Chapter 5 Orbital Filling Diagrams and Electron Dot

Chapter 5 Orbital Filling Diagrams and Electron Dot Diagrams
Orbital Filling Diagrams • Orbital filling diagrams illustrate the pairing of the electrons in an atom.

Remember how we illustrate the sublevels with boxes.
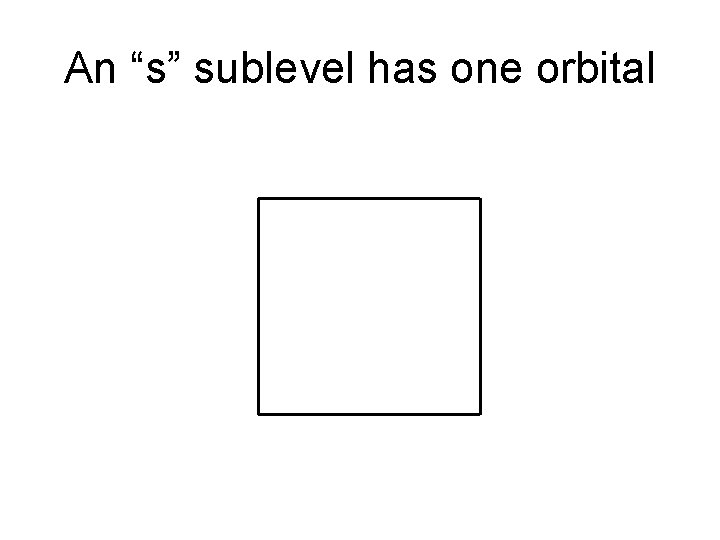
An “s” sublevel has one orbital
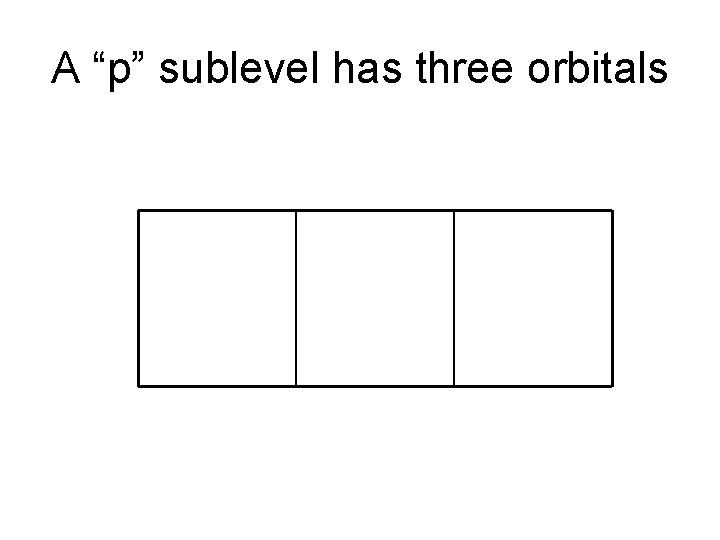
A “p” sublevel has three orbitals
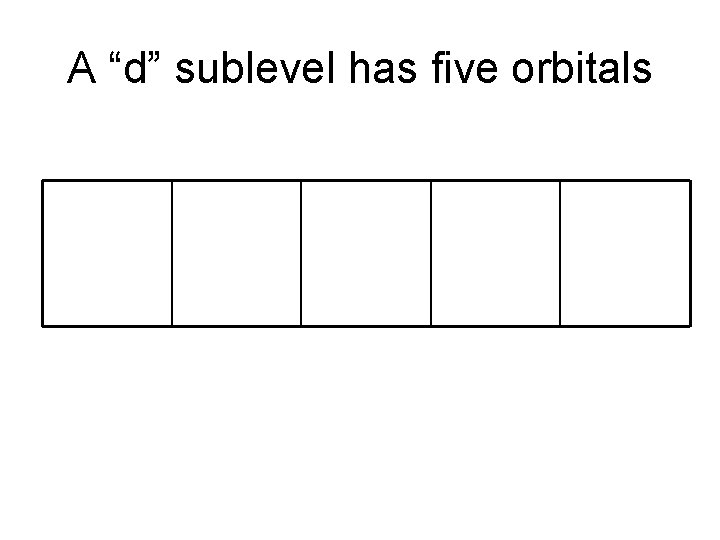
A “d” sublevel has five orbitals
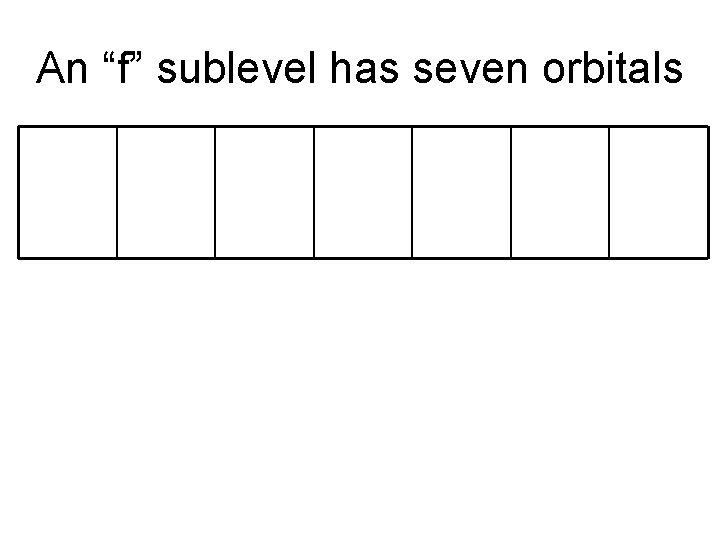
An “f” sublevel has seven orbitals
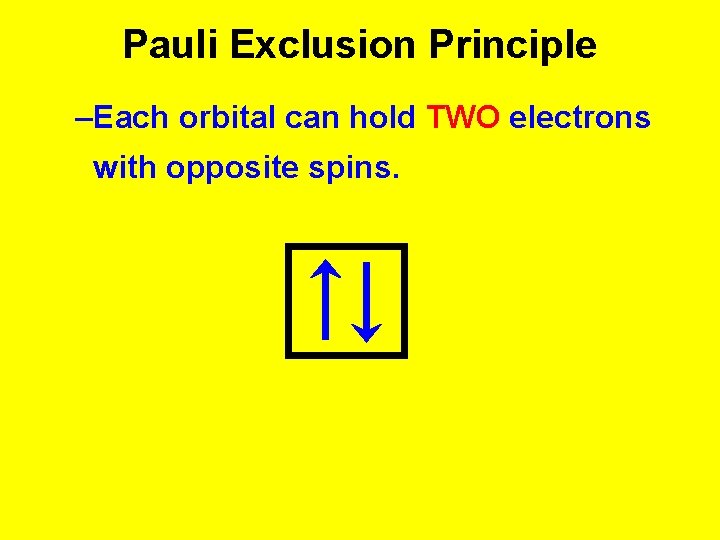
Pauli Exclusion Principle –Each orbital can hold TWO electrons with opposite spins.

Hund’s Rule • Within a sublevel, place one e- per orbital before pairing them. • All electrons in singly filled orbitals have the same direction of spin.
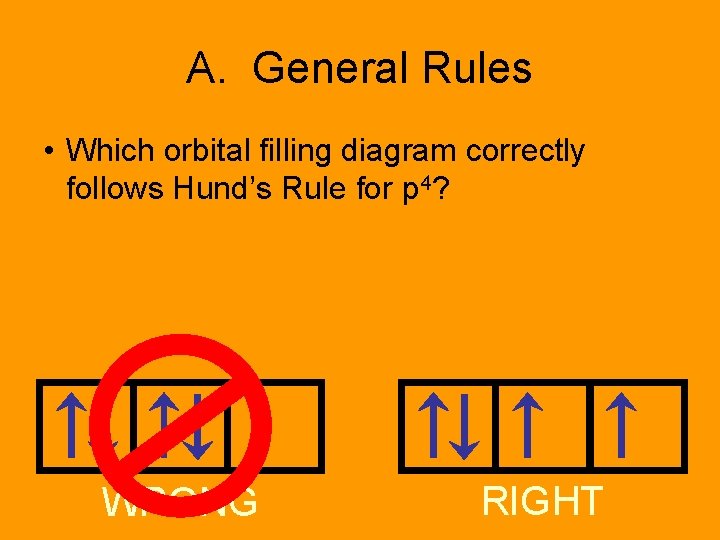
A. General Rules • Which orbital filling diagram correctly follows Hund’s Rule for p 4? WRONG RIGHT
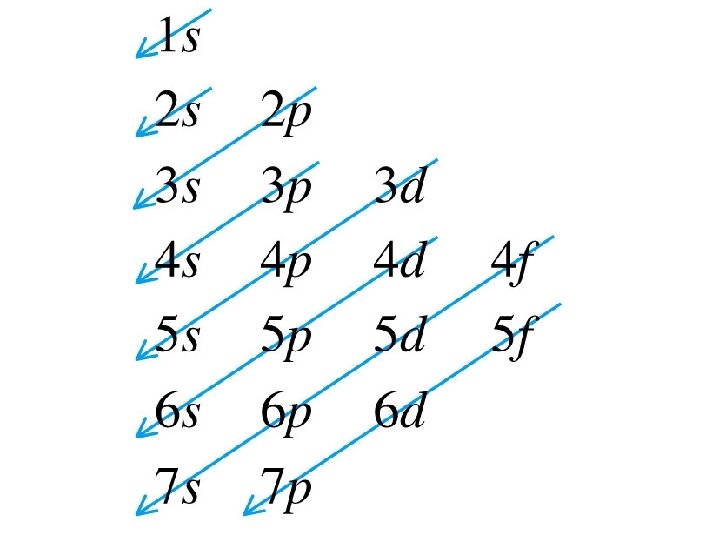
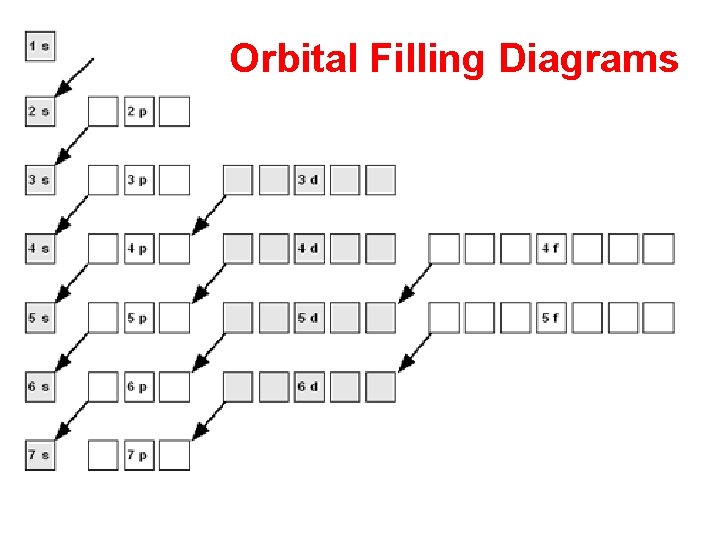
Orbital Filling Diagrams
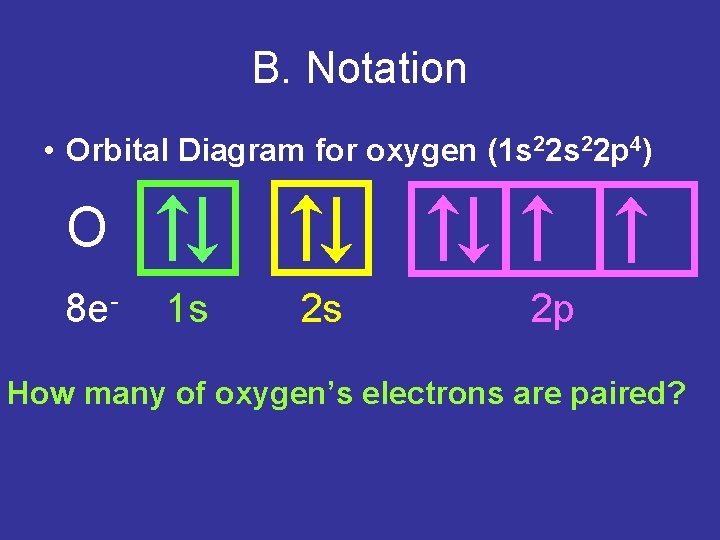
B. Notation • Orbital Diagram for oxygen (1 s 22 p 4) O 8 e- 1 s 2 s 2 p How many of oxygen’s electrons are paired?
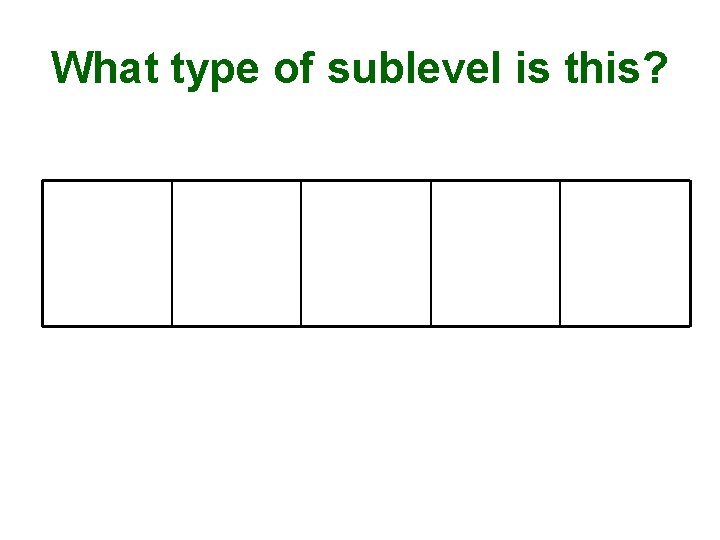
What type of sublevel is this?
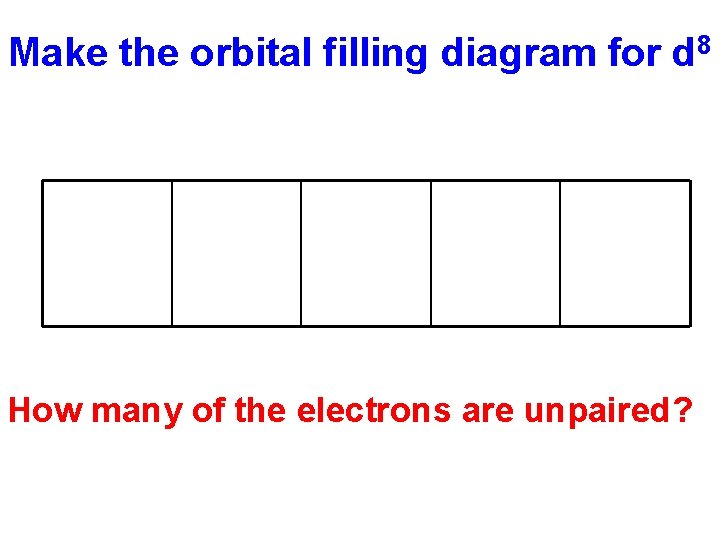
Make the orbital filling diagram for d 8 How many of the electrons are unpaired?
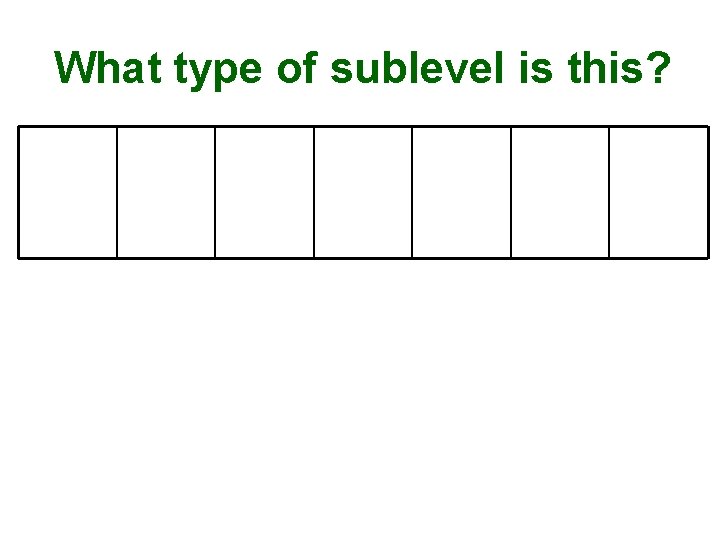
What type of sublevel is this?
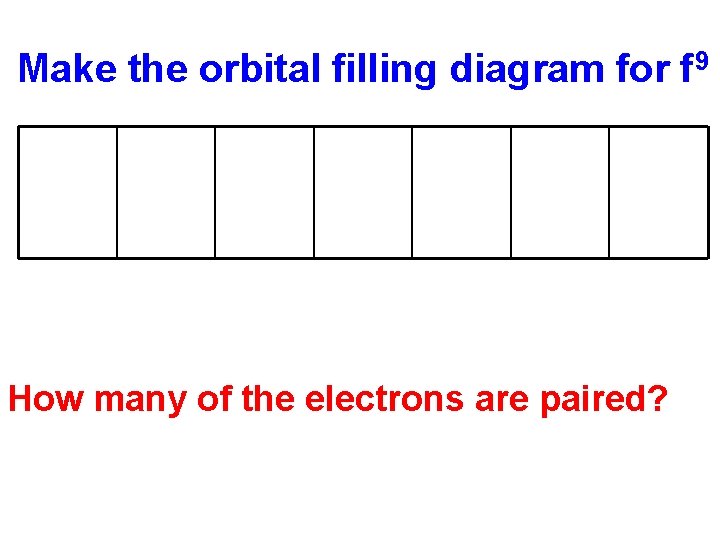
Make the orbital filling diagram for f 9 How many of the electrons are paired?
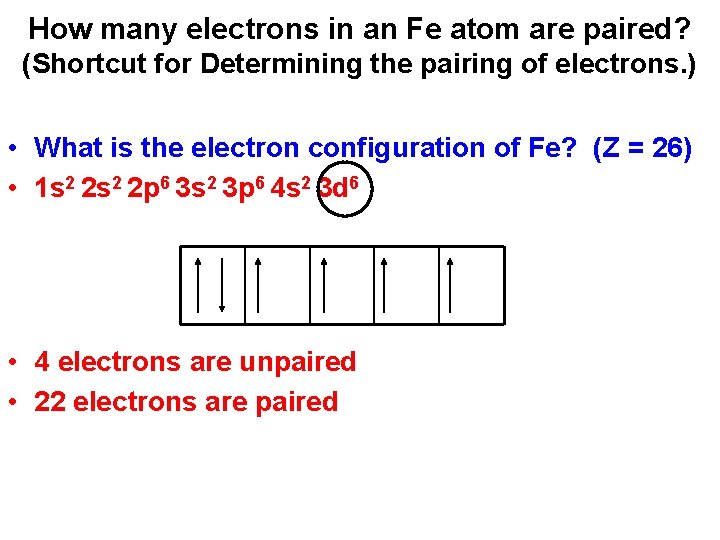
How many electrons in an Fe atom are paired? (Shortcut for Determining the pairing of electrons. ) • What is the electron configuration of Fe? (Z = 26) • 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 • 4 electrons are unpaired • 22 electrons are paired
Electron Dot Diagrams Step 1: Write the electron configuration
Electron Dot Diagrams Step 2: Determine the number of valence electrons. The valence electrons are the electrons in the outer energy level.
Electron Dot Diagrams Step 3: Write the symbol of the element surrounded by valence electrons. Place one electron on each side (top, bottom, right, left) before pairing them up.

• Draw the electron dot diagram for oxygen.

Draw the electron dot diagram for selenium. (Z = 34)
Draw the electron dot diagram for chlorine. (Z = 17)

Draw the electron dot diagram for hydrogen. (Z = 1)

HCl vs H 2 O • When hydrogen combines with chorine it does so in a 1: 1 ratio. • When hydrogen combines with oxygen it does so in a 2: 1 ratio. Why?
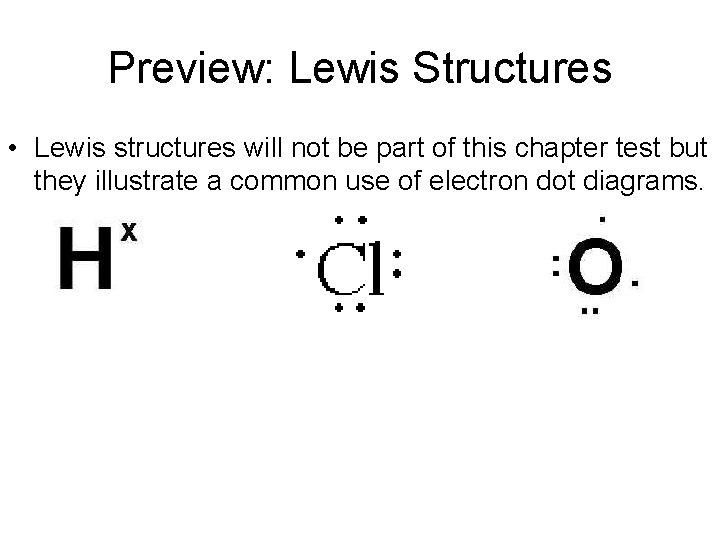
Preview: Lewis Structures • Lewis structures will not be part of this chapter test but they illustrate a common use of electron dot diagrams.
- Slides: 27