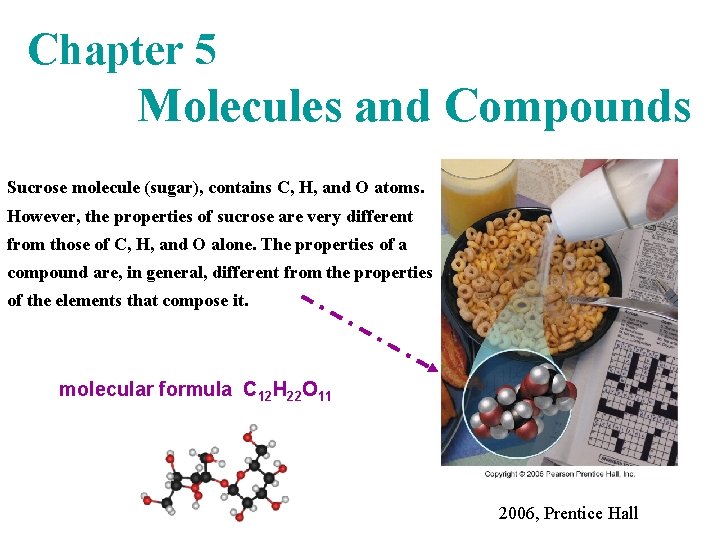
Chapter 5 Molecules and Compounds Sucrose molecule sugar












- Slides: 73

Chapter 5 Molecules and Compounds Sucrose molecule (sugar), contains C, H, and O atoms. However, the properties of sucrose are very different from those of C, H, and O alone. The properties of a compound are, in general, different from the properties of the elements that compose it. molecular formula C 12 H 22 O 11 2006, Prentice Hall

CHAPTER OUTLINE § § § § § Law of Constant Composition Octet Rule and Ions Ionic Charges Definitions of Molecules and Compounds Types of Compounds Binary Ionic Compounds (Type I and Type II) Polyatomic Ions Binary Molecular Compounds Naming Acids and Oxyacids Formula Mass 2

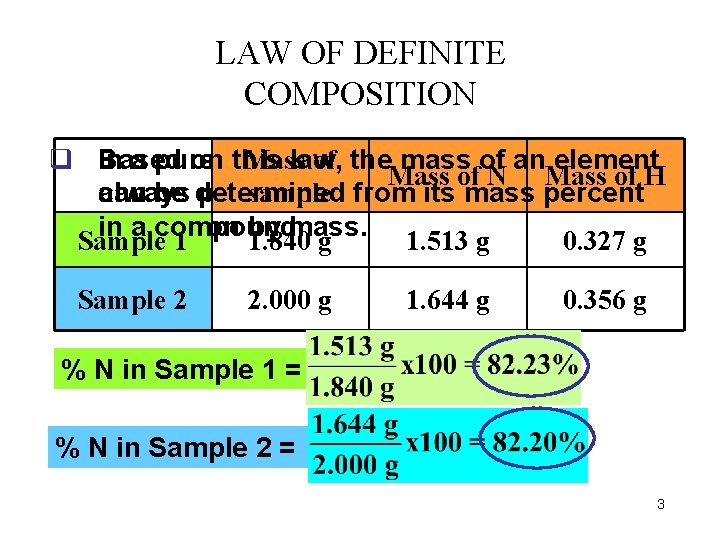
LAW OF DEFINITE COMPOSITION Mass of thethe q In Based a pure oncompound, this law, mass elements of an element are Mass of N Mass of H sample always can be determined present in the from same its mass definite percent proportion in a compound. by mass. Sample 1 1. 840 g 1. 513 g 0. 327 g Sample 2 2. 000 g 1. 644 g 0. 356 g % N in Sample 1 = % N in Sample 2 = 3

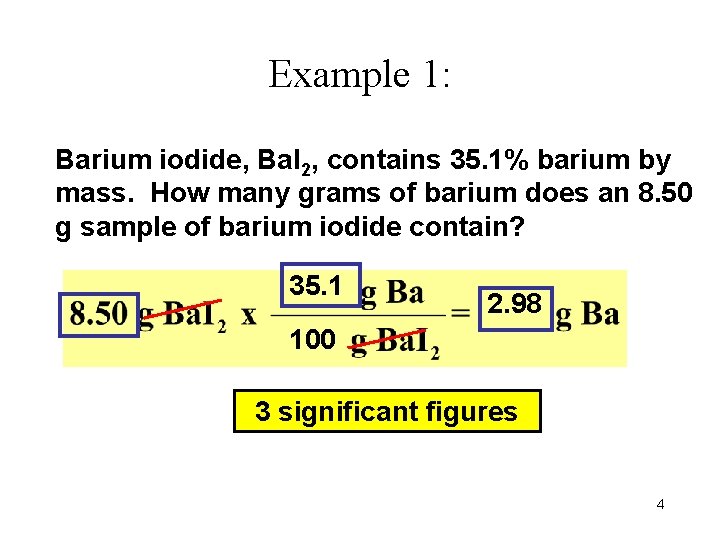
Example 1: Barium iodide, Ba. I 2, contains 35. 1% barium by mass. How many grams of barium does an 8. 50 g sample of barium iodide contain? 35. 1 2. 98 100 3 significant figures 4

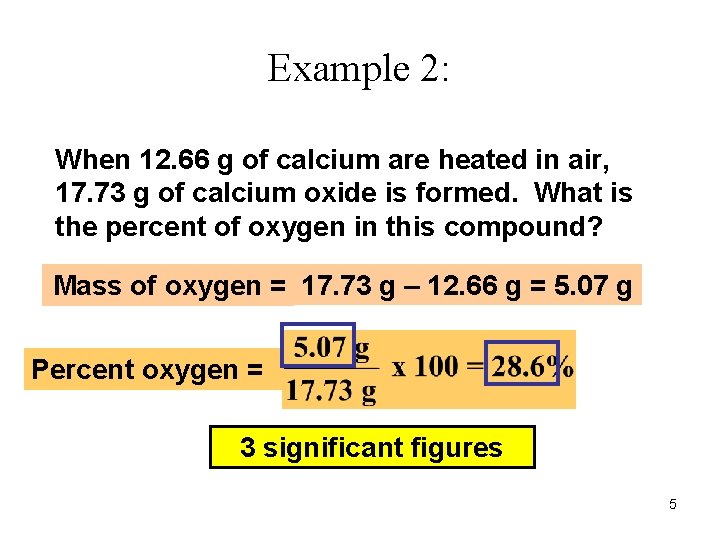
Example 2: When 12. 66 g of calcium are heated in air, 17. 73 g of calcium oxide is formed. What is the percent of oxygen in this compound? Mass of oxygen = 17. 73 g – 12. 66 g = 5. 07 g Percent oxygen = 3 significant figures 5

OCTET RULE & IONS q Most elements, except noble gases, combine to form compounds. Compounds are the result of the formation of chemical bonds between two or more different elements. q In the formation of a chemical bond, atoms lose, gain or share valence electrons to complete their outer shell and attain a noble gas configuration. q This tendency of atoms to have eight electrons in their outer shell is known as the octet rule. 6

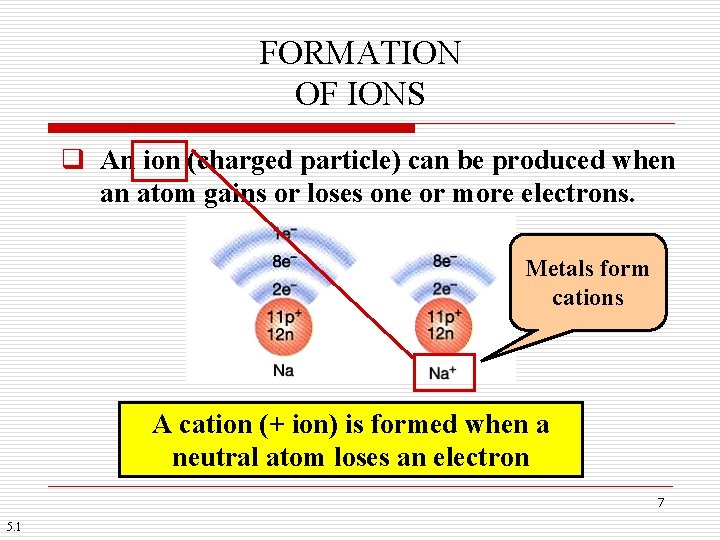
FORMATION OF IONS q An ion (charged particle) can be produced when an atom gains or loses one or more electrons. Metals form cations A cation (+ ion) is formed when a neutral atom loses an electron 7 5. 1

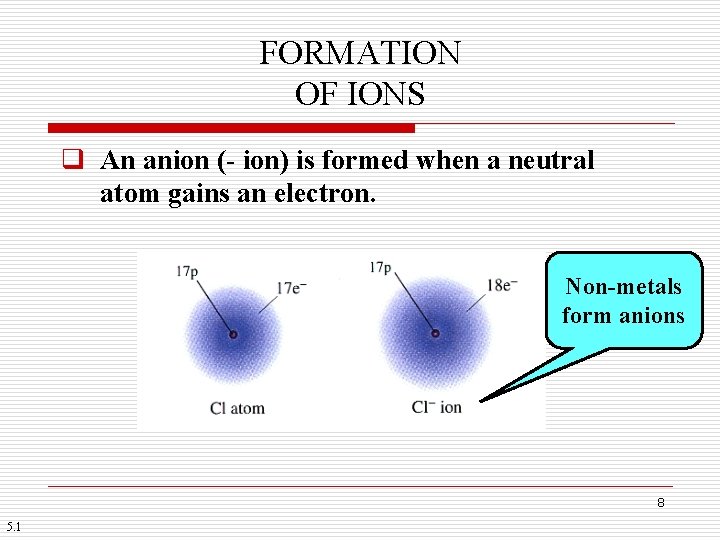
FORMATION OF IONS q An anion (- ion) is formed when a neutral atom gains an electron. Non-metals form anions 8 5. 1

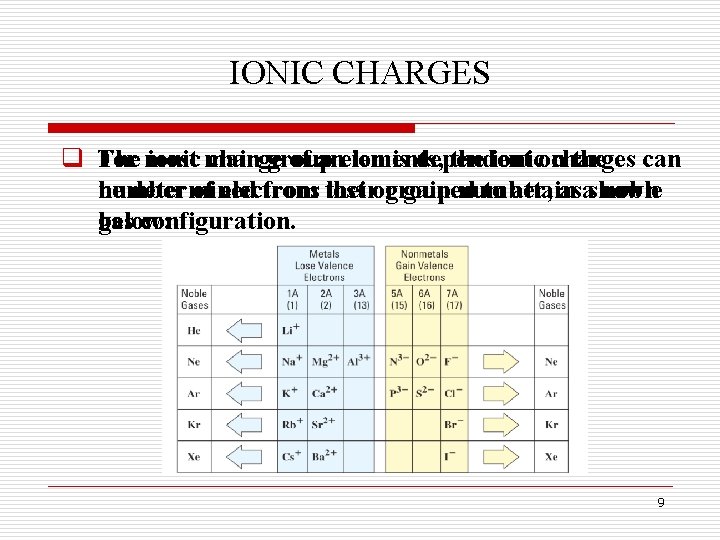
IONIC CHARGES q The For most ionic main charge group of anelements, ion is dependent the ionicon charges the can number be determined of electrons from their lost orgroup gained number, to attain asashown noble gas configuration. below: 9

Definition of Molecule and Compound • molecule - consist of at least two different atoms in a definite arrangement held together by very strong chemical bonds. • compound is a substance consisting of two or more elements in fixed and definite proportions

Formulas Describe Compounds • a compound is a distinct substance that is composed of atoms of two or more elements • a compound can be described by the number and type of each atom in the simplest unit of the compound – molecules or ions • each element is represented by its letter symbol • the number of atoms of each element is written to the right of the element as a subscript

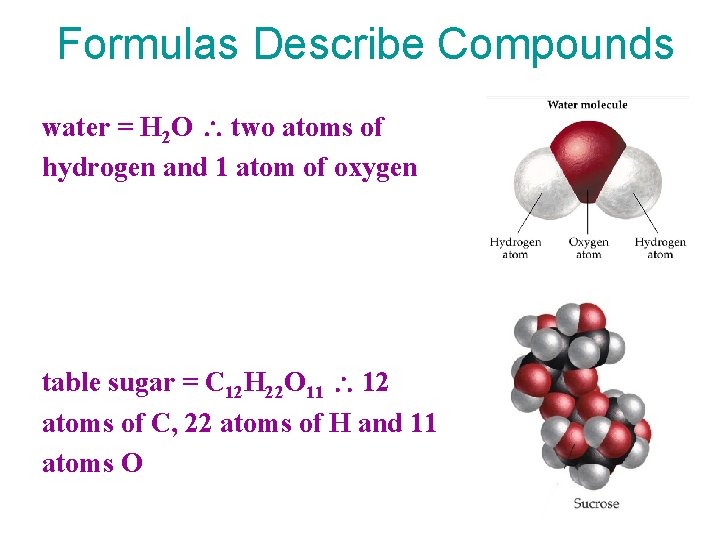
Formulas Describe Compounds water = H 2 O two atoms of hydrogen and 1 atom of oxygen table sugar = C 12 H 22 O 11 12 atoms of C, 22 atoms of H and 11 atoms O

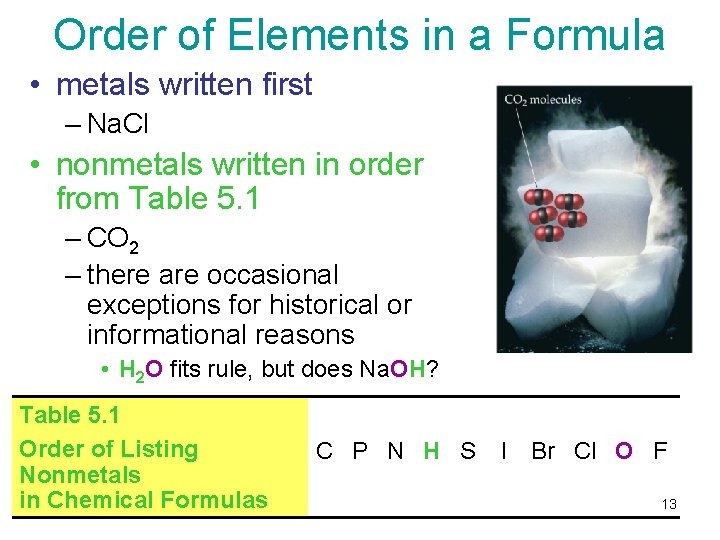
Order of Elements in a Formula • metals written first – Na. Cl • nonmetals written in order from Table 5. 1 – CO 2 – there are occasional exceptions for historical or informational reasons • H 2 O fits rule, but does Na. OH? Table 5. 1 Order of Listing C P N H S Nonmetals in Chemical Formulas Tro's Introductory Chemistry, Chapter 5 I Br Cl O F 13

Types of Chemical Formulas • An empirical formula gives the relative number of atoms of each element in a compound. • A molecular formula gives the actual number of atoms of each element in a molecule of the compound. • For example, the molecular formula for hydrogen peroxide is H 2 O 2, and its empirical formula is HO. • The molecular formula is always a whole number multiple of the empirical formula. • For many compounds, such as H 2 O, the molecular formula is the same as the empirical formula. • A structural formula uses lines to represent chemical bonds and shows how the atoms in a molecule are connected to each other.

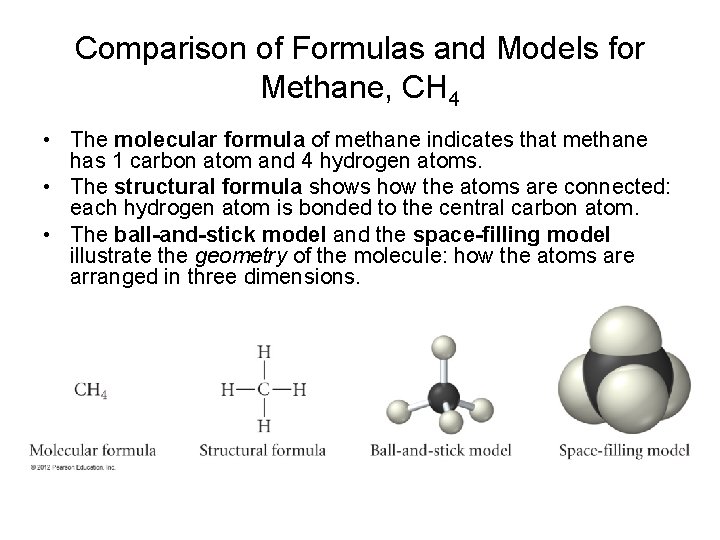
Comparison of Formulas and Models for Methane, CH 4 • The molecular formula of methane indicates that methane has 1 carbon atom and 4 hydrogen atoms. • The structural formula shows how the atoms are connected: each hydrogen atom is bonded to the central carbon atom. • The ball-and-stick model and the space-filling model illustrate the geometry of the molecule: how the atoms are arranged in three dimensions.

Classifying Materials • atomic elements = elements whose particles are single atoms • molecular elements = elements whose particles are multi-atom molecules • molecular compounds = compounds whose particles are molecules made of only nonmetals • ionic compounds = compounds whose particles are cations and Tro's Introductory Chemistry, anions Chapter 5

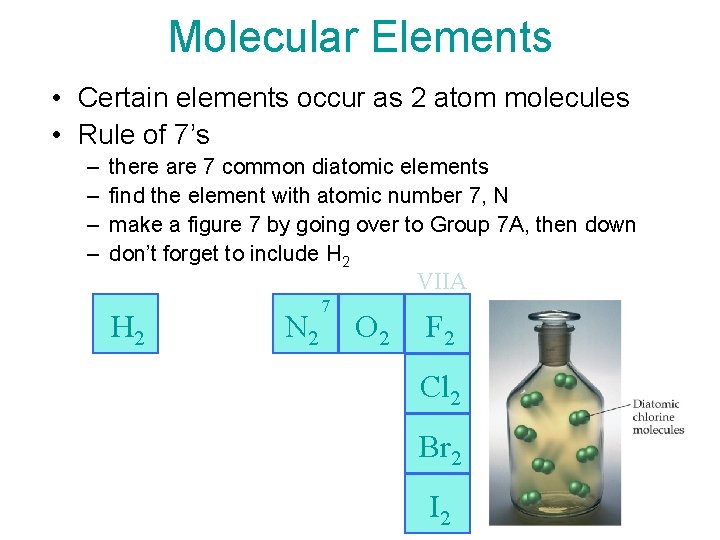
Molecular Elements • Certain elements occur as 2 atom molecules • Rule of 7’s – – there are 7 common diatomic elements find the element with atomic number 7, N make a figure 7 by going over to Group 7 A, then down don’t forget to include H 2 VIIA H 2 N 2 7 O 2 F 2 Cl 2 Br 2 I 2 17

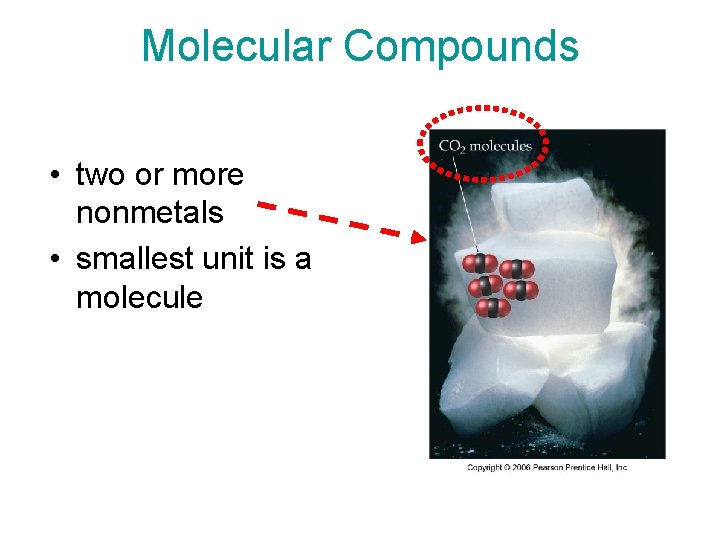
Molecular Compounds • two or more nonmetals • smallest unit is a molecule

Ionic Compounds • metals + nonmetals • no individual molecule, instead these have 3 dimensional array of cations and anions made of formula units (Na. Cl)

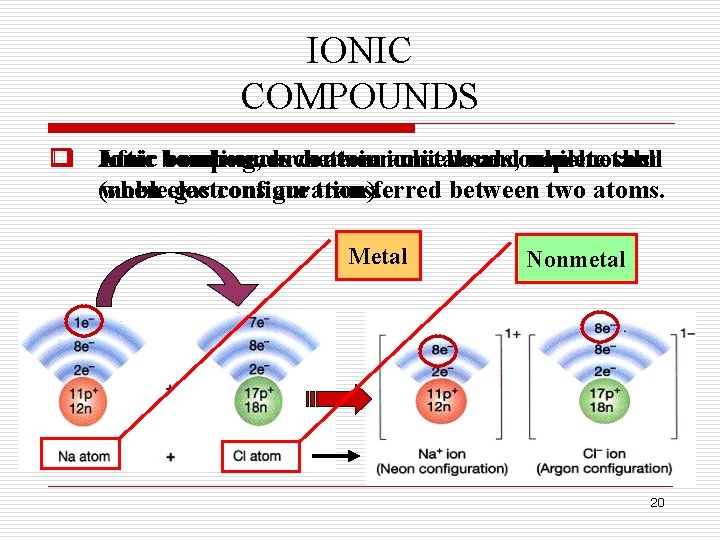
IONIC COMPOUNDS o After q Ionic bonds compounds bonding, occur each contain between atom ionic achieves metals bonds, and a complete which non-metals. occur shell (noble when electrons gas configuration). are transferred between two atoms. Metal Nonmetal 20

IONIC COMPOUNDS o Atoms q The smallest that gain lose particles electrons of ionic (metals) (non-metals) compounds form positive are negative ions (cations). (not ions atoms). (anions). Anion Cation 21

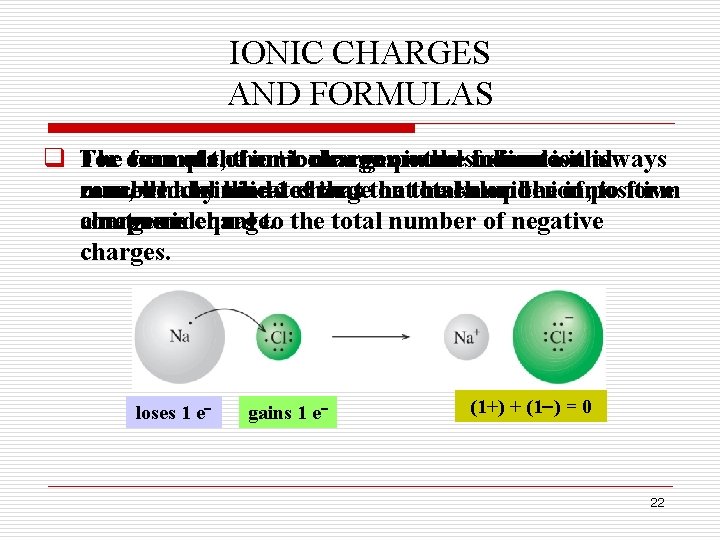
IONIC CHARGES AND FORMULAS q The For example, formula sum of the ionic an+1 ionic charges compound on inthe thesodium indicates formulaion isthe always is number zero, cancelled which and byindicates the kinds – 1 of charge that ions the that on total the make chloride number up theion, of ionic positive to form compound. acharges net zero is charge. equal to the total number of negative charges. loses 1 e gains 1 e (1+) + (1 ) = 0 22

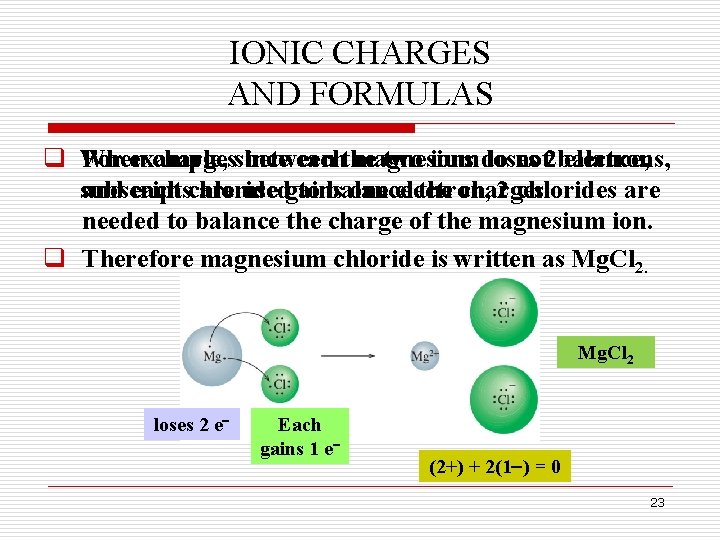
IONIC CHARGES AND FORMULAS q When For example, chargessince between eachthe magnesium two ions do loses not 2 balance, electrons, subscripts and each chloride are usedgains to balance one electron, the charges. 2 chlorides are needed to balance the charge of the magnesium ion. q Therefore magnesium chloride is written as Mg. Cl 2 loses 2 e Each gains 1 e (2+) + 2(1 ) = 0 23

Classify each of the following as either an atomic element, molecular compound or ionic compound • • • aluminum, Al aluminum chloride, Al. Cl 3 chlorine, Cl 2 acetone, C 3 H 6 O carbon monoxide, CO cobalt, Co = atomic element = ionic compound = molecular element = molecular compound = atomic element

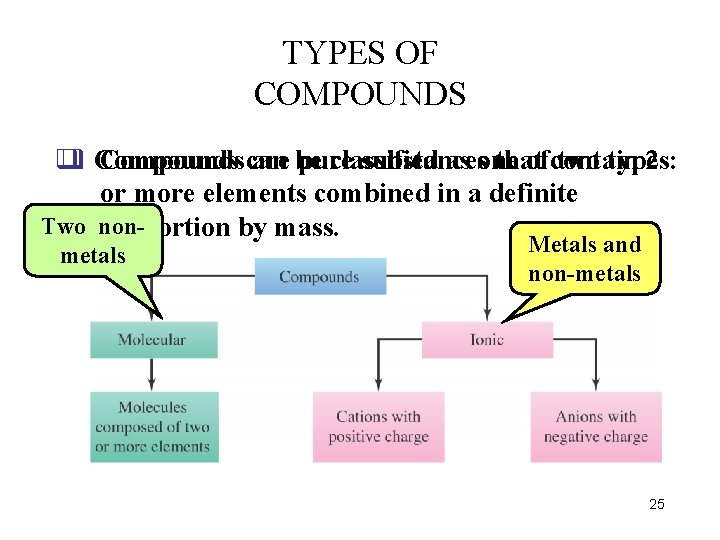
TYPES OF COMPOUNDS q q Compounds can are be pure classified substances as one that ofcontain two types: 2 or more elements combined in a definite Two nonproportion by mass. metals Metals and non-metals 25

Nomenclature of Compounds Common Names – Are Exceptions (like nicknames) H 2 O = water, steam, ice NH 3 = ammonia CH 4 = methane Na. Cl = table salt C 12 H 22 O 11 = table sugar

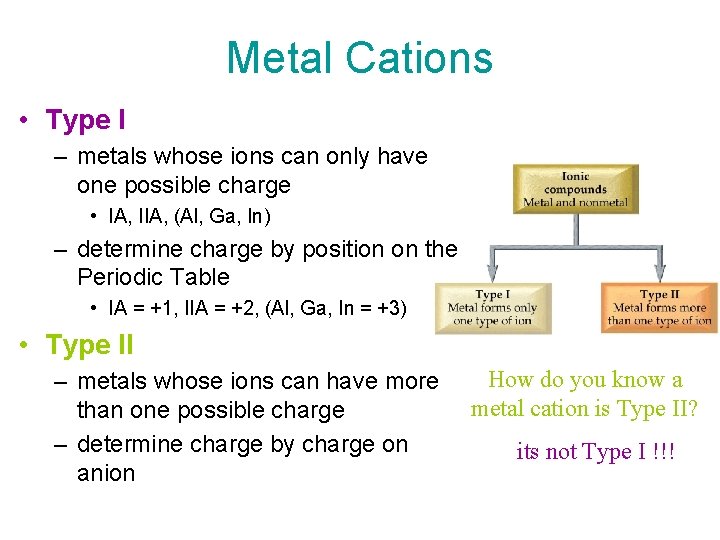
Metal Cations • Type I – metals whose ions can only have one possible charge • IA, IIA, (Al, Ga, In) – determine charge by position on the Periodic Table • IA = +1, IIA = +2, (Al, Ga, In = +3) • Type II – metals whose ions can have more than one possible charge – determine charge by charge on anion How do you know a metal cation is Type II? its not Type I !!!

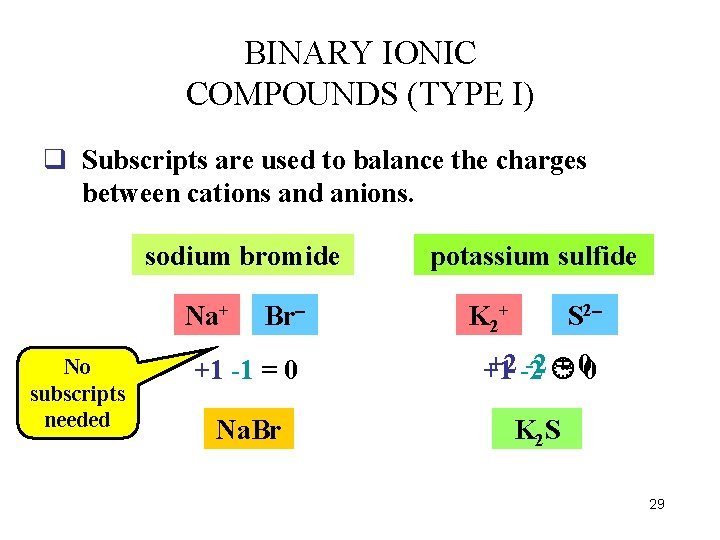
BINARY IONIC COMPOUNDS (TYPE I) q Binary compounds contain only two elements. q Ionic compounds are formed by combination of a metal and a non-metal. q Type I ions are those cations that form only one ion. q In these compounds, charges of the cations must equal the charges of the anions since the net charge is zero. 28

BINARY IONIC COMPOUNDS (TYPE I) q Subscripts are used to balance the charges between cations and anions. sodium bromide Na+ No subscripts needed Br potassium sulfide K K 2++ S 2 +1 -1 = 0 +2 -2 -2 = 00 +1 Na. Br K 2 S 29

Example 1: Write formulas for the following ionic compounds: calcium chloride Ca 2+ Cl Cl 2 sodium sulfide Na Na 2++ S 2 +2 -1 -2 = 00 +2 +2 -2 -2 = 00 +1 Ca. Cl 2 Na 2 S 30

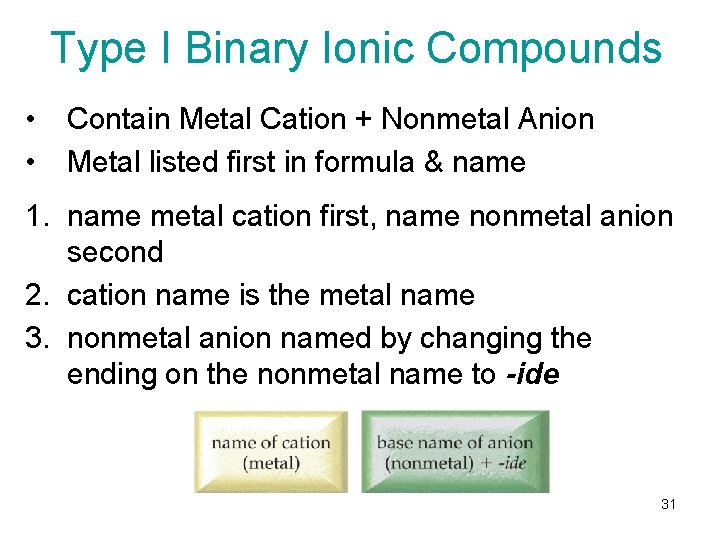
Type I Binary Ionic Compounds • • Contain Metal Cation + Nonmetal Anion Metal listed first in formula & name 1. name metal cation first, name nonmetal anion second 2. cation name is the metal name 3. nonmetal anion named by changing the ending on the nonmetal name to -ide 31

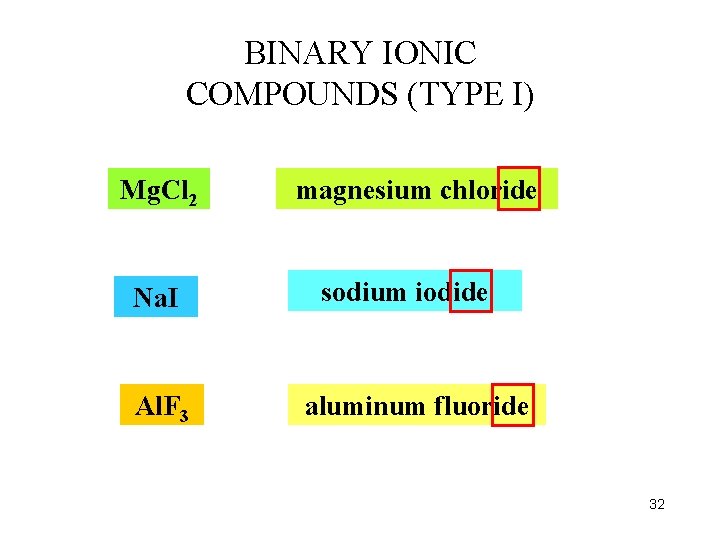
BINARY IONIC COMPOUNDS (TYPE I) Mg. Cl 2 Na. I Al. F 3 magnesium chloride sodium iodide aluminum fluoride 32

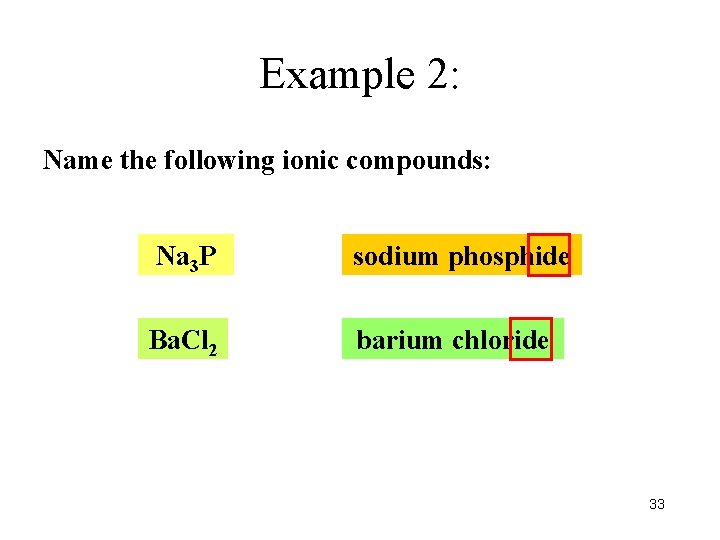
Example 2: Name the following ionic compounds: Na 3 P sodium phosphide Ba. Cl 2 barium chloride 33

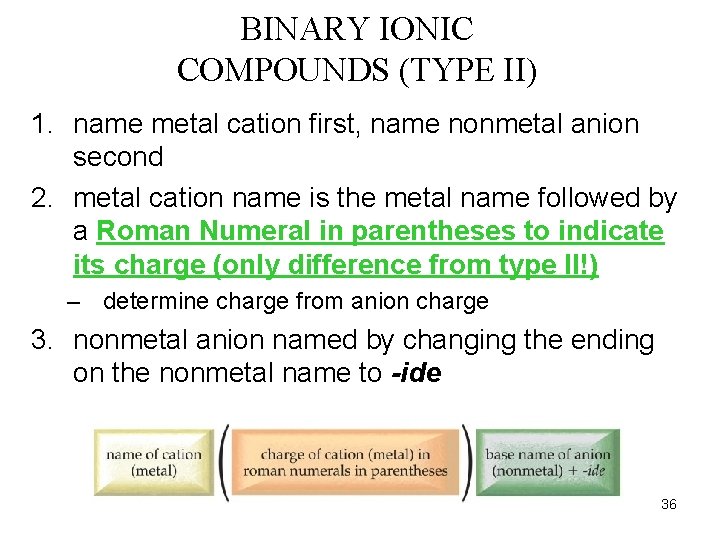
BINARY IONIC COMPOUNDS (TYPE II) q Type II ions are those cations that form more than one ion. q When naming compounds formed from these ions, include the ionic charge as Roman numeral, in parentheses, after the metal’s name. q This method of nomenclature is called the “stock” system. 34

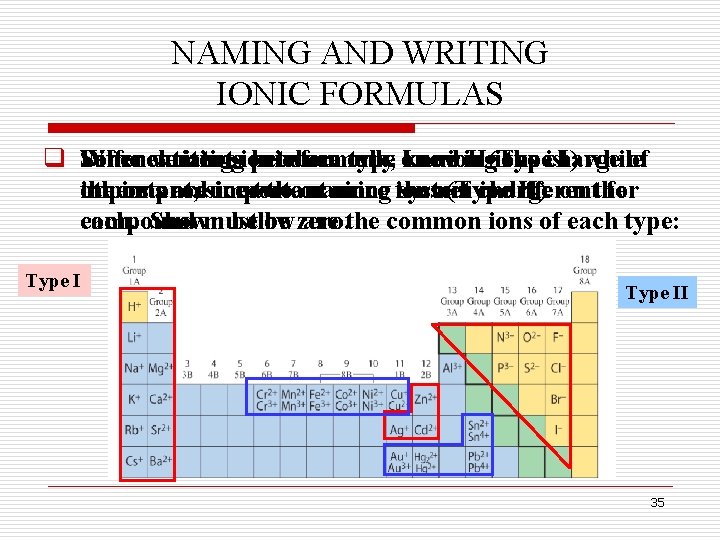
NAMING AND WRITING IONIC FORMULAS q Some Differentiating When elements writing ionic produce between formula, only type one Iknowing and ion II(Type ions the is charge I) while of the ionsproduce others important, aresince important two theor naming more since ions the system net (Type charge is different II). on the for compound each. Shown must below be zero. are the common ions of each type: Type II 35

BINARY IONIC COMPOUNDS (TYPE II) 1. name metal cation first, name nonmetal anion second 2. metal cation name is the metal name followed by a Roman Numeral in parentheses to indicate its charge (only difference from type II!) – determine charge from anion charge 3. nonmetal anion named by changing the ending on the nonmetal name to -ide 36

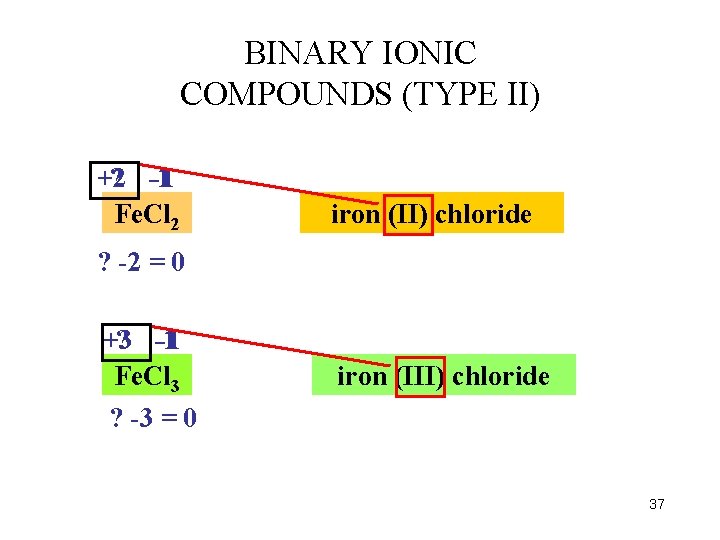
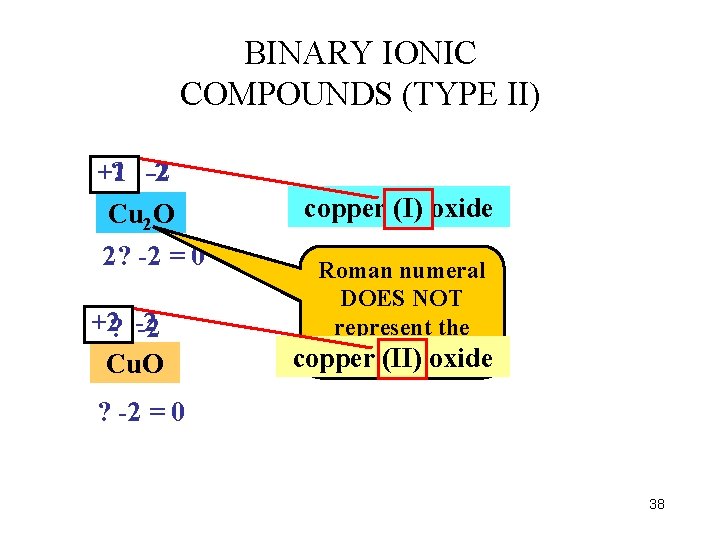
BINARY IONIC COMPOUNDS (TYPE II) +2 ? -1 -1 Fe. Cl 2 iron (II) chloride ? -2 = 0 +3 ? -1 -1 Fe. Cl 3 ? -3 = 0 iron (III) chloride 37

BINARY IONIC COMPOUNDS (TYPE II) +1 ? -2 -2 Cu 2 O 2? -2 = 0 +2? -2 -2 Cu. O copper (I) oxide Roman numeral DOES NOT represent the subscript copper (II) oxide ? -2 = 0 38

BINARY IONIC COMPOUNDS (TYPE II) q Type II cations can also be named by an older method (classical). q In this system, cations with the higher charge end in –ic, while cations with the lower charge end in –ous. q In this system, some cations are named based on their Latin roots. 39

CLASSICAL SYSTEM (DERIVED FROM LATIN) gold (aurum for Aurora the Roman goddess of the dawn) silver (argentum for 'bright') copper (cuprum for 'Cyprus' where the Romans first obtained copper) tin (stannum for alloys containing lead) lead (plumbum for 'lead') mercury (hydrargyrum for 'liquid silver' or quick silver) antimony (stibium for 'not alone') iron (ferrum for 'firmness‘) potassium (kalium via the Arabic qali for alkali) sodium (natrium for soda) 40

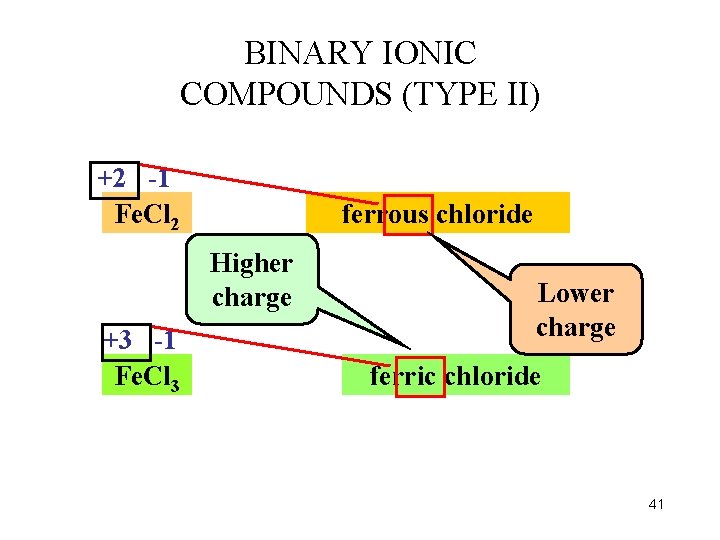
BINARY IONIC COMPOUNDS (TYPE II) +2 -1 Fe. Cl 2 ferrous chloride Higher charge +3 -1 Fe. Cl 3 Lower charge ferric chloride 41

BINARY IONIC COMPOUNDS (TYPE II) +1 -2 Cu 2 O cuprous oxide Higher charge +2 -2 Cu. O Lower charge cupric oxide 42

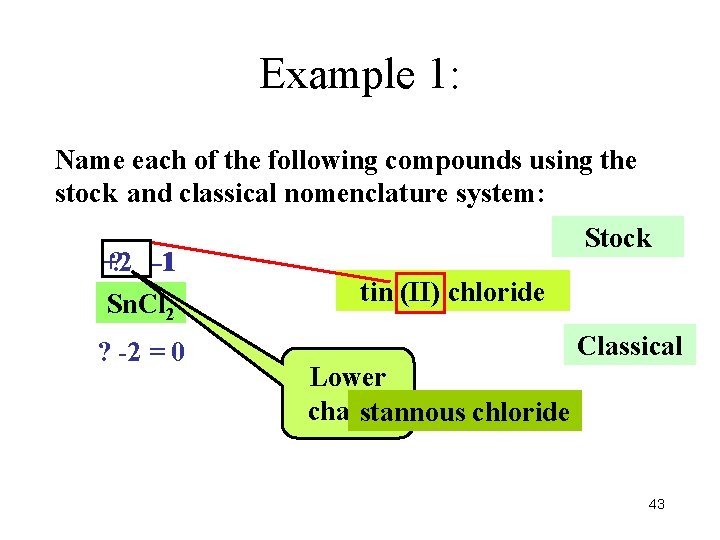
Example 1: Name each of the following compounds using the stock and classical nomenclature system: +2 ? -1 -1 Sn. Cl 2 ? -2 = 0 Stock tin (II) chloride Classical Lower charge stannous chloride 43

Example 1: Name each of the following compounds using the stock and classical nomenclature system: Stock +1 ? -2 -2 Cu 2 S 2? -2 = 0 copper (I) sulfide Lower charge cuprous sulfide Classical 44

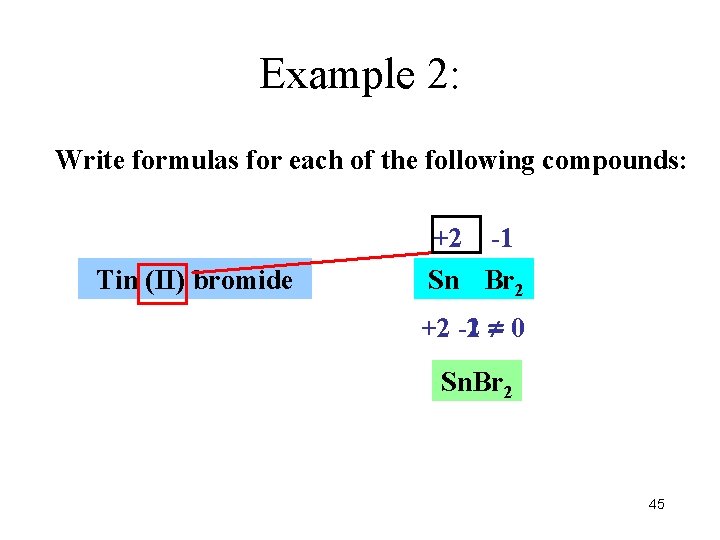
Example 2: Write formulas for each of the following compounds: +2 Tin (II) bromide -1 Sn Br Br 2 +2 -2 -1 = 0 Sn. Br 2 45

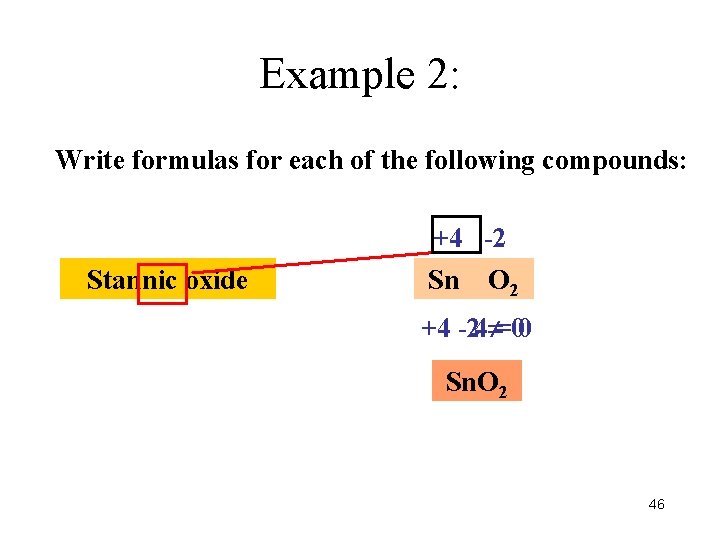
Example 2: Write formulas for each of the following compounds: +4 -2 Stannic oxide Sn O O 2 +4 --24 =00 Sn. O 2 46

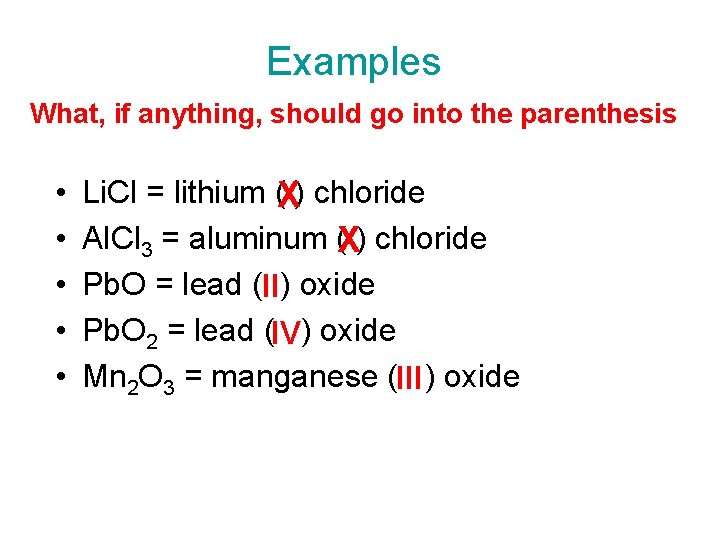
Examples What, if anything, should go into the parenthesis • • • Li. Cl = lithium (X) chloride Al. Cl 3 = aluminum (X) chloride Pb. O = lead (II) oxide Pb. O 2 = lead (IV) oxide Mn 2 O 3 = manganese (III ) oxide

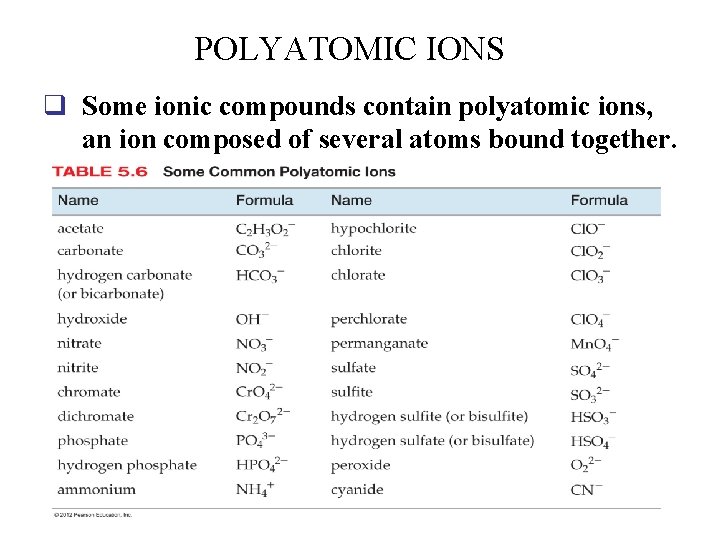
POLYATOMIC IONS q Some ionic compounds contain polyatomic ions, an ion composed of several atoms bound together. 48

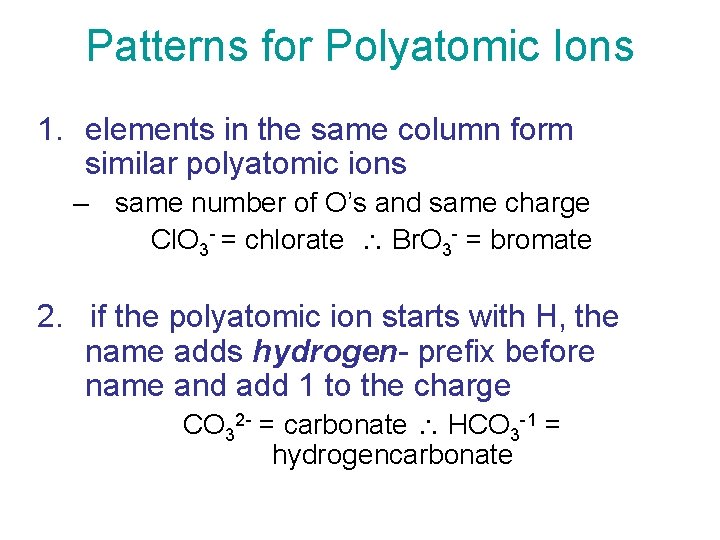
Patterns for Polyatomic Ions 1. elements in the same column form similar polyatomic ions – same number of O’s and same charge Cl. O 3 - = chlorate Br. O 3 - = bromate 2. if the polyatomic ion starts with H, the name adds hydrogen- prefix before name and add 1 to the charge CO 32 - = carbonate HCO 3 -1 = hydrogencarbonate

Periodic Pattern of Polyatomic Ions -ate groups IIIA -3 BO 3 IVA VA VIIA -2 CO 3 -1 NO 3 -2 Si. O 3 -3 PO 4 -2 SO 4 -1 Cl. O 3 -3 As. O 4 -2 Se. O 4 -1 Br. O 3 -2 Te. O 4 -1 IO 3

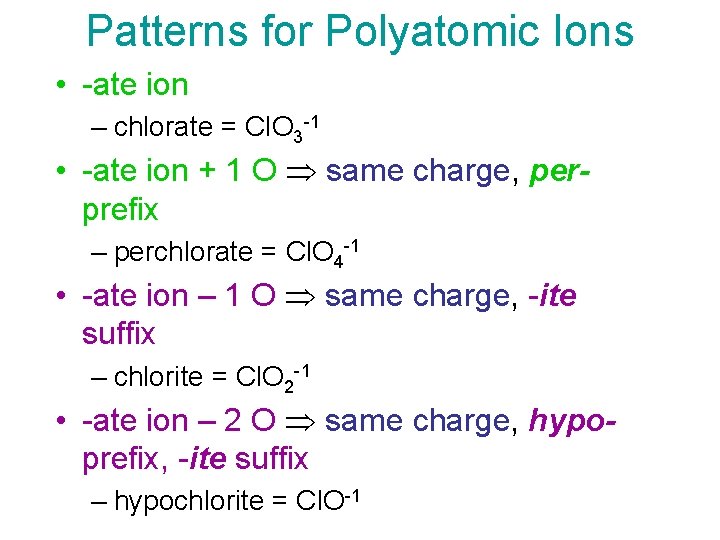
Patterns for Polyatomic Ions • -ate ion – chlorate = Cl. O 3 -1 • -ate ion + 1 O same charge, perprefix – perchlorate = Cl. O 4 -1 • -ate ion – 1 O same charge, -ite suffix – chlorite = Cl. O 2 -1 • -ate ion – 2 O same charge, hypoprefix, -ite suffix – hypochlorite = Cl. O-1

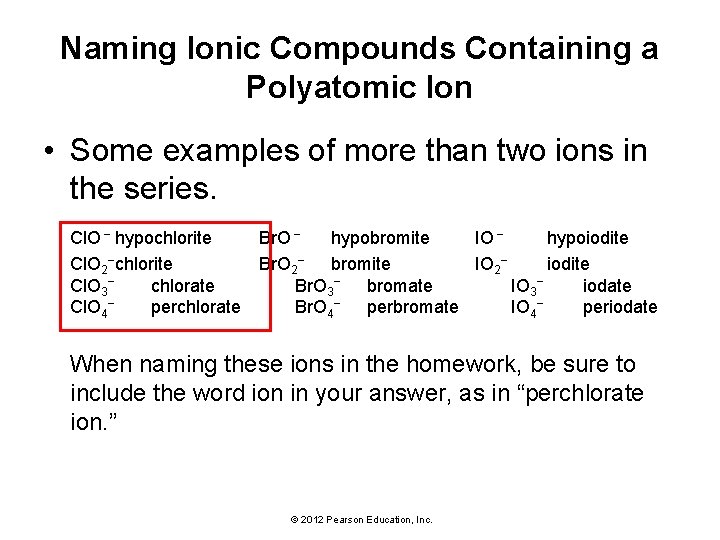
Naming Ionic Compounds Containing a Polyatomic Ion • Some examples of more than two ions in the series. Cl. O − hypochlorite Br. O − hypobromite IO − hypoiodite Cl. O 2−chlorite Br. O 2− bromite IO 2− iodite Cl. O 3− chlorate Br. O 3− bromate IO 3− iodate Cl. O 4− perchlorate Br. O 4− perbromate IO 4− periodate When naming these ions in the homework, be sure to include the word ion in your answer, as in “perchlorate ion. ” © 2012 Pearson Education, Inc.

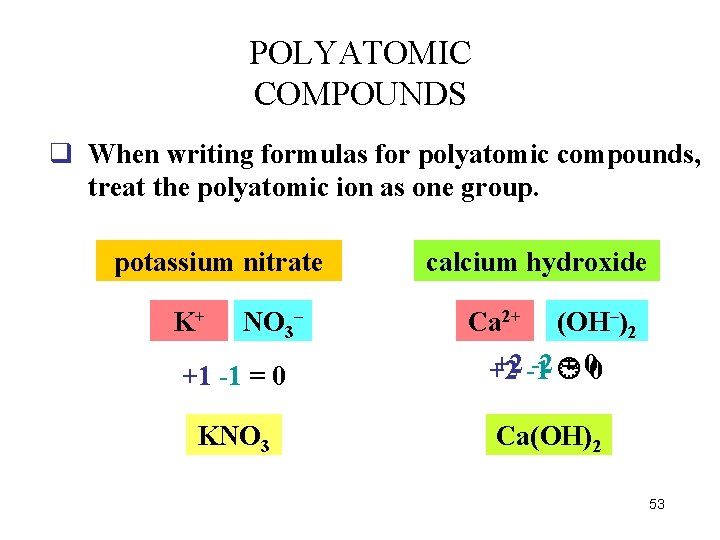
POLYATOMIC COMPOUNDS q When writing formulas for polyatomic compounds, treat the polyatomic ion as one group. potassium nitrate K+ NO 3– calcium hydroxide +1 -1 = 0 Ca 2+ (OH OH– )2 +2 -1 -2 = 00 +2 KNO 3 Ca(OH)2 53

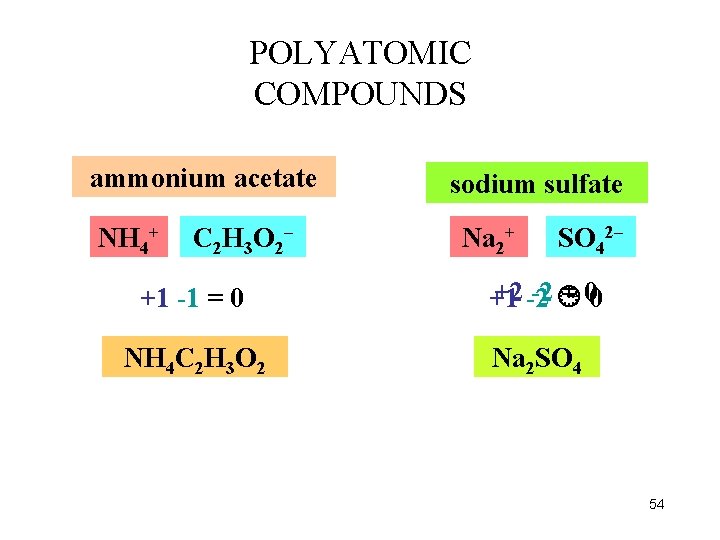
POLYATOMIC COMPOUNDS ammonium acetate NH 4+ C 2 H 3 O 2– +1 -1 = 0 NH 4 C 2 H 3 O 2 sodium sulfate Na Na 2++ SO 42– +2 -2 -2 = 00 +1 Na 2 SO 4 54

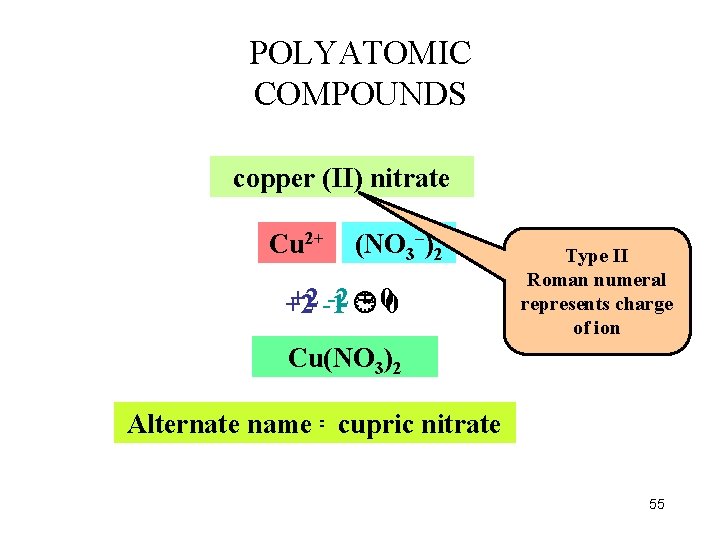
POLYATOMIC COMPOUNDS copper (II) nitrate Cu 2+ (NO NO 33––)2 +2 -1 -2 = 00 +2 Type II Roman numeral represents charge of ion Cu(NO 3)2 Alternate name =cupric nitrate 55

Example 2: Name the following polyatomic compounds: Mg(OH)2 Na. CN Fe 2(SO 4)3 +3 2 magnesium hydroxide Type II ion (Requires roman sodium Type I ioncyanide numeral) (Does not require roman numeral) iron (III) sulfate ferric sulfate 56

Subclasses • Compounds containing a metal and a nonmetal = binary ionic – Type I and II • Compounds containing a polyatomic ion = ionic with polyatomic ion

BINARY MOLECULAR COMPOUNDS q Molecular compounds are formed by combination of 2 or more non-metals. q The smallest particles of molecular compounds are molecules. q These compounds are named similar to ionic compounds, with the second element named based on its root and suffix “-ide”. 58

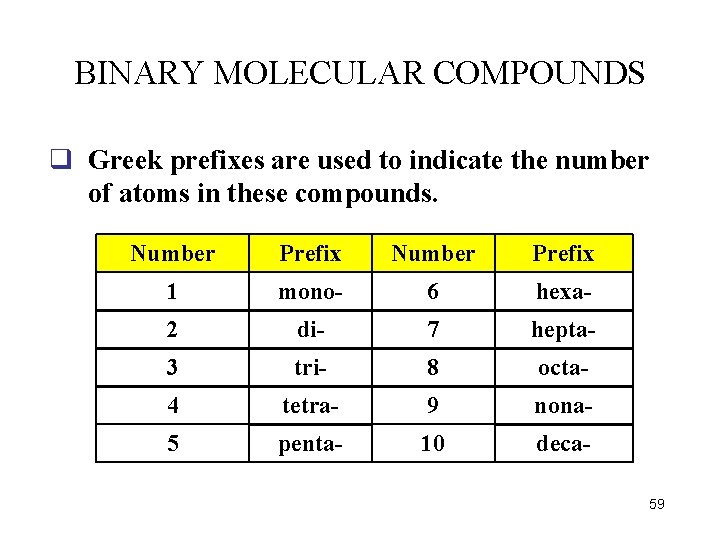
BINARY MOLECULAR COMPOUNDS q Greek prefixes are used to indicate the number of atoms in these compounds. Number Prefix 1 mono- 6 hexa- 2 di- 7 hepta- 3 tri- 8 octa- 4 tetra- 9 nona- 5 penta- 10 deca 59

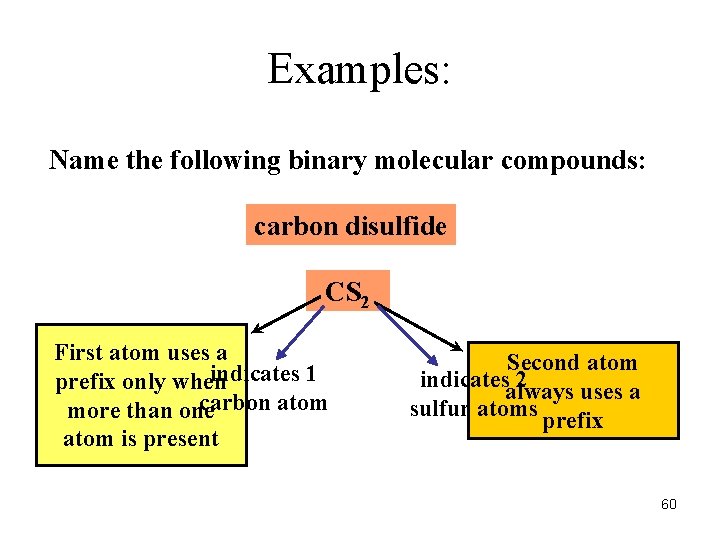
Examples: Name the following binary molecular compounds: carbon disulfide CS 2 First atom uses a indicates 1 prefix only when carbon atom more than one atom is present Second atom indicates 2 always uses a sulfur atoms prefix 60

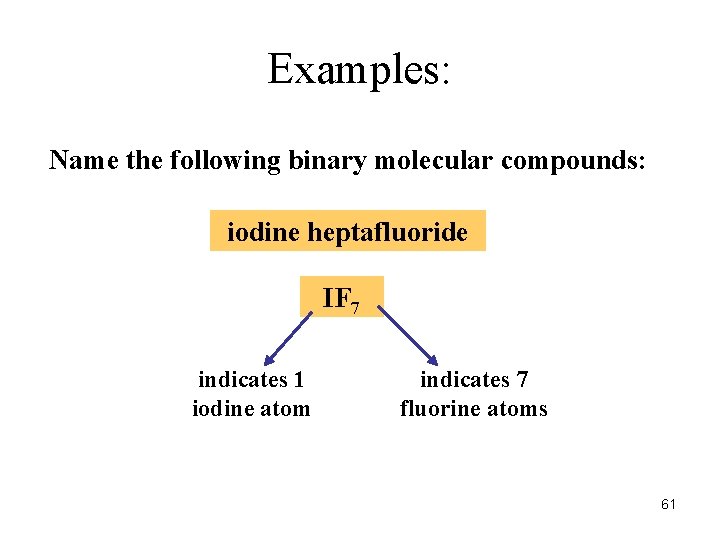
Examples: Name the following binary molecular compounds: iodine heptafluoride IF 7 indicates 1 iodine atom indicates 7 fluorine atoms 61

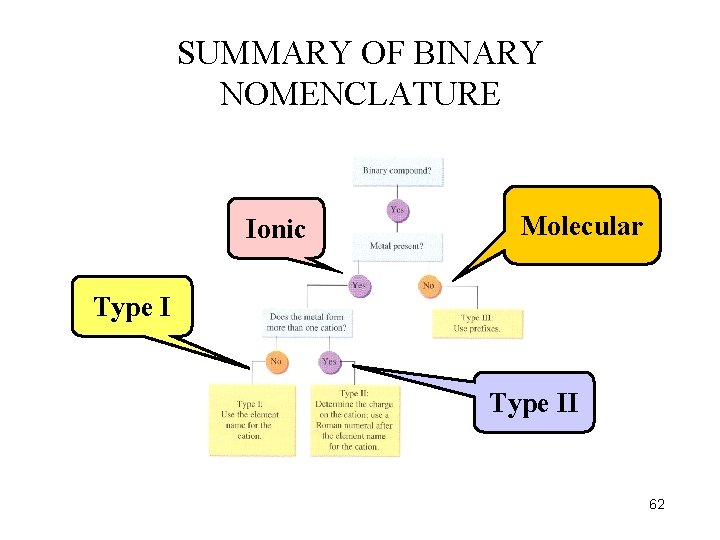
SUMMARY OF BINARY NOMENCLATURE Ionic Molecular Type II 62

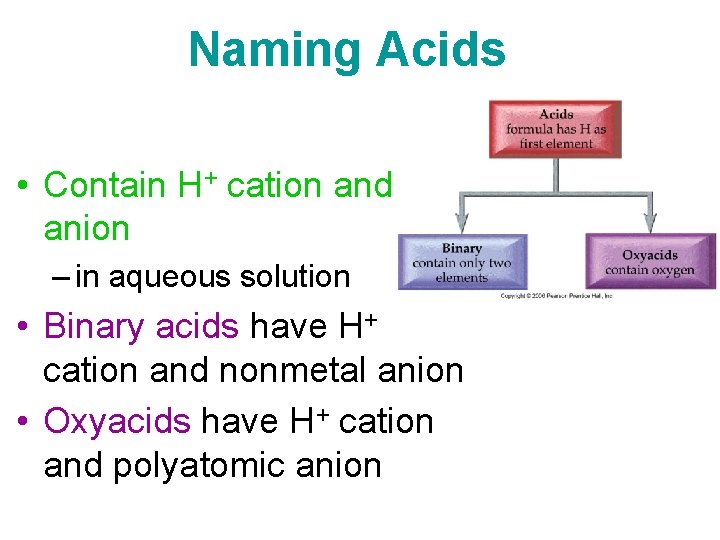
Naming Acids • Contain H+ cation and anion – in aqueous solution • Binary acids have H+ cation and nonmetal anion • Oxyacids have H+ cation and polyatomic anion

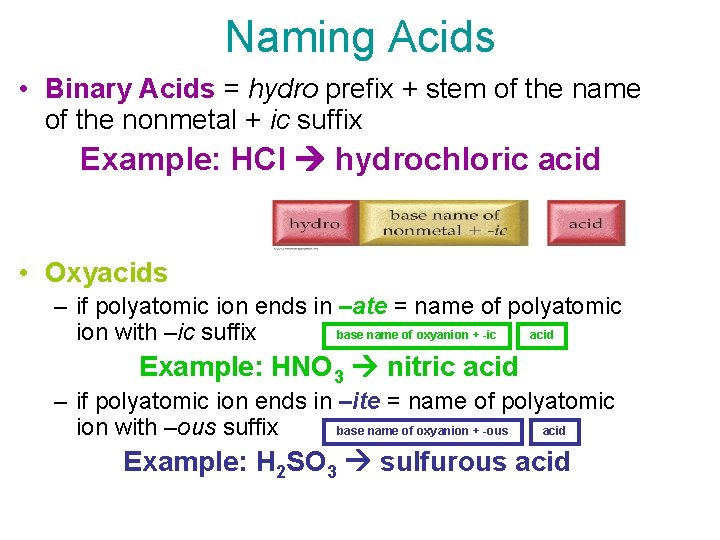
Naming Acids • Binary Acids = hydro prefix + stem of the name of the nonmetal + ic suffix Example: HCl hydrochloric acid • Oxyacids – if polyatomic ion ends in –ate = name of polyatomic base name of oxyanion + -ic acid ion with –ic suffix Example: HNO 3 nitric acid – if polyatomic ion ends in –ite = name of polyatomic ion with –ous suffix acid base name of oxyanion + -ous Example: H 2 SO 3 sulfurous acid

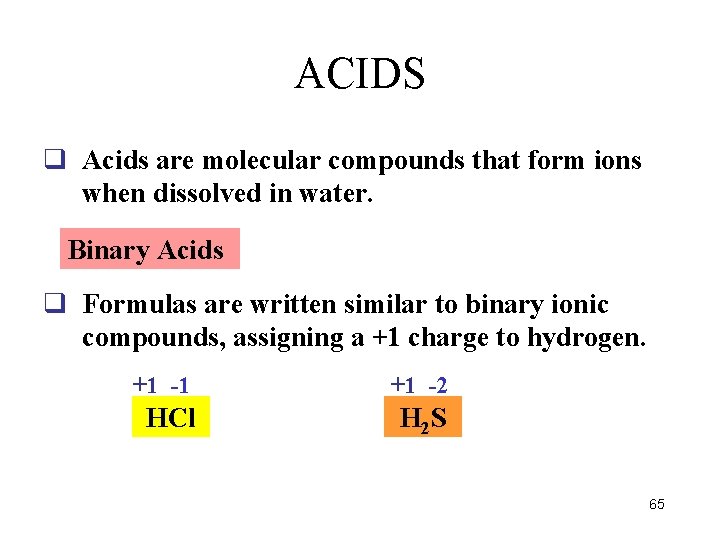
ACIDS q Acids are molecular compounds that form ions when dissolved in water. Binary Acids q Formulas are written similar to binary ionic compounds, assigning a +1 charge to hydrogen. +1 -1 +1 -2 HCl H 2 S 65

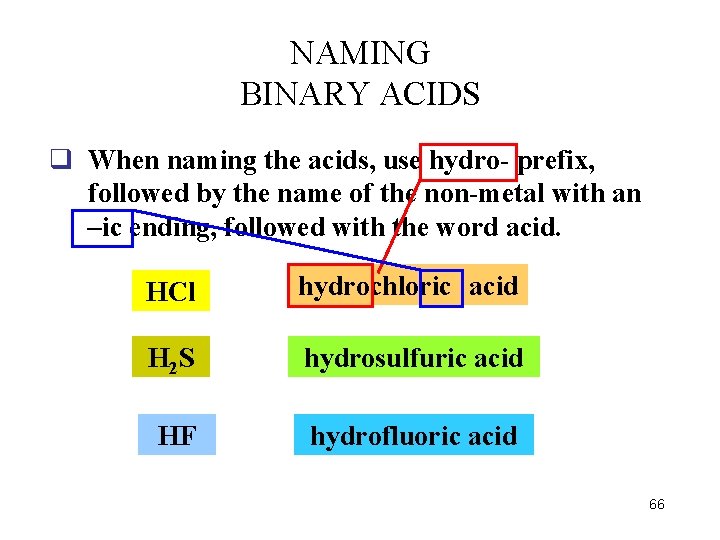
NAMING BINARY ACIDS q When naming the acids, use hydro- prefix, followed by the name of the non-metal with an –ic ending, followed with the word acid. HCl hydrochloric acid H 2 S hydrosulfuric acid HF hydrofluoric acid 66

POLYATOMIC ACIDS q Several polyatomic acids are important in the study of chemistry, and their names must be learned. q These acids and the polyatomic ions that form from their ionization are as follows: q Most of these are oxyacids 67

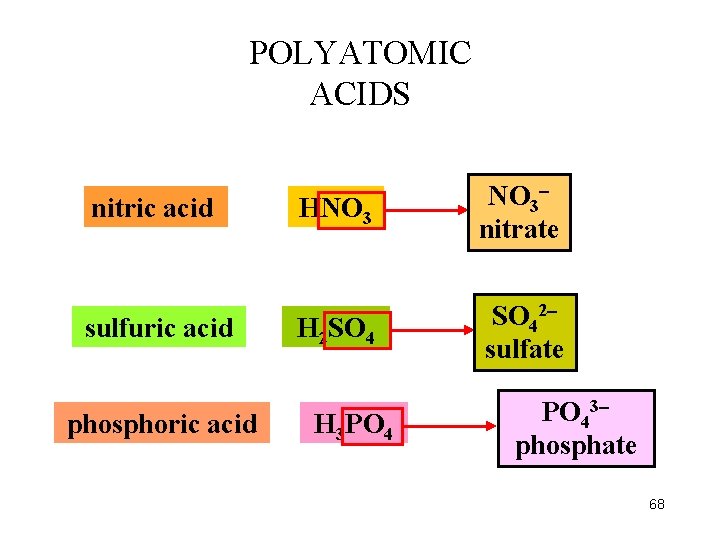
POLYATOMIC ACIDS nitric acid sulfuric acid phosphoric acid HNO 3 nitrate H 2 SO 42 sulfate H 3 PO 43 phosphate 68

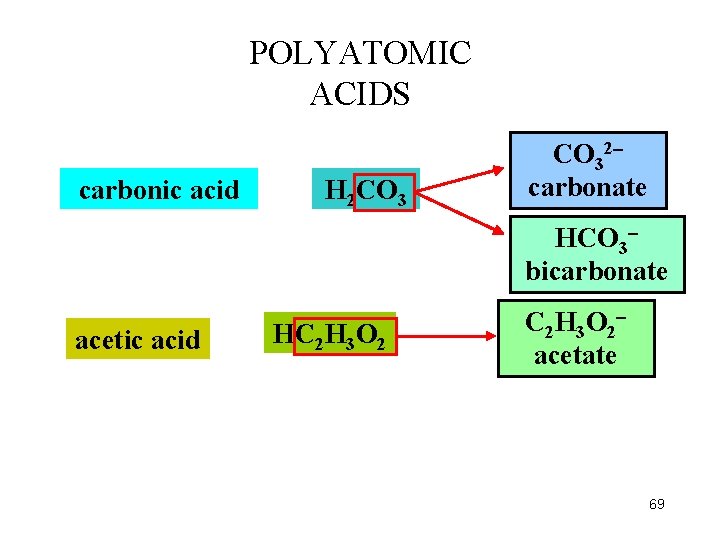
POLYATOMIC ACIDS carbonic acid H 2 CO 32 carbonate HCO 3 bicarbonate acetic acid HC 2 H 3 O 2 acetate 69

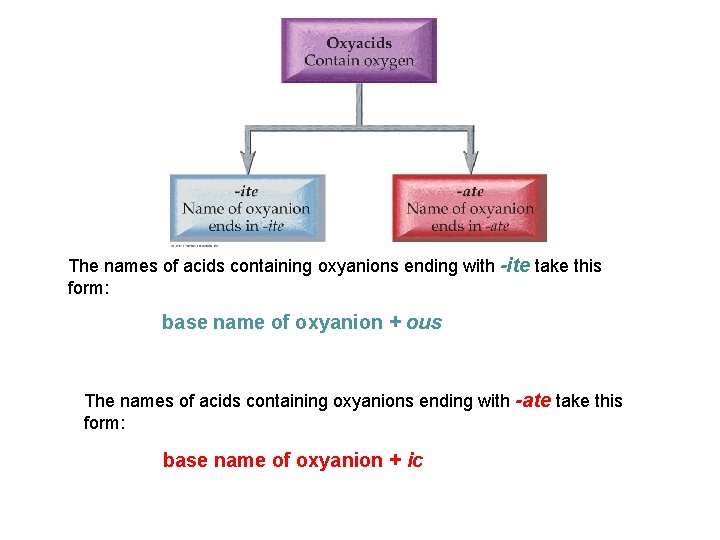
The names of acids containing oxyanions ending with -ite take this form: base name of oxyanion + ous The names of acids containing oxyanions ending with -ate take this form: base name of oxyanion + ic

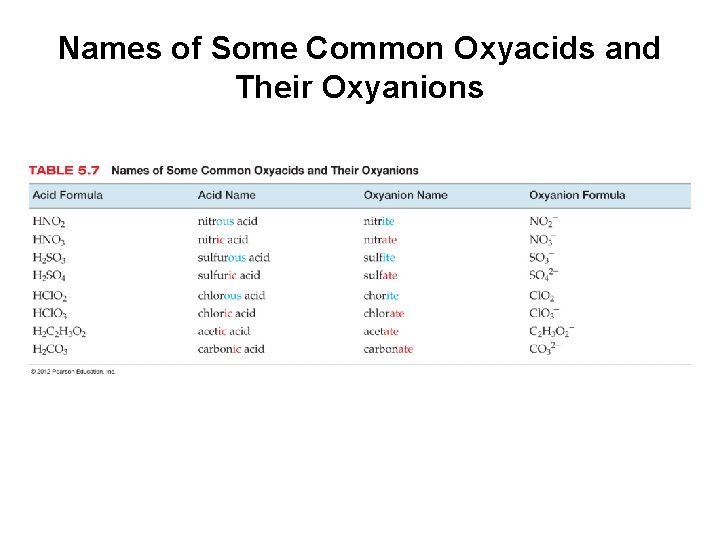
Names of Some Common Oxyacids and Their Oxyanions

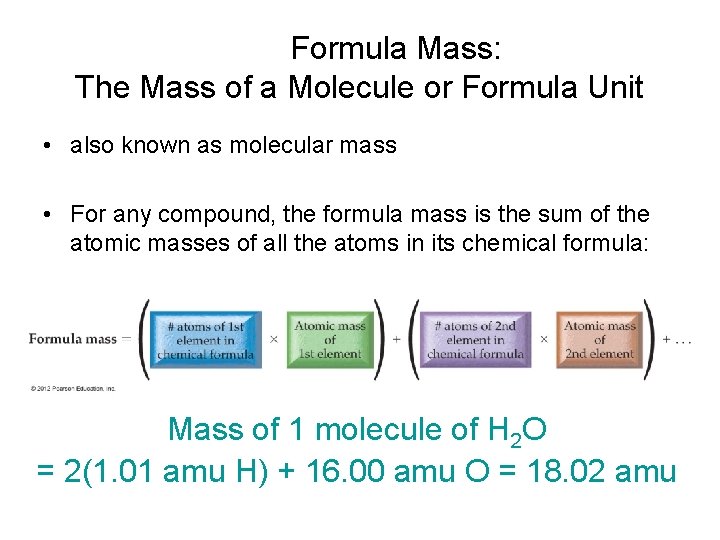
Formula Mass: The Mass of a Molecule or Formula Unit • also known as molecular mass • For any compound, the formula mass is the sum of the atomic masses of all the atoms in its chemical formula: Mass of 1 molecule of H 2 O = 2(1. 01 amu H) + 16. 00 amu O = 18. 02 amu

THE END 73