Chapter 5 is divided in 5 major areas





- Slides: 16

Chapter 5 is divided in 5 major areas. 1. 2. 3. 4. 5. Chemical Compounds. Ionic Bonding. Covalent Bonding. Electronegativity. Shapes of Molecules and Intermolecular forces.

Sigma bonds vs Pi bonds Sigma bonds. • A sigma bond is formed when electrons are shared in line with the nuclei. (a head-on overlap of orbitals. ) Pi bonds. • A Pi bond is formed when the shared orbitals are side on i. e. (not in line with the nuclei. ) N. B. Sigma bonds are stronger. In a covalent single bond it is a sigma bond, however, in a double or triple bond there is 1 sigma bond and the others are pi bonds.


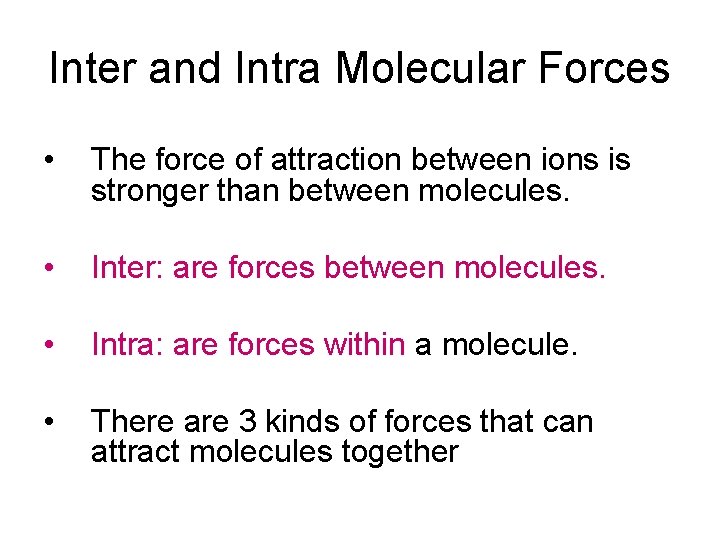
Polar and Non-Polar Covalent Bonding • A polar bond can be pictured using partial charges: + H Cl 2. 1 3. 0 = 0. 9

Inter and Intra Molecular Forces • The force of attraction between ions is stronger than between molecules. • Inter: are forces between molecules. • Intra: are forces within a molecule. • There are 3 kinds of forces that can attract molecules together

1. Van der Waal’s: • These are the weakest forces caused by the movement of e- within a molecule. The electrons move randomly within the bond so at 1 point in time they are nearer to 1 atom than the other. This induces a temporary dipole force. • Temporary dipoles will result in increased boiling points. • The greater number of e- in a molecule the greater the number of temporary dipoles.

2. Dipole-dipole: • The positively charged end of a polar molecule is attracted to the negative end of another molecule. • The dipoles in this case are permanent. • As a result they are stronger than Van der Waal’s forces.

3. Hydrogen Bonding • When H is bonded to F, O or N these elements are sufficiently electronegative to make the bond polar. • • H has only 1 e- in its atom, a strong partial positive charge will result. This means it is very strongly attracted to the negative atom and as a result H 2 O is a liquid at room temperature with a fairly high boiling point.

Shapes of Covalent Molecules

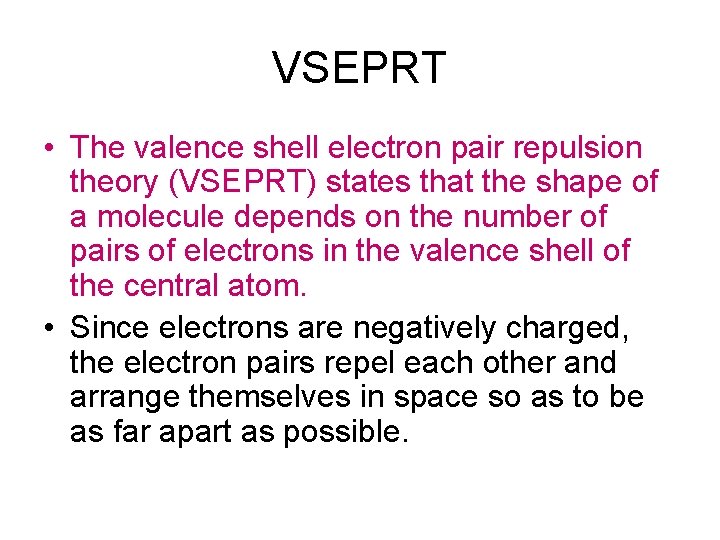
VSEPRT • The valence shell electron pair repulsion theory (VSEPRT) states that the shape of a molecule depends on the number of pairs of electrons in the valence shell of the central atom. • Since electrons are negatively charged, the electron pairs repel each other and arrange themselves in space so as to be as far apart as possible.

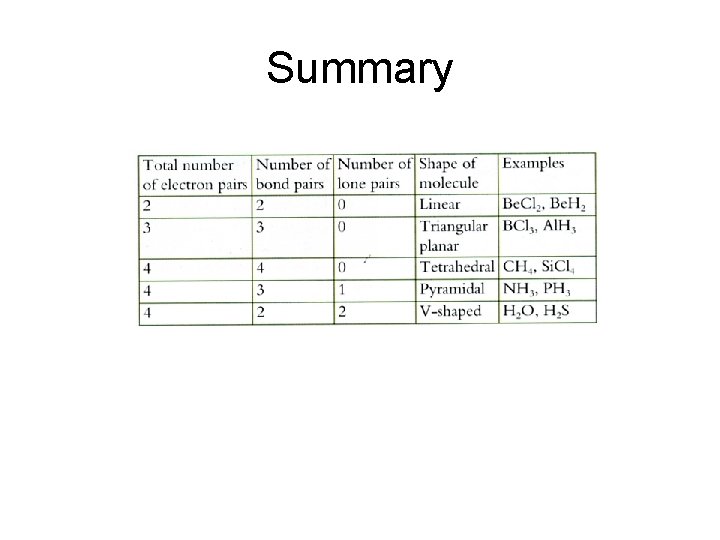
Linear • Beryllium Chloride (Be. Cl 2) has 2 bond pairs of electrons around the central atom. • These bond pairs repel each other as far as possible resulting in a linear shape. • The bond angle is 180 o.

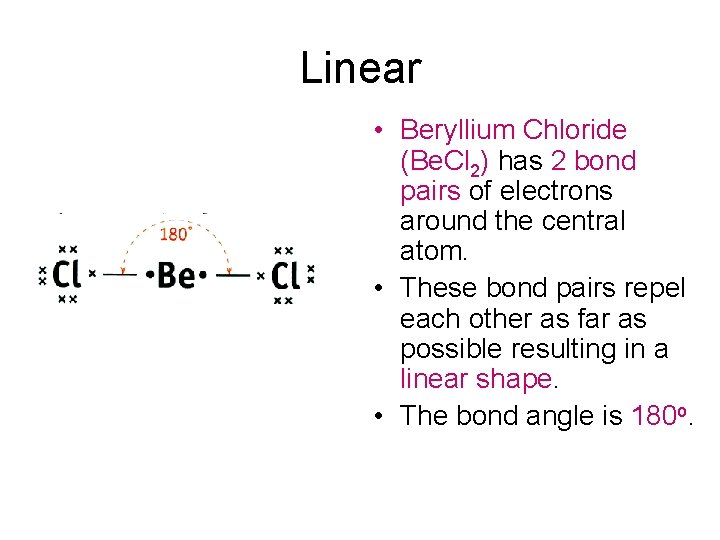
Triangular planar • Boron trichloride (BCl 3) has 3 bond pairs of electrons around the central atom. • The three pairs of electrons repel each other as far as possible resulting in a triangular planar shaped molecule. • The bond angle is 120 o.

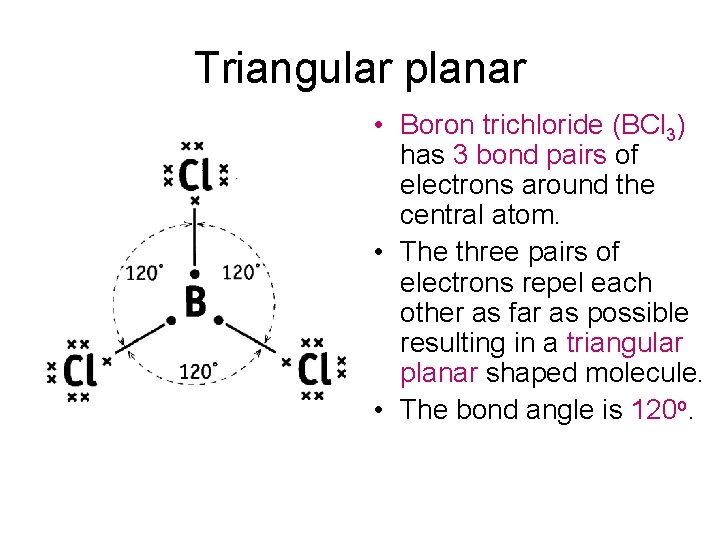
Tetrahedral • Methane (CH 4) has 4 bond pairs of electrons around the central atom. • The four pairs of electrons repel each other as far as possible resulting in a tetrahedral shaped molecule. • The bond angle is 109. 5 o.

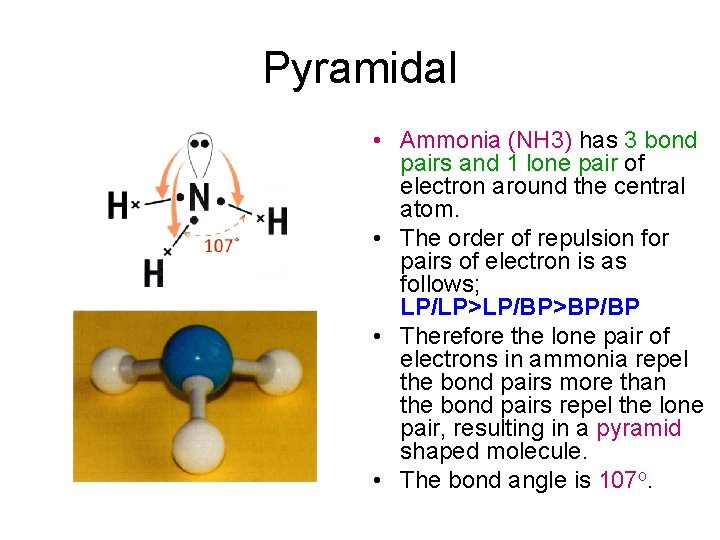
Pyramidal • Ammonia (NH 3) has 3 bond pairs and 1 lone pair of electron around the central atom. • The order of repulsion for pairs of electron is as follows; LP/LP>LP/BP>BP/BP • Therefore the lone pair of electrons in ammonia repel the bond pairs more than the bond pairs repel the lone pair, resulting in a pyramid shaped molecule. • The bond angle is 107 o.

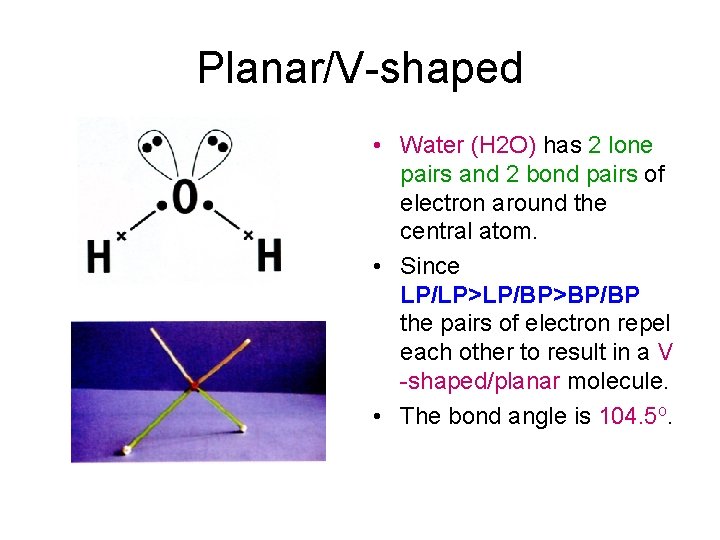
Planar/V-shaped • Water (H 2 O) has 2 lone pairs and 2 bond pairs of electron around the central atom. • Since LP/LP>LP/BP>BP/BP the pairs of electron repel each other to result in a V -shaped/planar molecule. • The bond angle is 104. 5 o.

Summary