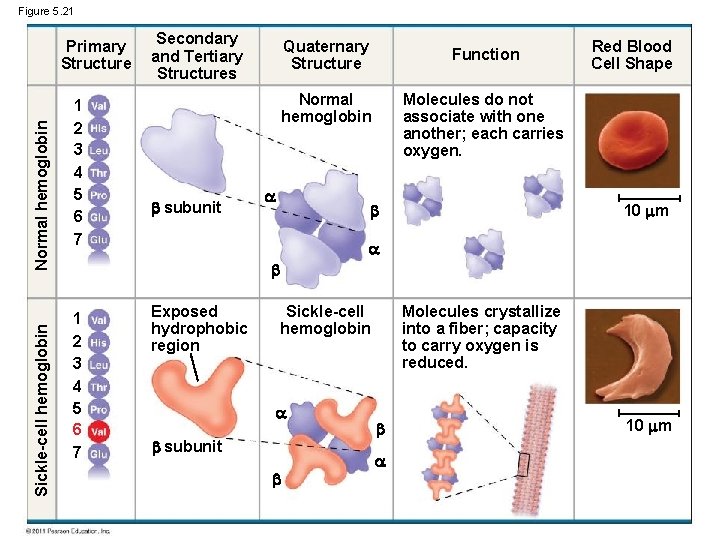
Sickle-Cell Disease: A Change in Primary Structure • A slight change in primary structure can affect a protein’s structure and ability to function • Sickle-cell disease, an inherited blood disorder, results from a single amino acid(valine) substitution (for glutamic acid) in the protein hemoglobin © 2011 Pearson Education, Inc.

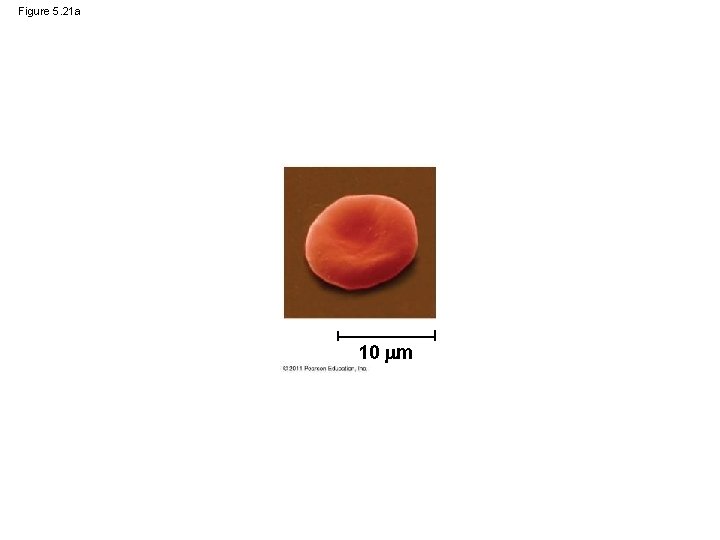
Figure 5. 21 Sickle-cell hemoglobin Normal hemoglobin Primary Structure 1 2 3 4 5 6 7 Secondary and Tertiary Structures Quaternary Structure Function Molecules do not associate with one another; each carries oxygen. Normal hemoglobin subunit Red Blood Cell Shape 10 m 1 2 3 4 5 6 7 Exposed hydrophobic region Sickle-cell hemoglobin subunit Molecules crystallize into a fiber; capacity to carry oxygen is reduced. 10 m

Figure 5. 21 a 10 m

Figure 5. 21 b 10 m

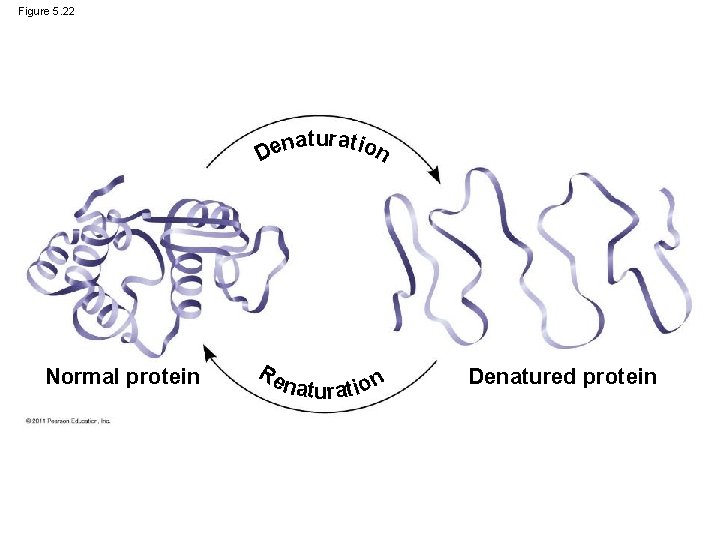
What Determines Protein Structure? • This loss of a protein’s native structure is called denaturation • A denatured protein is biologically inactive © 2011 Pearson Education, Inc.

Figure 5. 22 aturat ion n e D Normal protein Re naturat ion Denatured protein

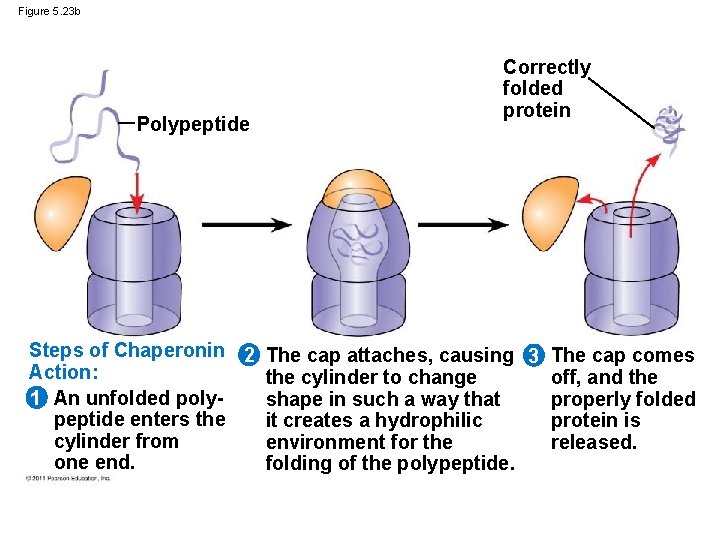
Figure 5. 23 b Polypeptide Correctly folded protein Steps of Chaperonin 2 The cap attaches, causing 3 The cap comes Action: the cylinder to change off, and the 1 An unfolded polyshape in such a way that properly folded peptide enters the it creates a hydrophilic protein is cylinder from environment for the released. one end. folding of the polypeptide.

Techniques to determine protein structure • X-ray crystallography • nuclear magnetic resonance (NMR) spectroscopy • Bioinformatics uses computer programs to predict protein structure from amino acid sequences © 2011 Pearson Education, Inc.

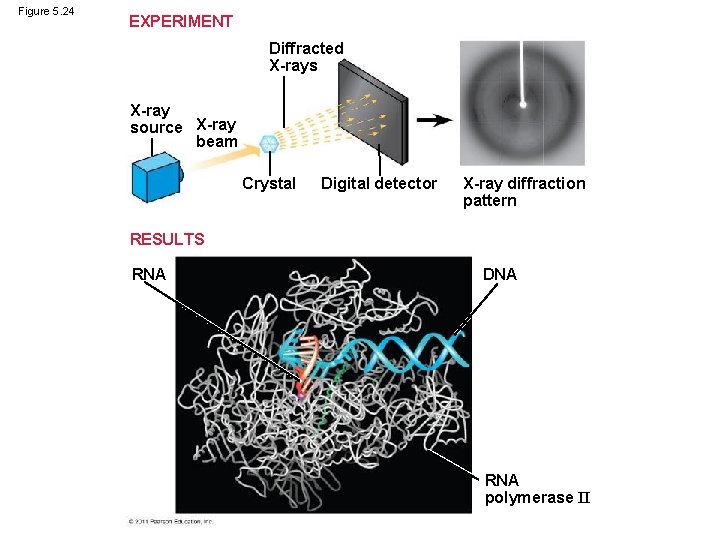
Figure 5. 24 EXPERIMENT Diffracted X-rays X-ray source X-ray beam Crystal Digital detector X-ray diffraction pattern RESULTS RNA DNA RNA polymerase II

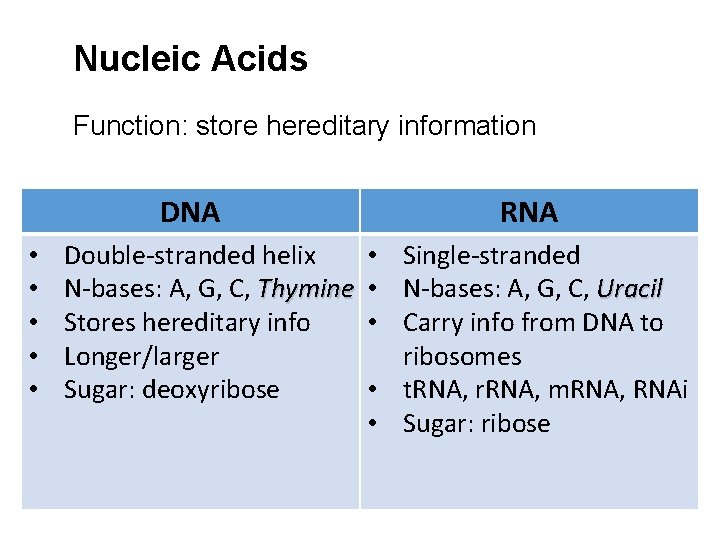
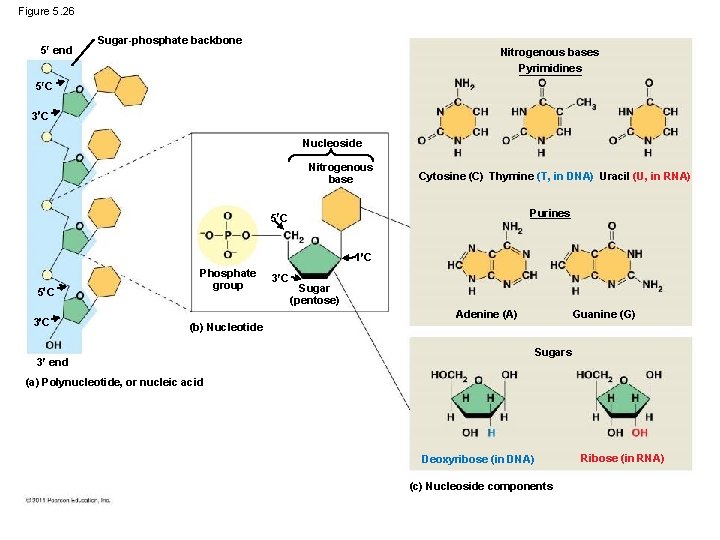
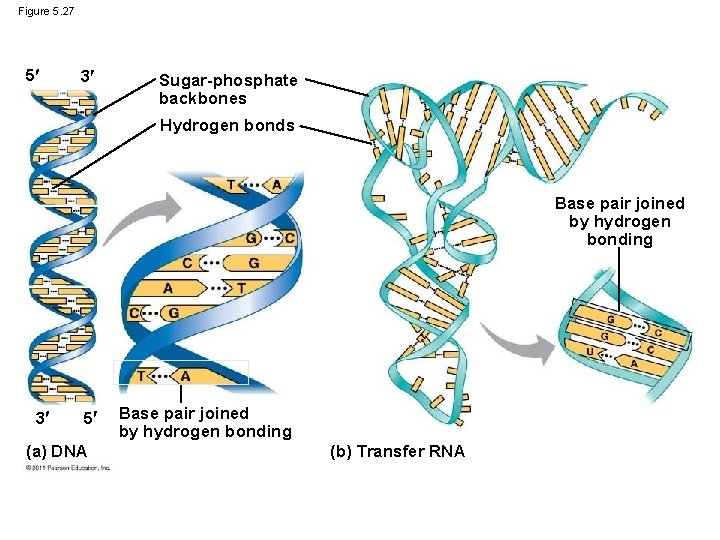
Nucleic Acids Function: store hereditary information DNA • • • Double-stranded helix N-bases: A, G, C, Thymine Stores hereditary info Longer/larger Sugar: deoxyribose RNA • Single-stranded • N-bases: A, G, C, Uracil • Carry info from DNA to ribosomes • t. RNA, r. RNA, m. RNA, RNAi • Sugar: ribose

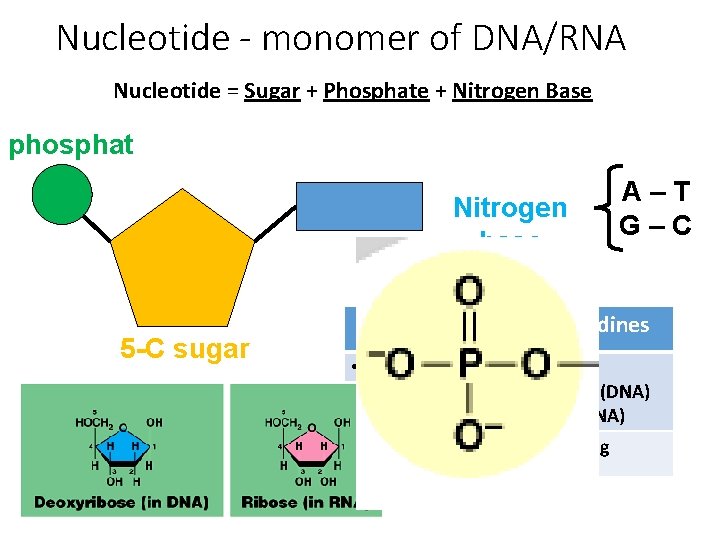
Nucleotide - monomer of DNA/RNA Nucleotide = Sugar + Phosphate + Nitrogen Base phosphat e 5 -C sugar Nitrogen base Purines A–T G–C Pyrimidines • Adenine • Guanine • Cytosine • Thymine (DNA) • Uracil (RNA) • Double ring • Single ring

Figure 5. 26 5 end Sugar-phosphate backbone Nitrogenous bases Pyrimidines 5 C 3 C Nucleoside Nitrogenous base Cytosine (C) Thymine (T, in DNA) Uracil (U, in RNA) Purines 5 C 1 C 5 C 3 C Phosphate group 3 C Sugar (pentose) Guanine (G) Adenine (A) (b) Nucleotide 3 end Sugars (a) Polynucleotide, or nucleic acid Deoxyribose (in DNA) (c) Nucleoside components Ribose (in RNA)

Figure 5. 27 5 3 Sugar-phosphate backbones Hydrogen bonds Base pair joined by hydrogen bonding 3 5 (a) DNA Base pair joined by hydrogen bonding (b) Transfer RNA

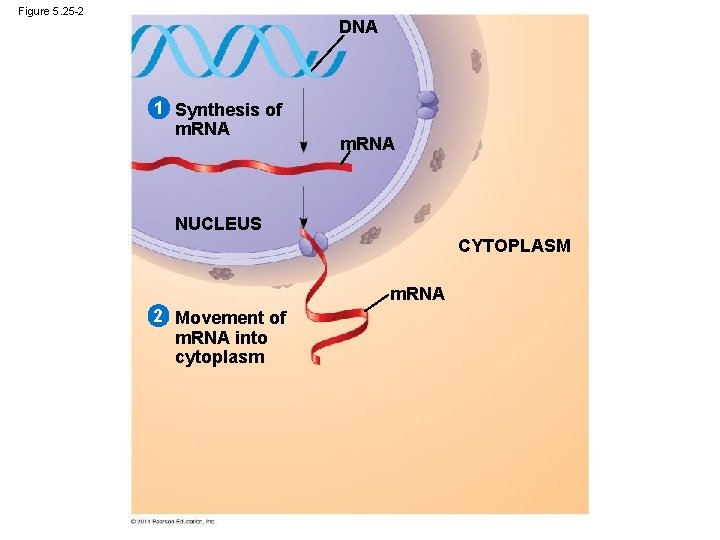
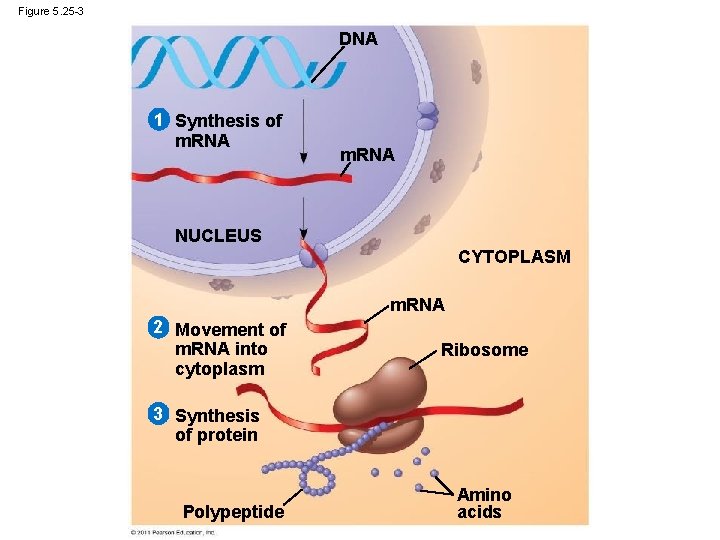
Information flow in a cell: DNA RNA protein

Figure 5. 25 -1 DNA 1 Synthesis of m. RNA NUCLEUS CYTOPLASM

Figure 5. 25 -2 DNA 1 Synthesis of m. RNA NUCLEUS CYTOPLASM m. RNA 2 Movement of m. RNA into cytoplasm

Figure 5. 25 -3 DNA 1 Synthesis of m. RNA NUCLEUS CYTOPLASM m. RNA 2 Movement of m. RNA into cytoplasm Ribosome 3 Synthesis of protein Polypeptide Amino acids

DNA and Proteins as Tape Measures of Evolution • The linear sequences of nucleotides in DNA molecules are passed from parents to offspring • Two closely related species are more similar in DNA than are more distantly related species • Molecular biology can be used to assess evolutionary kinship © 2011 Pearson Education, Inc.

The Theme of Emergent Properties in the Chemistry of Life: A Review • Higher levels of organization result in the emergence of new properties • Organization is the key to the chemistry of life © 2011 Pearson Education, Inc.

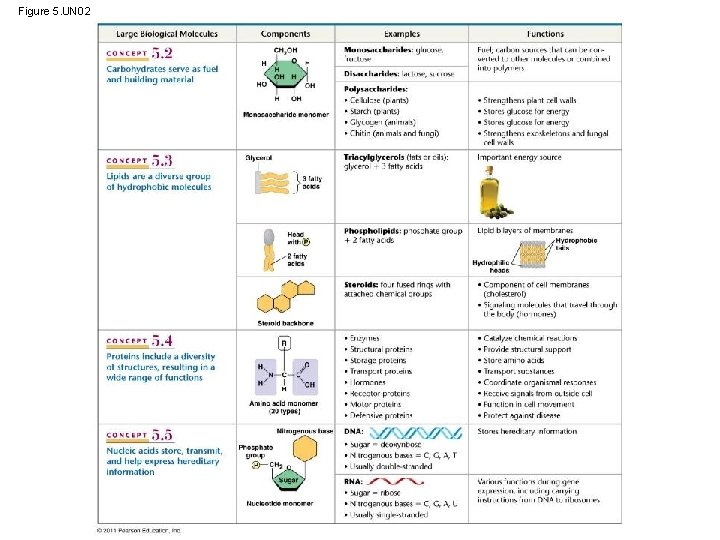
Figure 5. UN 02

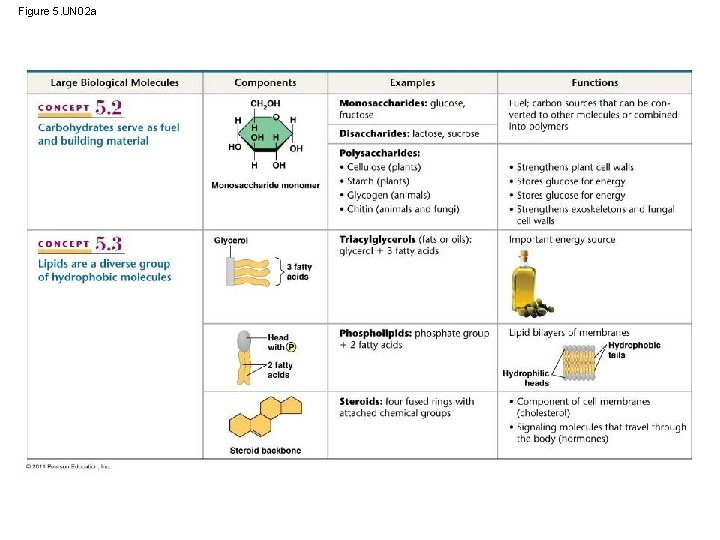
Figure 5. UN 02 a

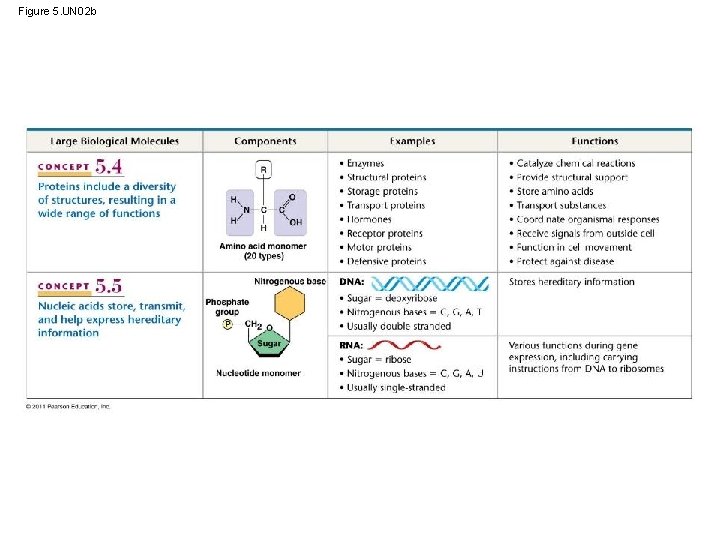
Figure 5. UN 02 b