Chapter 5 OxidationReduction Reactions Chemistry The Molecular Nature




- Slides: 19

Chapter 5 Oxidation–Reduction Reactions Chemistry: The Molecular Nature of Matter, 7 E Jespersen/Hyslop

Oxidation Reduction Reaction Oxidizing Agent (Oxidizer) • Substance that accepts electrons – Accepts electrons from another substance – Substance that is reduced – Cl 2 + 2 e– 2 Cl– Reducing Agent (Reducer) • Substance that donates electrons – Releases electrons to another substance – Substance that is oxidized – Na Na+ + e– 2

REDOX in Aqueous Solution Example: Mix solutions of K 2 Cr 2 O 7 and Fe. SO 4 – Dichromate ion, Cr 2 O 72–, oxidizes Fe 2+ to Fe 3+ – Cr 2 O 72– is reduced to form Cr 3+ – Acidity of mixture decreases as H+ reacts with oxygen to form water Skeletal Eqn. Cr 2 O 72– + Fe 2+ Cr 3+ + Fe 3+ 3

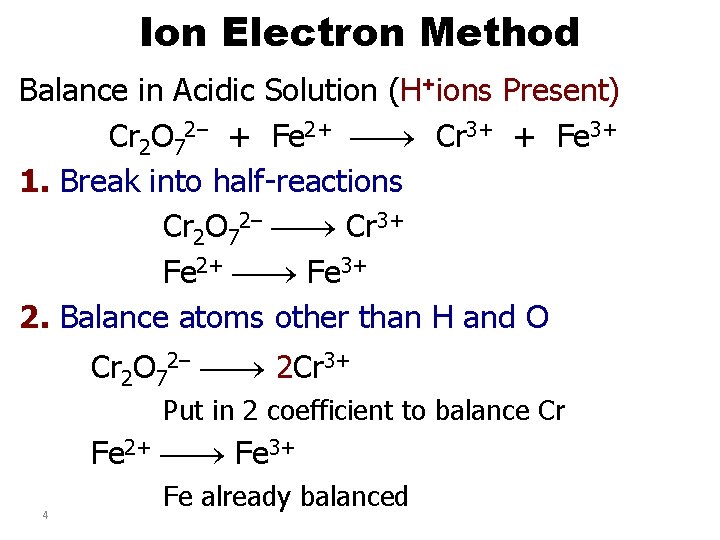
Ion Electron Method Balance in Acidic Solution (H+ions Present) Cr 2 O 72– + Fe 2+ Cr 3+ + Fe 3+ 1. Break into half-reactions Cr 2 O 72– Cr 3+ Fe 2+ Fe 3+ 2. Balance atoms other than H and O Cr 2 O 72– 2 Cr 3+ Put in 2 coefficient to balance Cr Fe 2+ Fe 3+ 4 Fe already balanced

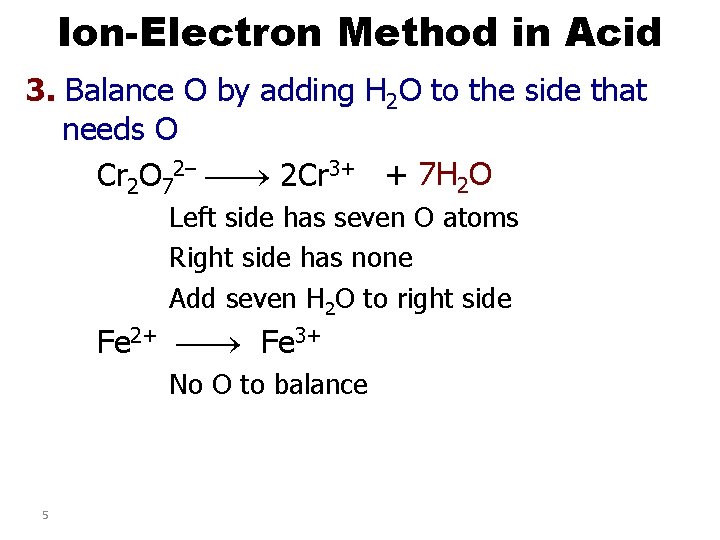
Ion-Electron Method in Acid 3. Balance O by adding H 2 O to the side that needs O Cr 2 O 72– 2 Cr 3+ + 7 H 2 O Left side has seven O atoms Right side has none Add seven H 2 O to right side Fe 2+ Fe 3+ No O to balance 5

Ion-Electron Method in Acid 4. Balance H by adding H+ to side that needs H 14 H+ + Cr 2 O 72– 2 Cr 3+ + 7 H 2 O Right side has fourteen H atoms Left side has none Add fourteen H+ to left side Fe 2+ Fe 3+ No H to balance 6

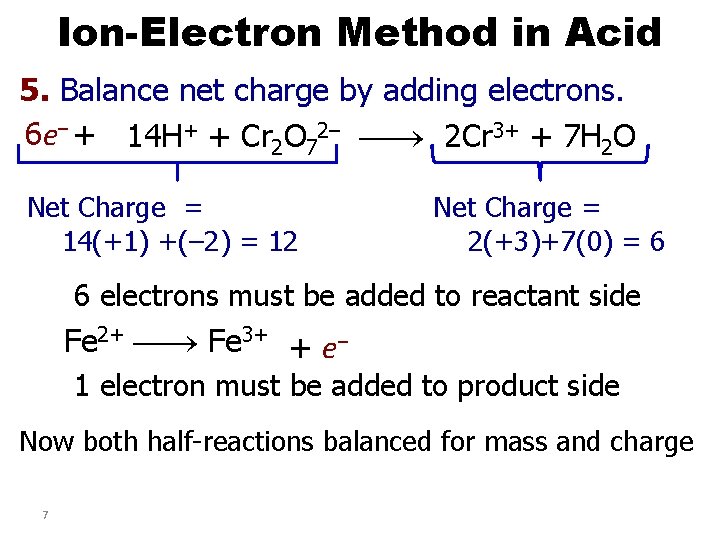
Ion-Electron Method in Acid 5. Balance net charge by adding electrons. 6 e– + 14 H+ + Cr 2 O 72– 2 Cr 3+ + 7 H 2 O Net Charge = 14(+1) +(– 2) = 12 Net Charge = 2(+3)+7(0) = 6 6 electrons must be added to reactant side Fe 2+ Fe 3+ + e– 1 electron must be added to product side Now both half-reactions balanced for mass and charge 7

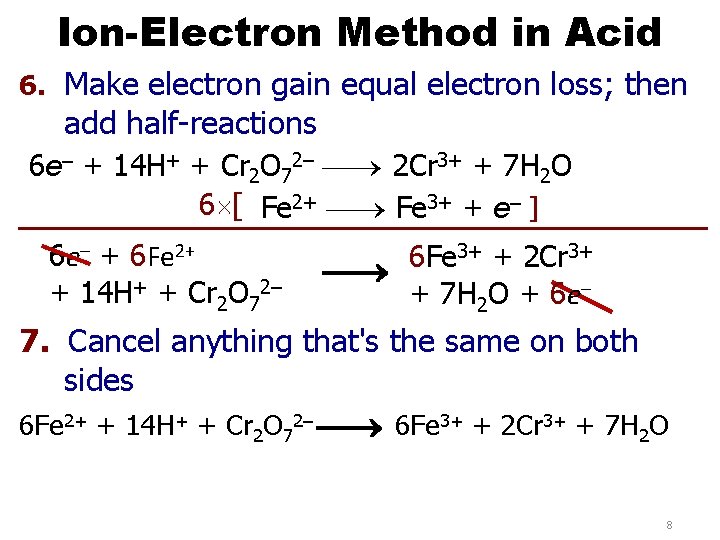
Ion-Electron Method in Acid 6. Make electron gain equal electron loss; then add half-reactions 6 e– + 14 H+ + Cr 2 O 72– 2 Cr 3+ + 7 H 2 O 6 [ Fe 2+ Fe 3+ + e– ] 6 e– + 6 Fe 2+ + 14 H+ + Cr 2 O 72– 3+ + 2 Cr 3+ 6 Fe + 7 H 2 O + 6 e– 7. Cancel anything that's the same on both sides 6 Fe 2+ + 14 H+ + Cr 2 O 72– 6 Fe 3+ + 2 Cr 3+ + 7 H 2 O 8

Ion-Electron in Basic Solution • The simplest way to balance an equation in basic solution Use steps 1 – 7 above, then 8. Add the same number of OH– to both sides of the equation as there are H+ 9. Combine H+ and OH– to form H 2 O 10. Cancel any H 2 O that you can from both sides 9

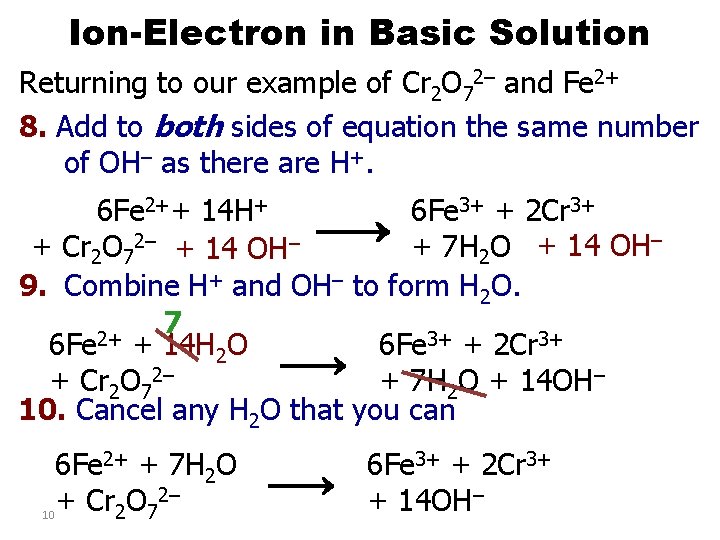
Ion-Electron in Basic Solution Returning to our example of Cr 2 O 72– and Fe 2+ 8. Add to both sides of equation the same number of OH– as there are H+. 6 Fe 2++ 14 H+ 6 Fe 3+ + 2 Cr 3+ 2– – + 7 H 2 O + 14 OH– + Cr 2 O 7 + 14 OH 9. Combine H+ and OH– to form H 2 O. 7 2+ 6 Fe + 14 H 2 O 6 Fe 3+ + 2 Cr 3+ 2– + Cr 2 O 7 + 7 H 2 O + 14 OH– 10. Cancel any H 2 O that you can 6 Fe 2+ + 7 H 2 O + Cr 2 O 72– 10 6 Fe 3+ + 2 Cr 3+ + 14 OH–

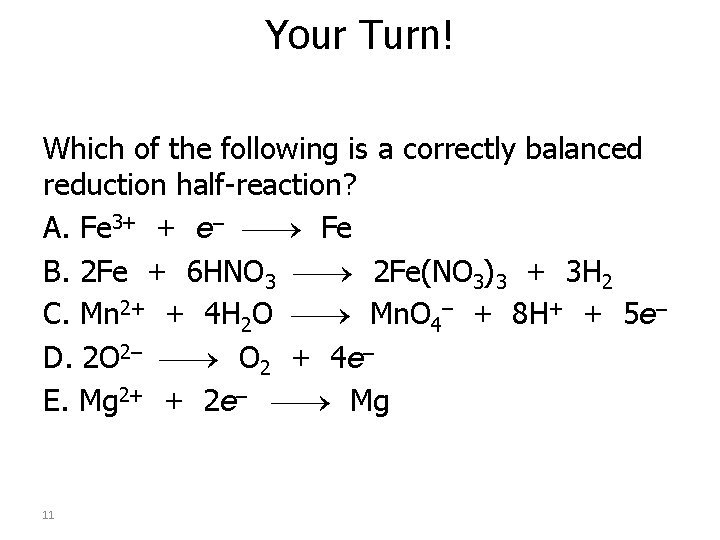
Your Turn! Which of the following is a correctly balanced reduction half-reaction? A. Fe 3+ + e– Fe B. 2 Fe + 6 HNO 3 2 Fe(NO 3)3 + 3 H 2 C. Mn 2+ + 4 H 2 O Mn. O 4– + 8 H+ + 5 e– D. 2 O 2– O 2 + 4 e– E. Mg 2+ + 2 e– Mg 11

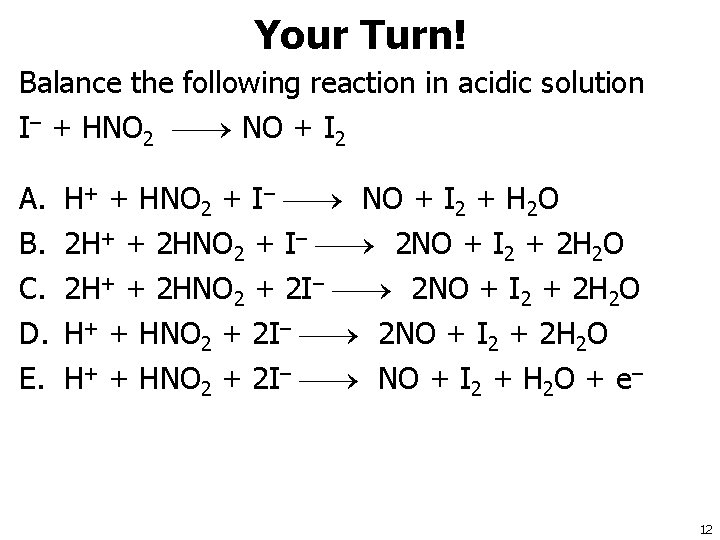
Your Turn! Balance the following reaction in acidic solution I– + HNO 2 NO + I 2 A. B. C. D. E. H+ + HNO 2 + I– NO + I 2 + H 2 O 2 H+ + 2 HNO 2 + I– 2 NO + I 2 + 2 H 2 O 2 H+ + 2 HNO 2 + 2 I– 2 NO + I 2 + 2 H 2 O H+ + HNO 2 + 2 I– NO + I 2 + H 2 O + e– 12

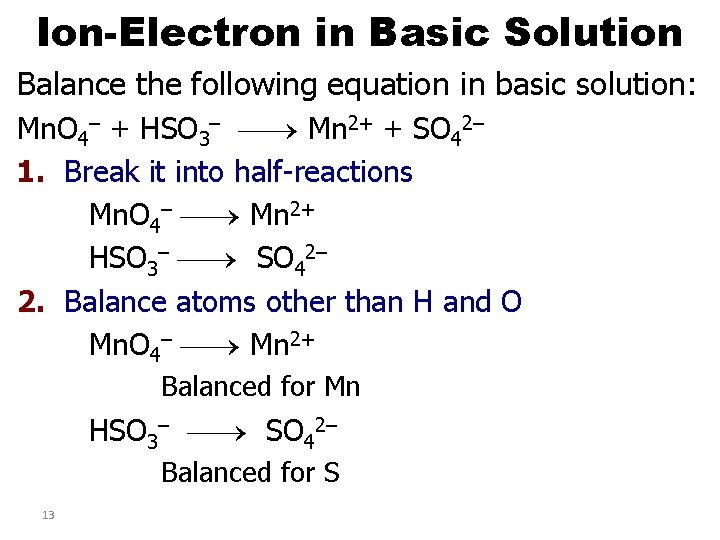
Ion-Electron in Basic Solution Balance the following equation in basic solution: Mn. O 4– + HSO 3– Mn 2+ + SO 42– 1. Break it into half-reactions Mn. O 4– Mn 2+ HSO 3– SO 42– 2. Balance atoms other than H and O Mn. O 4– Mn 2+ Balanced for Mn HSO 3– SO 42– Balanced for S 13

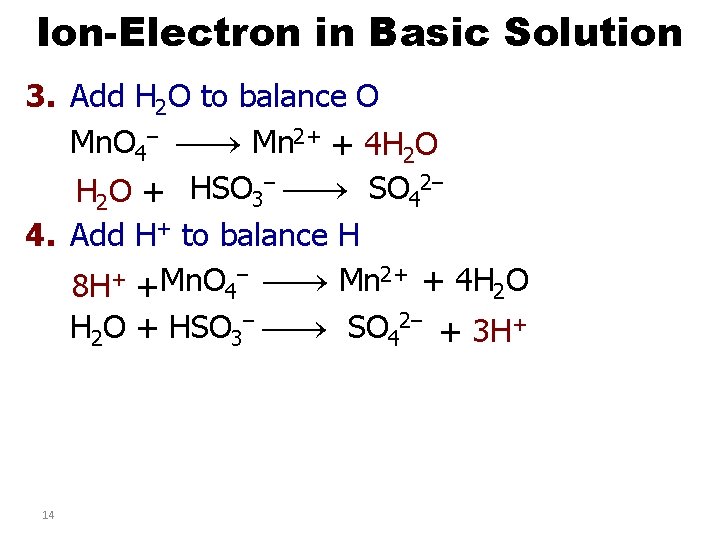
Ion-Electron in Basic Solution 3. Add H 2 O to balance O Mn. O 4– Mn 2+ + 4 H 2 O + HSO 3– SO 42– 4. Add H+ to balance H – Mn 2+ + 4 H O + Mn. O 8 H + 4 2 H 2 O + HSO 3– SO 42– + 3 H+ 14

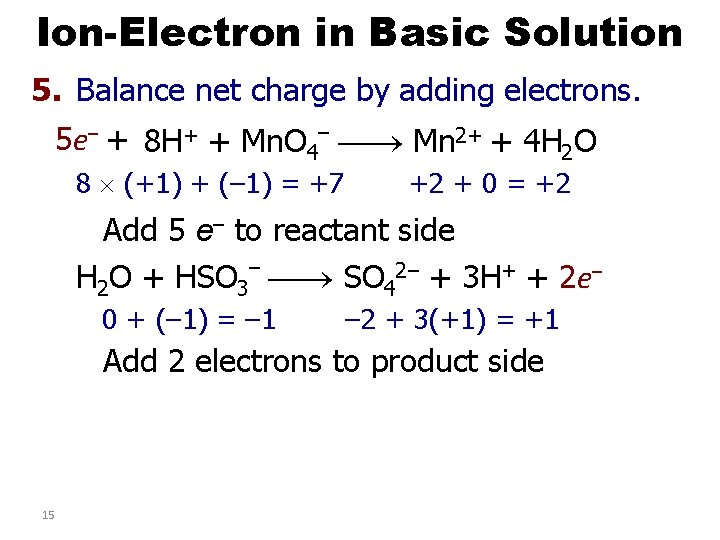
Ion-Electron in Basic Solution 5. Balance net charge by adding electrons. 5 e– + 8 H+ + Mn. O 4– Mn 2+ + 4 H 2 O 8 (+1) + (– 1) = +7 +2 + 0 = +2 Add 5 e– to reactant side H 2 O + HSO 3– SO 42– + 3 H+ + 2 e– 0 + (– 1) = – 1 – 2 + 3(+1) = +1 Add 2 electrons to product side 15

Ion-Electron in Basic Solution 6. Make electron gain equal electron loss 2 [ 5 e– + 8 H+ + Mn. O 4– Mn 2+ + 4 H 2 O – 2 + 3 H+ + 2 e– H O + HSO SO 5 [ 2 3 4 – Must multiply Mn half-reaction by 2 – Must multiply S half-reaction by 5 – Now have 10 electrons on each side 16 ] ]

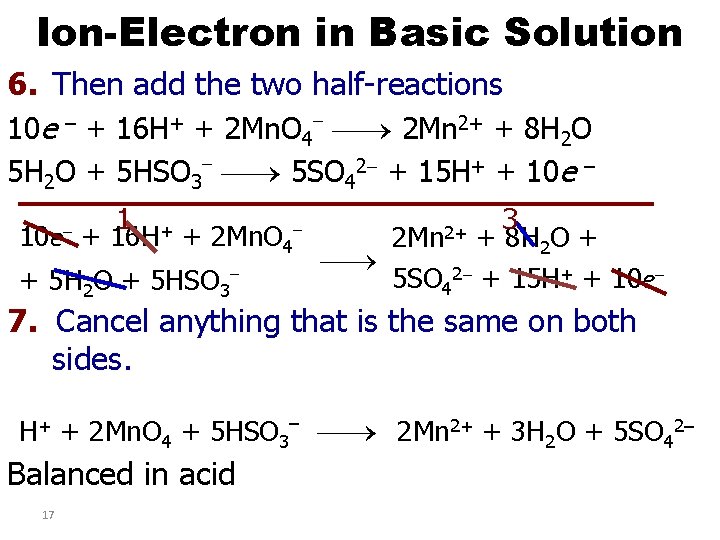
Ion-Electron in Basic Solution 6. Then add the two half-reactions 10 e – + 16 H+ + 2 Mn. O 4 2 Mn 2+ + 8 H 2 O 5 H 2 O + 5 HSO 3 5 SO 42 + 15 H+ + 10 e – 10 e– 1 + + 16 H + 2 Mn. O 4 + 5 H 2 O + 5 HSO 3 3 + 8 H 2 O + 5 SO 42 + 15 H+ + 10 e– 2 Mn 2+ 7. Cancel anything that is the same on both sides. H+ + 2 Mn. O 4 + 5 HSO 3– 2 Mn 2+ + 3 H 2 O + 5 SO 42– Balanced in acid 17

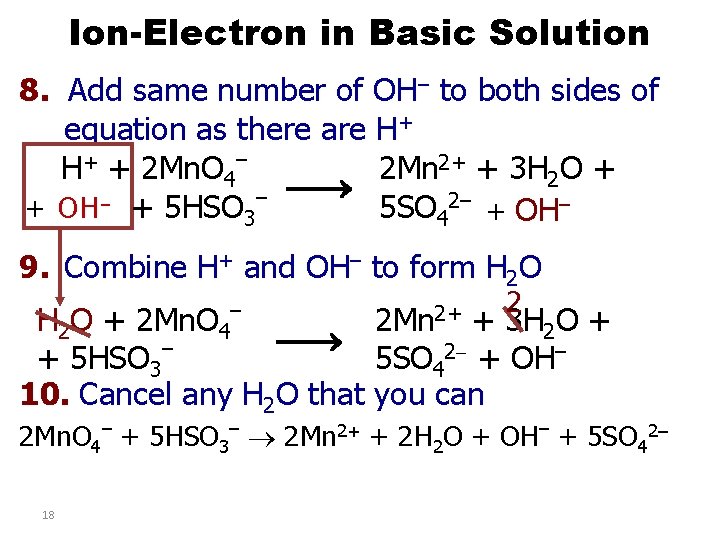
Ion-Electron in Basic Solution 8. Add same number of OH– to both sides of equation as there are H+ H+ + 2 Mn. O 4– 2 Mn 2+ + 3 H 2 O + – – 5 SO 42– + OH + 5 HSO 3 9. Combine H+ and OH– to form H 2 O – 2 Mn 2+ 2 H 2 O + 2 Mn. O 4 + 3 H 2 O + – + 5 HSO 3 5 SO 42 + OH– 10. Cancel any H 2 O that you can 2 Mn. O 4– + 5 HSO 3– 2 Mn 2+ + 2 H 2 O + OH– + 5 SO 42– 18

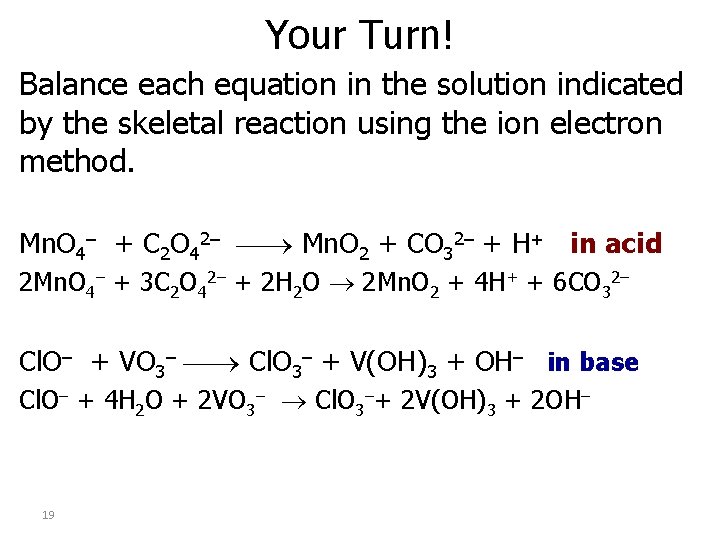
Your Turn! Balance each equation in the solution indicated by the skeletal reaction using the ion electron method. Mn. O 4– + C 2 O 42– Mn. O 2 + CO 32– + H+ in acid 2 Mn. O 4– + 3 C 2 O 42– + 2 H 2 O 2 Mn. O 2 + 4 H+ + 6 CO 32– Cl. O– + VO 3– Cl. O 3– + V(OH)3 + OH– in base Cl. O– + 4 H 2 O + 2 VO 3– Cl. O 3–+ 2 V(OH)3 + 2 OH– 19