Chapter 5 Online Resource Naming Type I Type













![Acid Naming Summary NO Oxygen Always: Hydro[anion root]ic acid Ex’s: HF, HS, HN hydrofluoric Acid Naming Summary NO Oxygen Always: Hydro[anion root]ic acid Ex’s: HF, HS, HN hydrofluoric](https://slidetodoc.com/presentation_image_h2/067aab096040a374204b7273fc211a29/image-20.jpg)
- Slides: 20

Chapter 5 Online Resource Naming Type I, Type III and Type IV Compounds Naming Acids

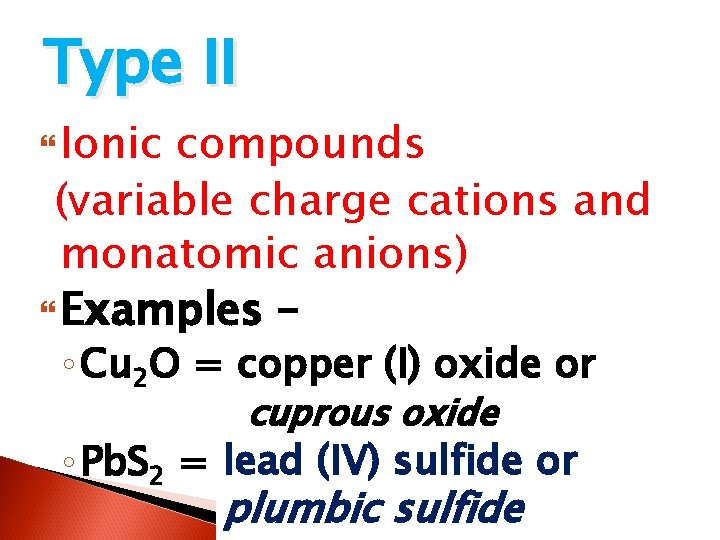
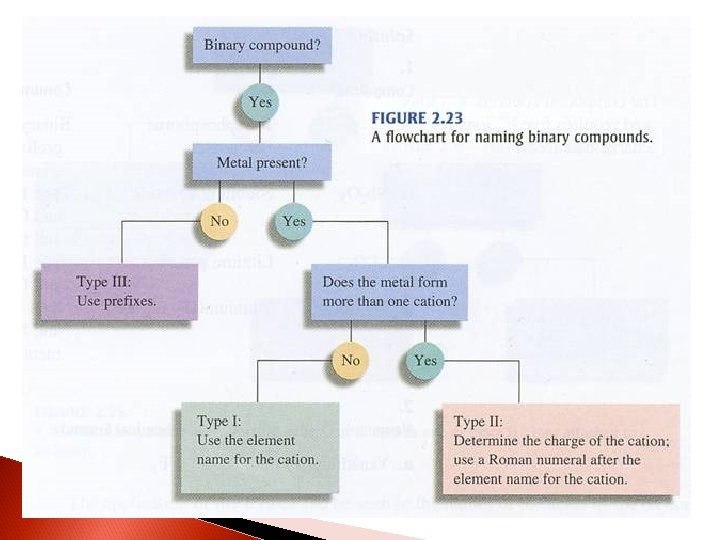
Type I Ionic compounds (monatomic cations & anions) Examples – ◦ Li 2 O = lithium oxide aluminum nitride ◦ Al. N = ? ? ?

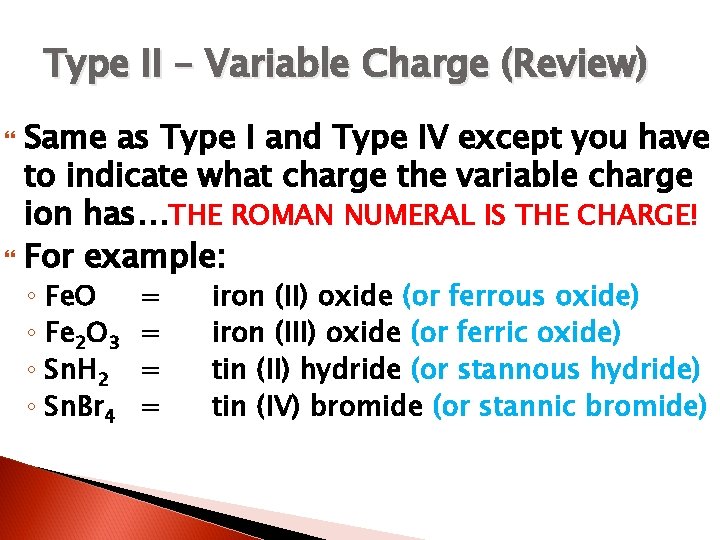
Type II Ionic compounds (variable charge cations and monatomic anions) Examples – ◦ Cu 2 O = copper (I) oxide or cuprous oxide lead (IV) sulfide or ◦ Pb. S 2 = ? ? ? plumbic sulfide

Type II – Variable Charge (Review) Same as Type I and Type IV except you have to indicate what charge the variable charge ion has…THE ROMAN NUMERAL IS THE CHARGE! For example: ◦ Fe. O ◦ Fe 2 O 3 ◦ Sn. H 2 ◦ Sn. Br 4 = = iron (II) oxide (or ferrous oxide) iron (III) oxide (or ferric oxide) tin (II) hydride (or stannous hydride) tin (IV) bromide (or stannic bromide)

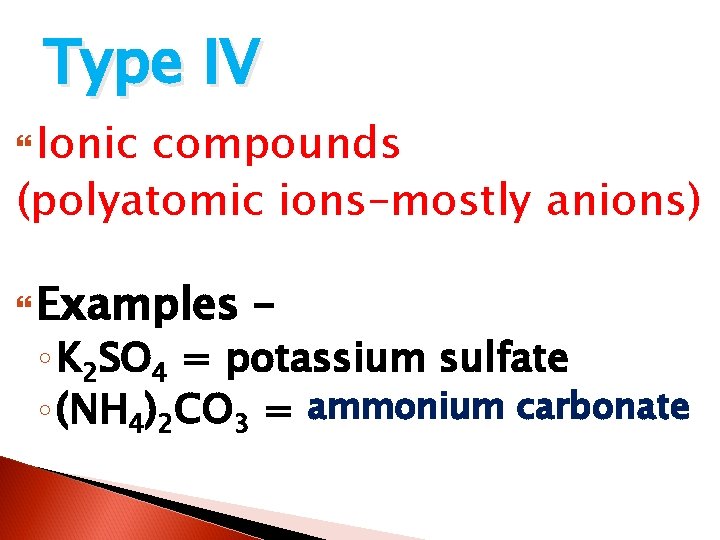
Type IV Ionic compounds (polyatomic ions–mostly anions) Examples – ◦ K 2 SO 4 = potassium sulfate ammonium carbonate ◦ (NH 4)2 CO 3 = ? ? ?

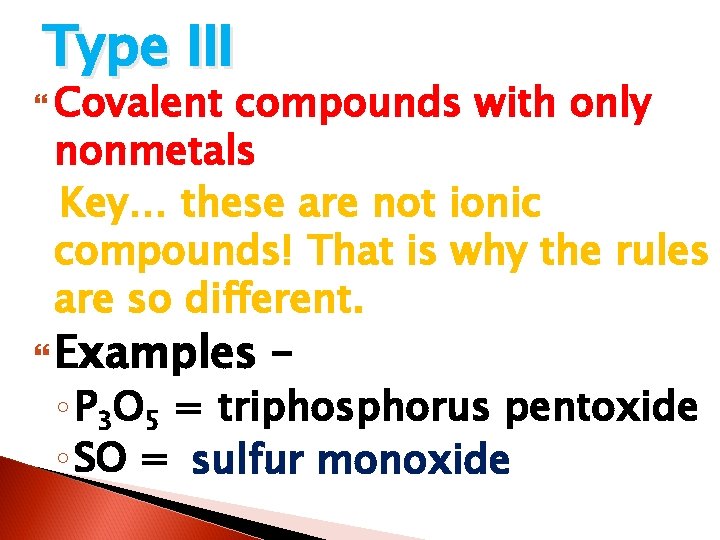
Type III Covalent compounds with only nonmetals Key… these are not ionic compounds! That is why the rules are so different. Examples – ◦ P 3 O 5 = triphosphorus pentoxide ◦ SO = ? ? ? sulfur monoxide

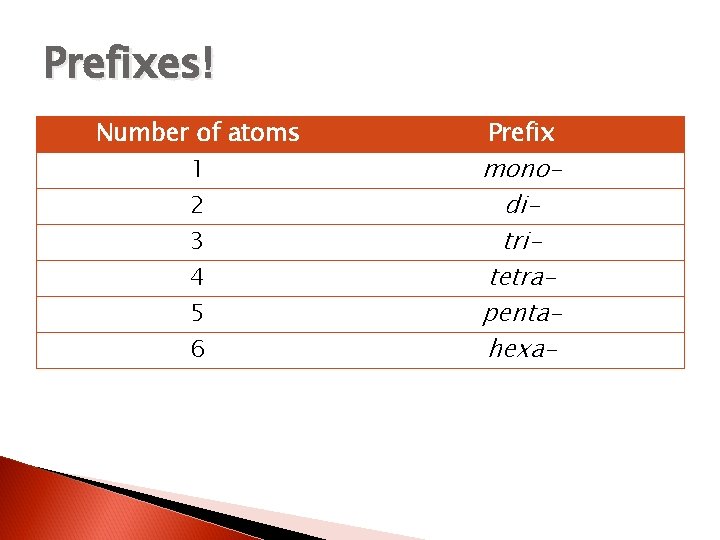
Prefixes! Number of atoms 1 2 3 4 5 6 Prefix monoditritetrapentahexa-

Some TYPE III tips… Never use “mono” at the beginning of the compound name, if there’s only 1 of the first atom no prefix is necessary. If the vowel at the end of the prefix plus the vowel at the start of the element name is awkward, (e. g. mono- and oxide) the vowel on the prefix gets dropped. (monoxide, tetroxide)

Name NO 1. Name 1 st element: nitrogen 2. Name 2 nd element like an anion: oxide 3. Use prefixes to denote numbers of atoms ◦ 1 nitrogen: if only 1 of the first atom, no prefix ◦ 1 oxygen: mono◦ nitrogen monoxide

Name BF 3 1. 2. 3. Name the first element using the element name: boron Name the second element as if it were an anion: fluoride (instead of fluorine) Use prefixes to denote numbers of atoms: ◦ 1 boron: if only 1 of the first atom, no prefix ◦ 3 fluorine: tri◦ boron trifluoride


Two super awesome websites for polyatomic ion practice! Charge practice: http: //www. chemfiles. com/flash/polyions 1. h tml Name/formula practice: http: //www. chemfiles. com/flash/polyions. ht ml (Really tough ion/compound practice: http: //www. chemfiles. com/flash/polyatomic_ ions. html)

Acid Naming (our last type!) Acids = molecules that produce H+ ions in water First recognized for the sour taste of their solutions: e. g. citric acid in lemons and limes is responsible for that sour taste An acid is an anion with one or more H+ cations bonded to it that it can let go of when dissolved in water.

Acid Naming Rule #1 If the anion does NOT contain oxygen… HCl 1. Use the prefix hydro- and the suffix –ic after the “root” of the anion: chloride hydrochloric acid How do you know it’s an acid? It starts with H!

More Practice… Acid Naming Rule #1 If the anion does NOT contain oxygen… HCN 1. Use the prefix hydro- and the suffix –ic after the “root” of the anion: cyanide hydrocyanic acid

Acid Naming Rule #2 If the anion DOES contain oxygen… H 2 SO 4 2. Take the root name of the central anion and add a suffix: -ic when the anion ends in –ate -ous when anion names in –ite hydrogen sulfate should be called… …sulfuric acid

Acid Naming Rule #2 If the anion DOES contain oxygen… HNO 2 2. Take the root name of the central anion and add a suffix: -ic when the anion ends in –ate -ous when anion names in –ite hydrogen nitrite should be called… …nitrous acid

Acid Naming Rule #3 If the anion DOES contain oxygen, but isn’t a simple “-ate” or an “-ite”… perchlorate chlorite hypochlorite HCl. O 4 HCl. O 3 HCl. O 2 HCl. O perchloric acid chlorous acid hypochlorous acid per - used for anions with one more oxygen than an ‘-ate” hypo - used for anions with one less oxygen than an ‘-ite”

More Practice…Acid Naming Rule #3 If the anion DOES contain oxygen, but isn’t a simple “-ate” or an “-ite”… perbromate bromite hypobromite HBr. O 4 HBr. O 3 HBr. O 2 HBr. O perbromic acid bromous acid hypobromous acid per - used for anions with one more oxygen than an ‘-ate” hypo - used for anions with one less oxygen than an ‘-ite”
![Acid Naming Summary NO Oxygen Always Hydroanion rootic acid Exs HF HS HN hydrofluoric Acid Naming Summary NO Oxygen Always: Hydro[anion root]ic acid Ex’s: HF, HS, HN hydrofluoric](https://slidetodoc.com/presentation_image_h2/067aab096040a374204b7273fc211a29/image-20.jpg)
Acid Naming Summary NO Oxygen Always: Hydro[anion root]ic acid Ex’s: HF, HS, HN hydrofluoric acid, hydrosulfuric acid, hydronitric acid CONTAINS Oxygen What is the key anion? How many oxygens does it have compared to the “ -ate” or “-ite”? (prefix)[anion root](suffix) acid Ex’s: HNO 3, HNO 2, HNO 4 nitric acid, nitrous acid, hyponitrous acid, pernitric acid