Chapter 5 Ions and Ionic Compounds 5 1






































- Slides: 65

Chapter 5: Ions and Ionic Compounds

5. 1 Ions

Ion � Ion: an atom or group of atoms that has a charge as a result of losing or gaining one or more electrons

Cation � Ca+ion: positively charged ion � Formed from a metal atom losing electrons

Anion � Anion: negatively charged ion � Formed from a nonmetal atom gaining electrons

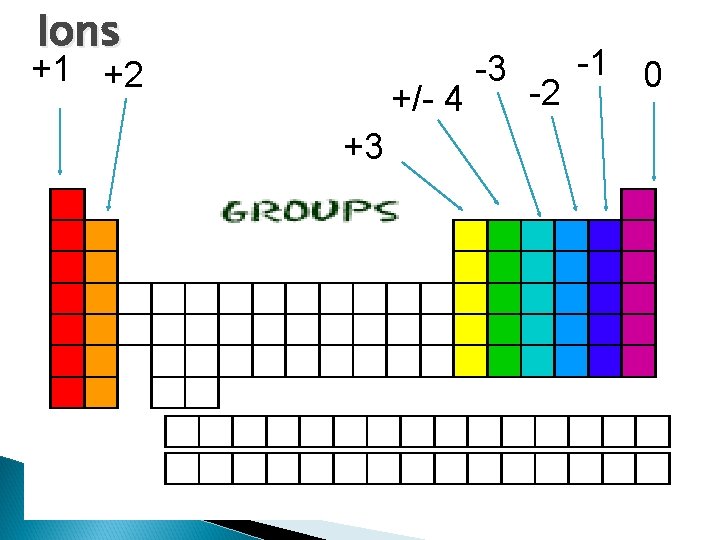
Ions +1 +2 +/- 4 +3 -3 -1 0 -2

Metals and Roman Numerals � Metals can have various charges, so you MUST specify which one you have! � The charge of a metal is indicated by a Roman Numeral. � Exceptions: Groups 1 & 2, Silver (Ag), and Zinc (Zn) have only one charge, so they do not get Roman numerals in their names! � Examples: 1. ) Copper (II) = Cu+2 2. ) Iron (III) = Fe+2

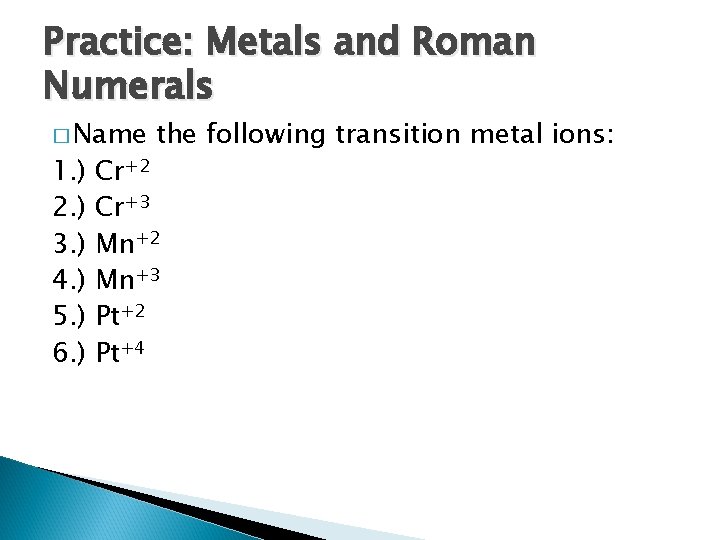
Practice: Metals and Roman Numerals � Name 1. ) 2. ) 3. ) 4. ) 5. ) 6. ) the following transition metal ions: Cr+2 Cr+3 Mn+2 Mn+3 Pt+2 Pt+4

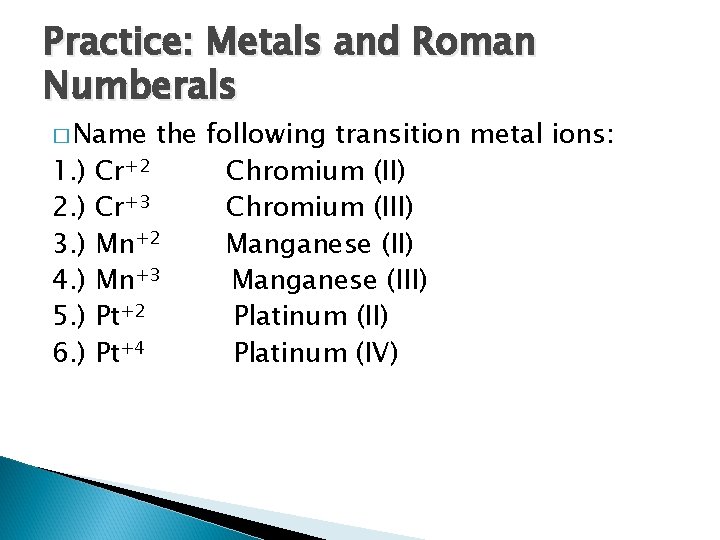
Practice: Metals and Roman Numberals � Name 1. ) 2. ) 3. ) 4. ) 5. ) 6. ) the following transition metal ions: Cr+2 Chromium (II) Cr+3 Chromium (III) Mn+2 Manganese (II) Mn+3 Manganese (III) Pt+2 Platinum (II) Pt+4 Platinum (IV)

Practice: Ions � Determine if the following ions are cations (metal) or anions (nonmetal): 1. ) Calcium ion 2. ) Nitrogen ion 3. ) Potassium ion 4. ) Bromine ion 5. ) Oxygen ion 6. ) Lead (IV) ion

Practice: Ions � Determine if the following ions are cations or anions: 1. ) Calcium ion: cation 2. ) Nitrogen ion: anion 3. ) Potassium ion: cation 4. ) Bromine ion: anion 5. ) Oxygen ion: anion 6. ) Lead (IV) ion: cation

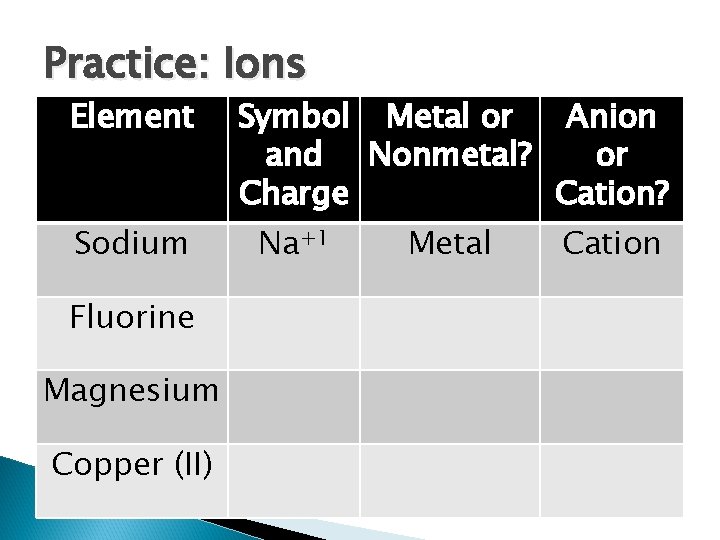
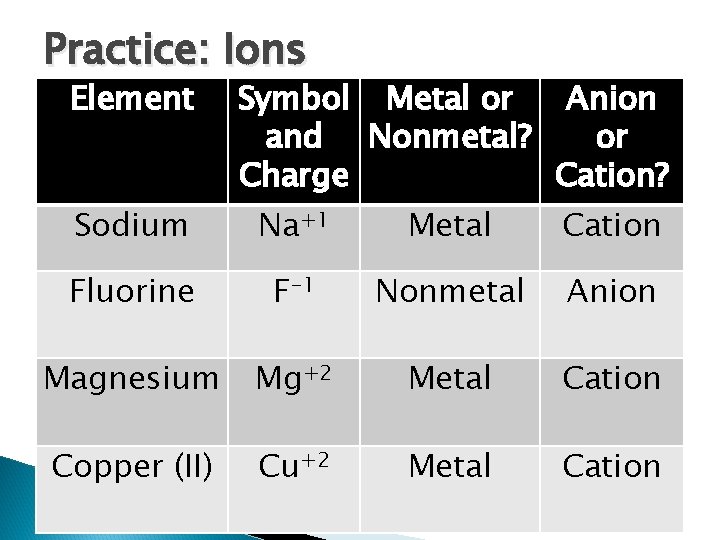
Practice: Ions Element Sodium Fluorine Magnesium Copper (II) Symbol Metal or Anion and Nonmetal? or Charge Cation? Na+1 Metal Cation

Practice: Ions Element Symbol Metal or Anion and Nonmetal? or Charge Cation? Sodium Na+1 Metal Cation Fluorine F-1 Nonmetal Anion Magnesium Mg+2 Metal Cation Copper (II) Cu+2 Metal Cation

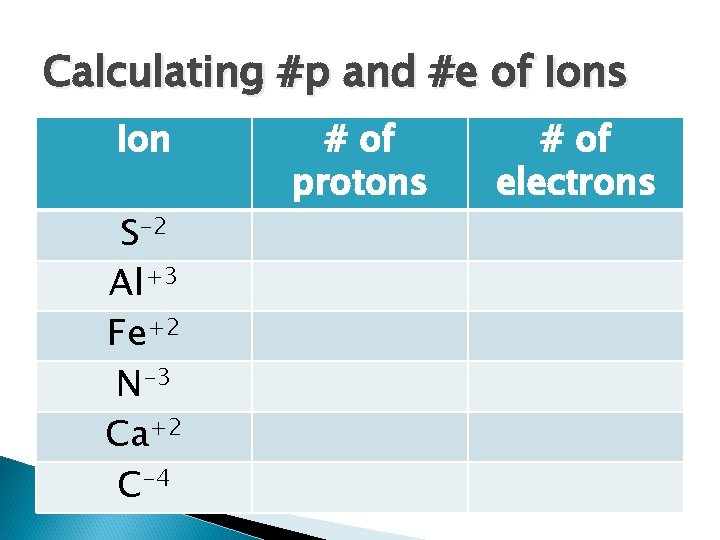
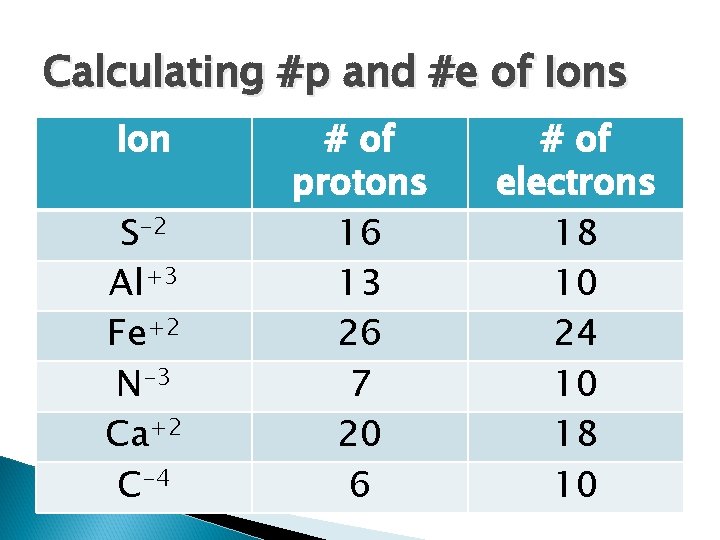
Calculating #p and #e of Ions Ion S-2 Al+3 Fe+2 N-3 Ca+2 C-4 # of protons # of electrons

Calculating #p and #e of Ions Ion S-2 Al+3 Fe+2 N-3 Ca+2 C-4 # of protons 16 13 26 7 20 6 # of electrons 18 10 24 10 18 10

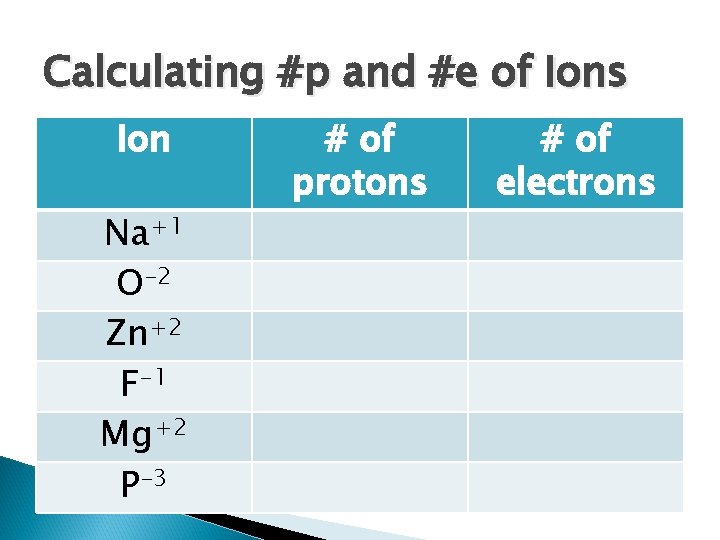
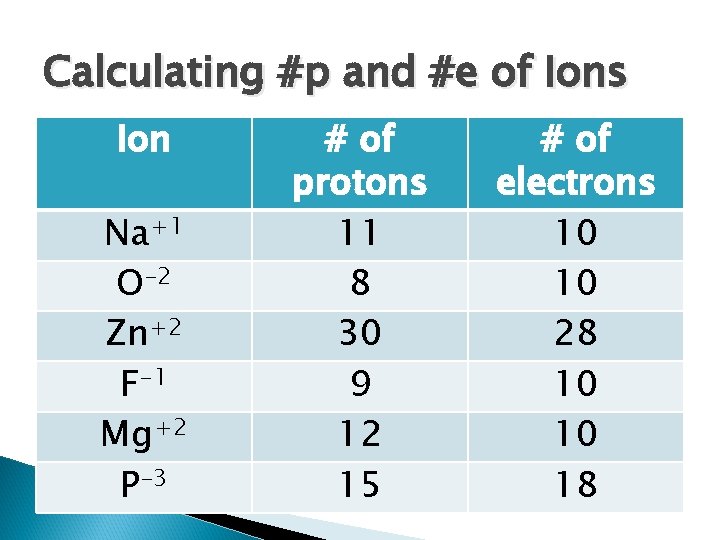
Calculating #p and #e of Ions Ion Na+1 O-2 Zn+2 F-1 Mg+2 P-3 # of protons # of electrons

Calculating #p and #e of Ions Ion Na+1 O-2 Zn+2 F-1 Mg+2 P-3 # of protons 11 8 30 9 12 15 # of electrons 10 10 28 10 10 18

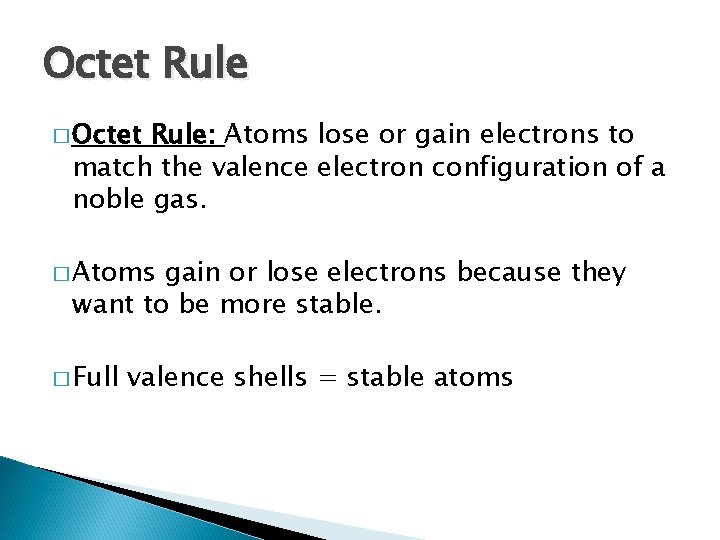
Octet Rule � Octet Rule: Atoms lose or gain electrons to match the valence electron configuration of a noble gas. � Atoms gain or lose electrons because they want to be more stable. � Full valence shells = stable atoms

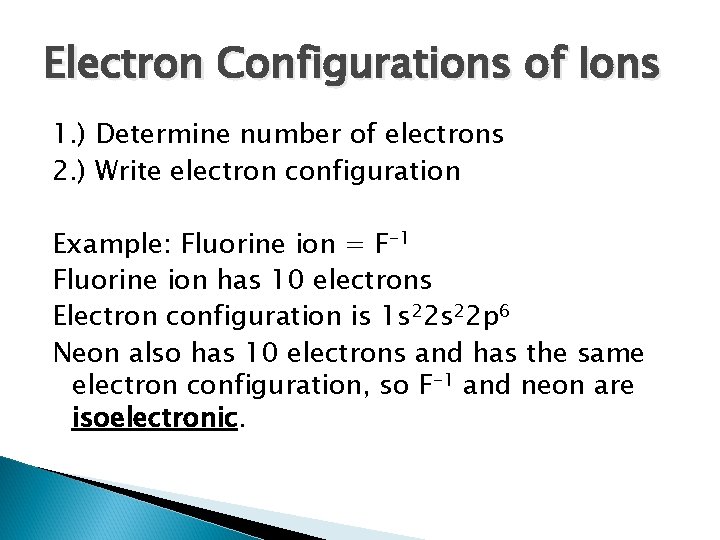
Electron Configurations of Ions 1. ) Determine number of electrons 2. ) Write electron configuration Example: Fluorine ion = F-1 Fluorine ion has 10 electrons Electron configuration is 1 s 22 p 6 Neon also has 10 electrons and has the same electron configuration, so F-1 and neon are isoelectronic.

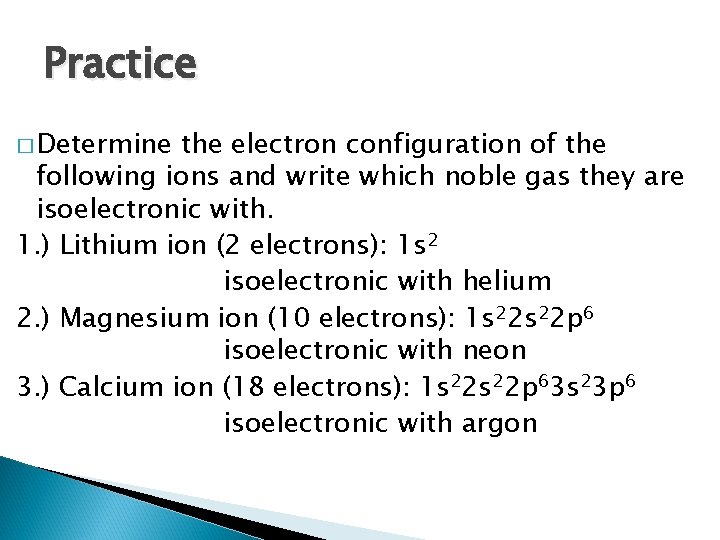
Practice � Determine the electron configuration of the following ions and write which noble gas they are isoelectronic with. 1. ) Lithium ion 2. ) Magnesium ion 3. ) Calcium ion

Practice � Determine the electron configuration of the following ions and write which noble gas they are isoelectronic with. 1. ) Lithium ion (2 electrons): 1 s 2 isoelectronic with helium 2. ) Magnesium ion (10 electrons): 1 s 22 p 6 isoelectronic with neon 3. ) Calcium ion (18 electrons): 1 s 22 p 63 s 23 p 6 isoelectronic with argon

5. 2 Ionic Bonds and Ionic Compounds

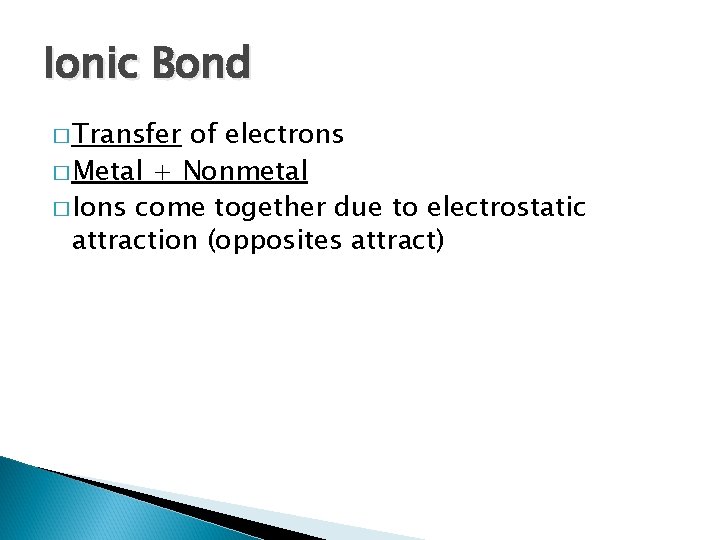
Ionic Bond � Transfer of electrons � Metal + Nonmetal � Ions come together due to electrostatic attraction (opposites attract)

Examples Determine if the following compounds are ionic: � Na. Cl : metal and nonmetal = ionic � CO 2 : metal and nonmetal = not ionic

Practice � Determine ionic: 1. ) CO 2. ) Mg. Cl 2 3. ) Ca. Br 2 4. ) H 2 O 5. ) Li. F if the following compounds are

Practice � Determine if the following compounds are ionic: 1. ) CO = nonmetal + nonmetal = not ionic 2. ) Mg. Cl 2 = metal + nonmetal = ionic 3. ) Ca. Br 2 = metal + nonmetal = ionic 4. ) H 2 O = metal + metal = not ionic 5. ) Li. F = metal + nonmental = ionic

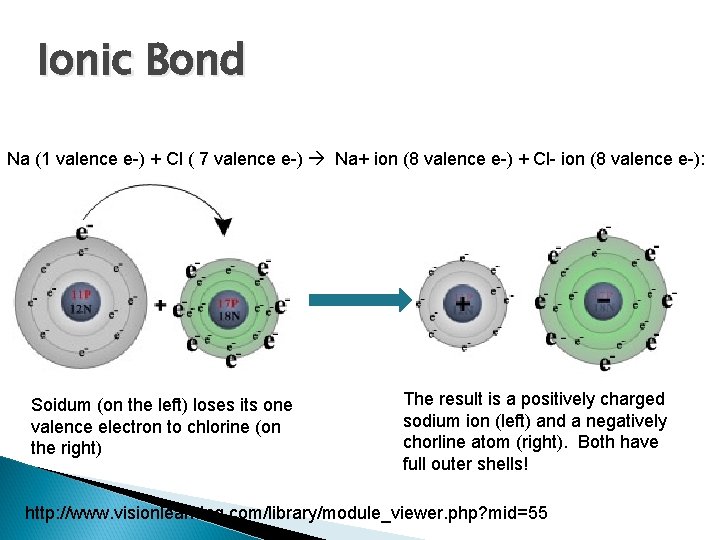
Ionic Bond Na (1 valence e-) + Cl ( 7 valence e-) Na+ ion (8 valence e-) + Cl- ion (8 valence e-): Soidum (on the left) loses its one valence electron to chlorine (on the right) The result is a positively charged sodium ion (left) and a negatively chorline atom (right). Both have full outer shells! http: //www. visionlearning. com/library/module_viewer. php? mid=55

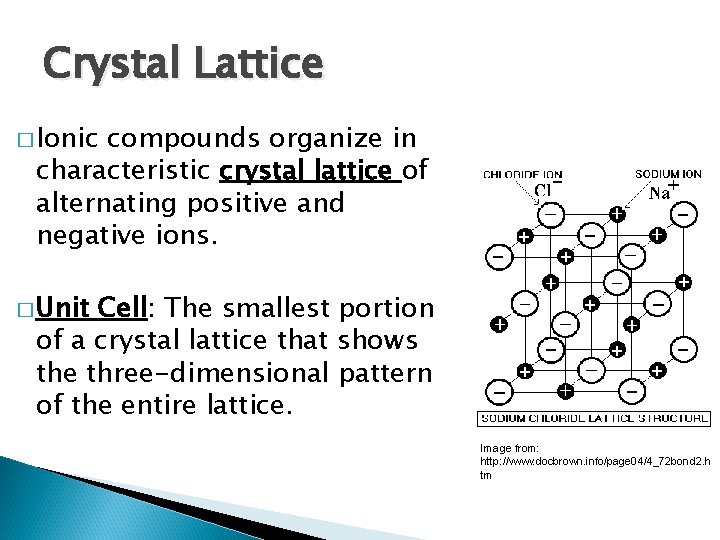
Crystal Lattice � Ionic compounds organize in characteristic crystal lattice of alternating positive and negative ions. � Unit Cell: The smallest portion of a crystal lattice that shows the three-dimensional pattern of the entire lattice. Image from: http: //www. docbrown. info/page 04/4_72 bond 2. h tm

Lattice Energy � When ionic bonds are formed, the energy that is released is called the lattice energy. � Alternatively, you can think of it as the amount of energy required to break the ionic bonds of a compound.

Examples � Sodium chloride (Na. Cl) dissolves in water: low lattice energy � Magnesium oxide (Mg. O) does not dissolve in water: high lattice energy

Properties of Ionic Compounds � Solid at room temperature � High boiling/melting points � Dissolve in water � Conduct electricity � Hard (the crystal is able to resist a large force applied to it) � Brittle (applied force results in fracture, not dents)

How to Identify an Ionic Compound 1. ) Identify the state of matter -should be a solid at room temperature 2. ) Tap it -will not break (hard) OR -will fracture if it breaks (brittle) 3. ) Heat it -will only melt at high temperatures 4. ) Shock it -will conduct electricity 5. ) Dissolve it in water -will dissolve in water

Is it Ionic? � You tap the crystal and they shatter but still retain their sharp edges. � You heat the substance and after 2 -3 minutes of heating it does not melt. � It dissolves in water and conducts electricity.

Is it Ionic? � You tap the crystal and they shatter but still retain their sharp edges. Yes � You heat the substance and after 2 -3 minutes of heating it does not melt. � It dissolves in water and conducts electricity.

Is it Ionic? � You tap the crystal and they shatter but still retain their sharp edges. Yes � You heat the substance and after 2 -3 minutes of heating it does not melt. Yes � It dissolves in water and conducts electricity.

Is it Ionic? � You tap the crystal and they shatter but still retain their sharp edges. Yes � You heat the substance and after 2 -3 minutes of heating it does not melt. Yes � It dissolves in water and conducts electricity. Yes

5. 3 Names and Formulas of Ionic Compounds

Writing Formulas Binary Ionic Compounds

Ionic Compounds � Metal + Nonmetal � Cation = positive charge � Anion = negative charge

Writing Ionic Formulas 1. Write the symbol of the cation. 2. Write the charge of the cation. 3. Write the symbol of the anion. 4. Write the charge of the anion.

5. Criss-cross the charges and write as subscripts without the signs. 6. Rewrite the formula. 7. Reduce the subscripts. 8. Check your answer (the compound should be neutral).

Examples 1. Sodium oxide 2. Aluminum sulfide 3. Barium chloride 4. Magnesium oxide

Examples 1. Sodium oxide = Na 2 O 2. Aluminum sulfide = Al 2 S 3 3. Barium chloride = Ba. Cl 2 4. Magnesium oxide = Mg. O

Recall: Metals & The Stock System � The metal will have its charge listed behind its name as a roman numeral in parentheses. � Example: Iron(III) bromide = Fe. Br 3 � Exceptions: Groups 1&2, Ag, and Zn don’t have Roman numerals because they have only one charge!

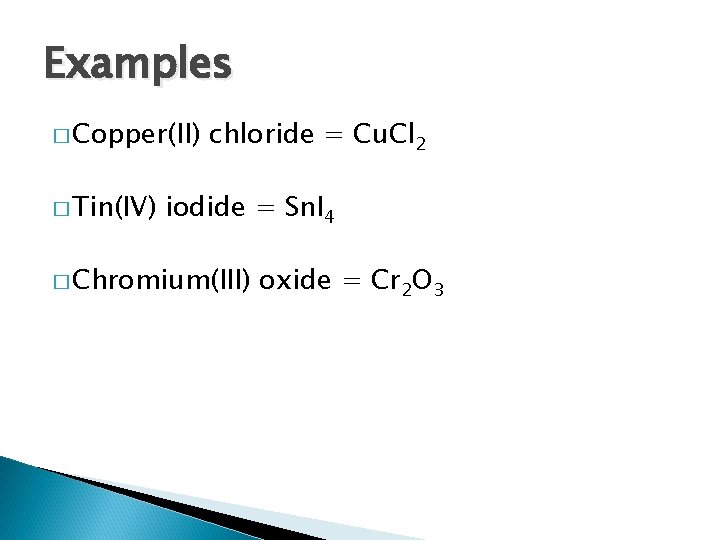
Examples � Copper(II) � Tin(IV) chloride iodide � Chromium(III) oxide

Examples � Copper(II) � Tin(IV) chloride = Cu. Cl 2 iodide = Sn. I 4 � Chromium(III) oxide = Cr 2 O 3

Writing Formulas Polyatomic Ionic Compounds

Monatomic Ion � An ion made up of one atom.

Polyatomic Ion �A charged group of 2 or more covalently bonded atoms.

Writing Formulas with Polyatomic Ions � When writing the polyatomic ion, put parentheses around the formula. � When criss-crossing the charges, drop the subscript behind the parentheses.

� NEVER change any subscripts inside the parenthesis.

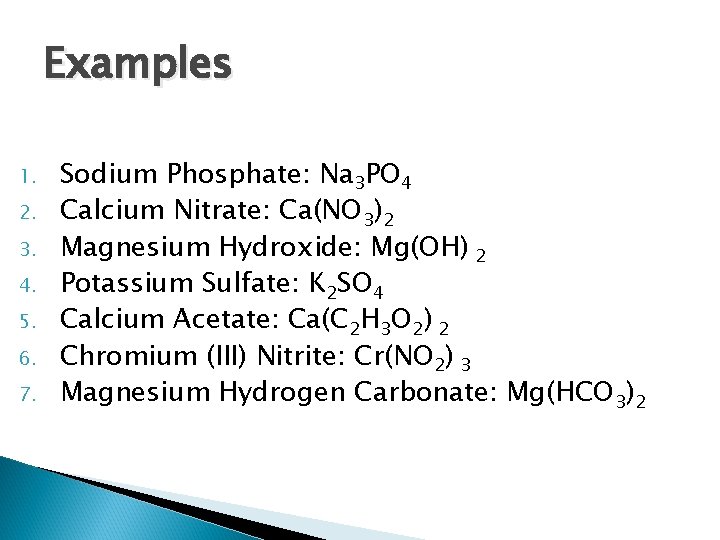
Examples 1. 2. 3. 4. 5. 6. 7. Sodium Phosphate Calcium Nitrate Magnesium Hydroxide Potassium Sulfate Calcium Acetate Chromium (III) Nitrite Magnesium Hydrogen Carbonate

Examples 1. 2. 3. 4. 5. 6. 7. Sodium Phosphate: Na 3 PO 4 Calcium Nitrate: Ca(NO 3)2 Magnesium Hydroxide: Mg(OH) 2 Potassium Sulfate: K 2 SO 4 Calcium Acetate: Ca(C 2 H 3 O 2) 2 Chromium (III) Nitrite: Cr(NO 2) 3 Magnesium Hydrogen Carbonate: Mg(HCO 3)2

Naming Compounds Binary Ionic Compounds

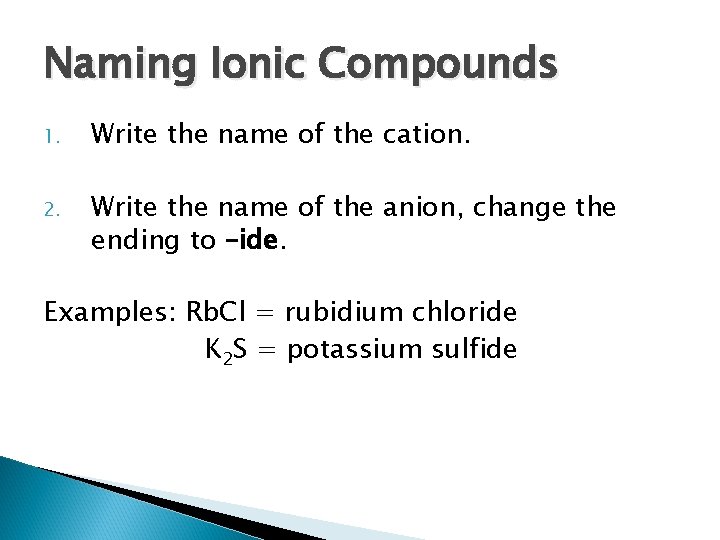
Naming Ionic Compounds 1. Write the name of the cation. 2. Write the name of the anion, change the ending to –ide. Examples: Rb. Cl = rubidium chloride K 2 S = potassium sulfide

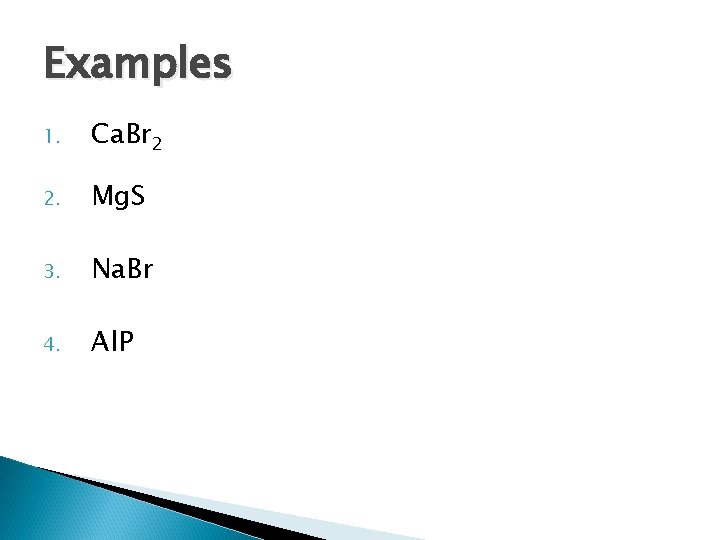
Examples 1. Ca. Br 2 2. Mg. S 3. Na. Br 4. Al. P

Examples 1. Ca. Br 2 = calcium bromide 2. Mg. S = magnesium sulfide 3. Na. Br = sodium bromide 4. Al. P = aluminum phosphide

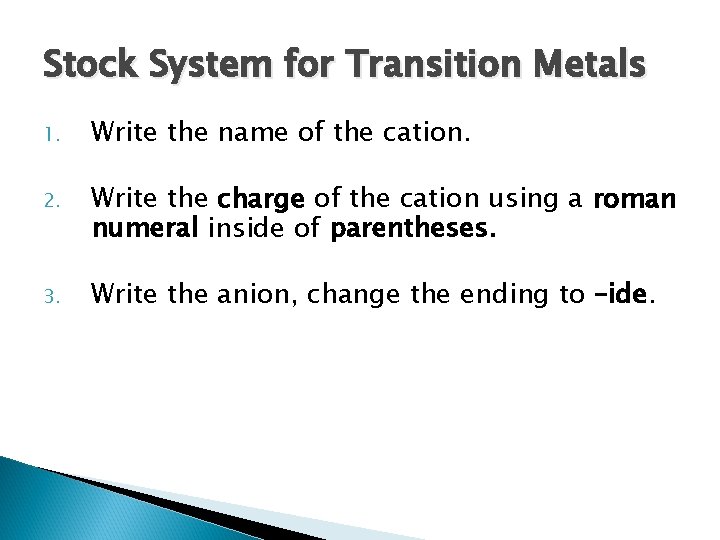
Stock System for Transition Metals 1. Write the name of the cation. 2. Write the charge of the cation using a roman numeral inside of parentheses. 3. Write the anion, change the ending to –ide.

Exceptions � Metals in Groups 1 and 2, Zinc and Silver are the only metals that will not follow the stock system because they only ever have one charge.

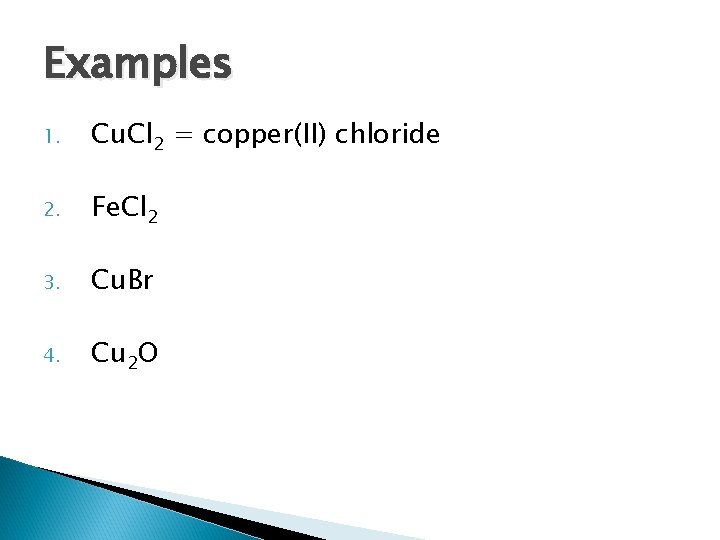
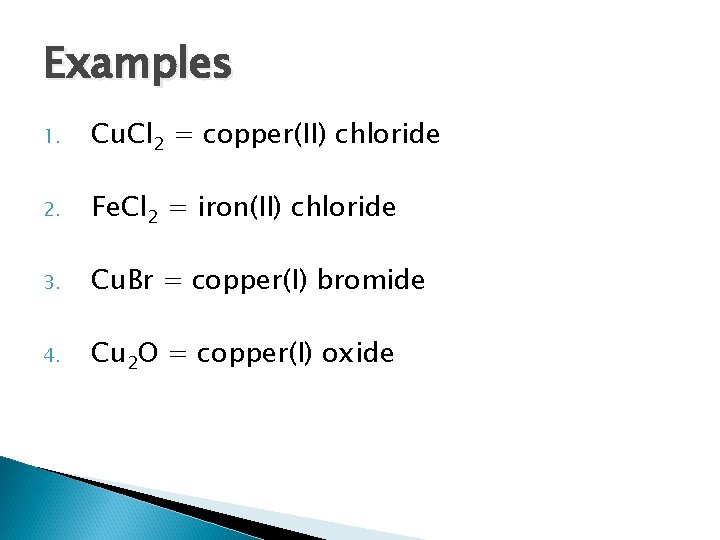
Examples 1. Cu. Cl 2 = copper(II) chloride 2. Fe. Cl 2 3. Cu. Br 4. Cu 2 O

Examples 1. Cu. Cl 2 = copper(II) chloride 2. Fe. Cl 2 = iron(II) chloride 3. Cu. Br = copper(I) bromide 4. Cu 2 O = copper(I) oxide

Naming Compounds Polyatomic Ionic Compounds

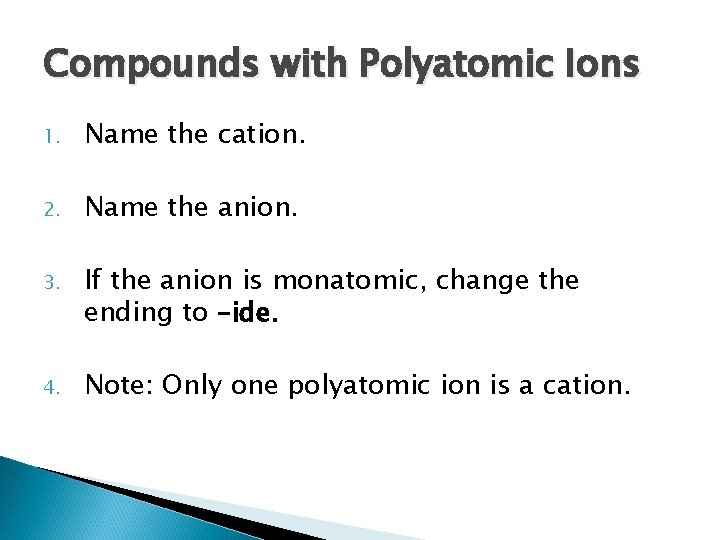
Compounds with Polyatomic Ions 1. Name the cation. 2. Name the anion. 3. If the anion is monatomic, change the ending to –ide. 4. Note: Only one polyatomic ion is a cation.

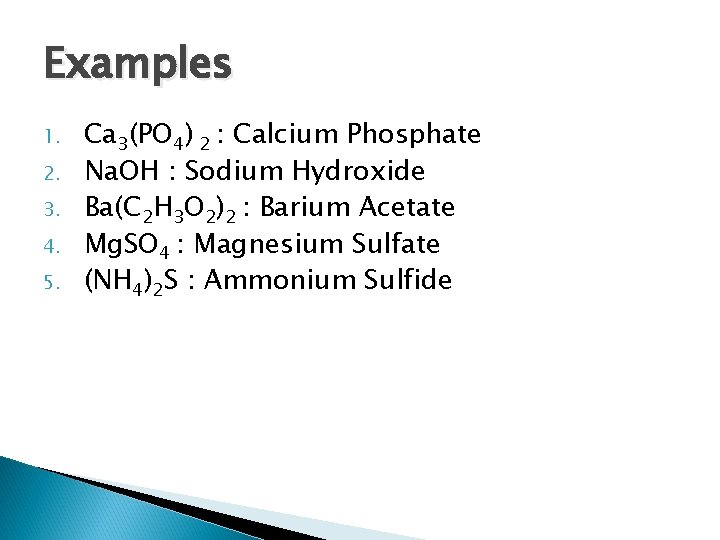
Examples 1. 2. 3. 4. 5. Ca 3(PO 4) 2 Na. OH Ba(C 2 H 3 O 2)2 Mg. SO 4 (NH 4)2 S

Examples 1. 2. 3. 4. 5. Ca 3(PO 4) 2 : Calcium Phosphate Na. OH : Sodium Hydroxide Ba(C 2 H 3 O 2)2 : Barium Acetate Mg. SO 4 : Magnesium Sulfate (NH 4)2 S : Ammonium Sulfide