Chapter 5 Interactions of Ionizing Radiation Ionization The






- Slides: 35

Chapter 5 Interactions of Ionizing Radiation

Ionization • The process by which a neutral atom acquires a positive or a negative charge • Directly ionizing radiation – electrons, protons, and particles – sufficient kinetic energy to produce ionization • ray • excitation • Indirectly ionizing radiation – neutrons and photons – to release directly ionizing particles from matter when they interact with matter

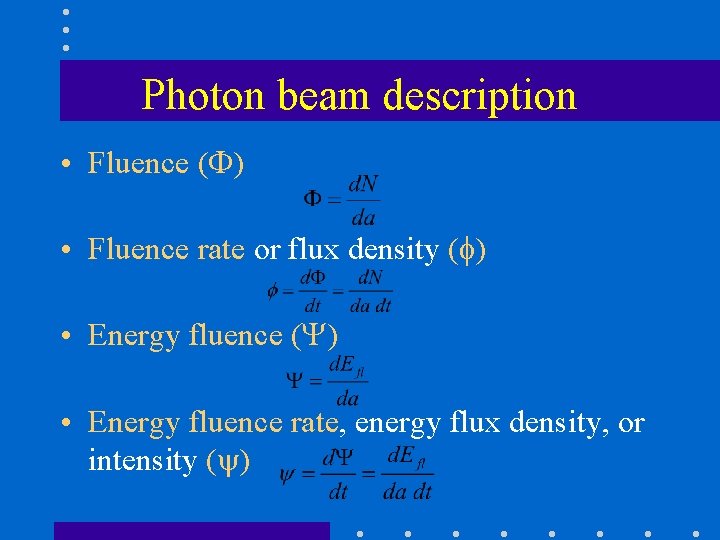
Photon beam description • Fluence ( ) • Fluence rate or flux density ( ) • Energy fluence rate, energy flux density, or intensity ( )

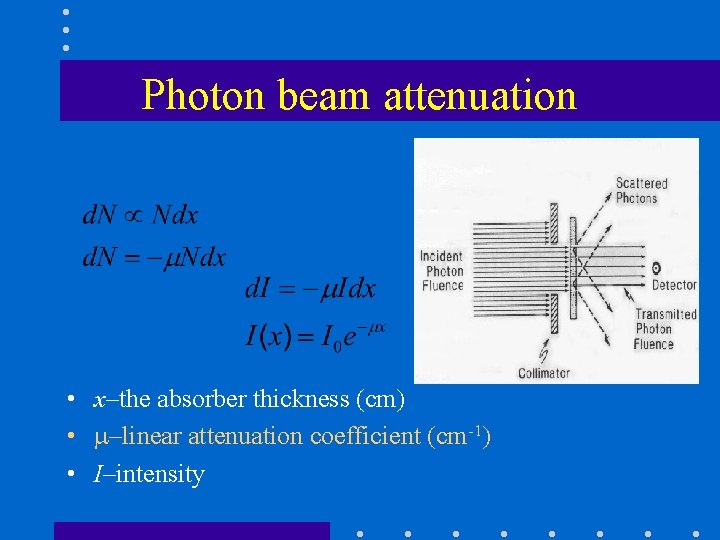
Photon beam attenuation • x–the absorber thickness (cm) • –linear attenuation coefficient (cm-1) • I–intensity

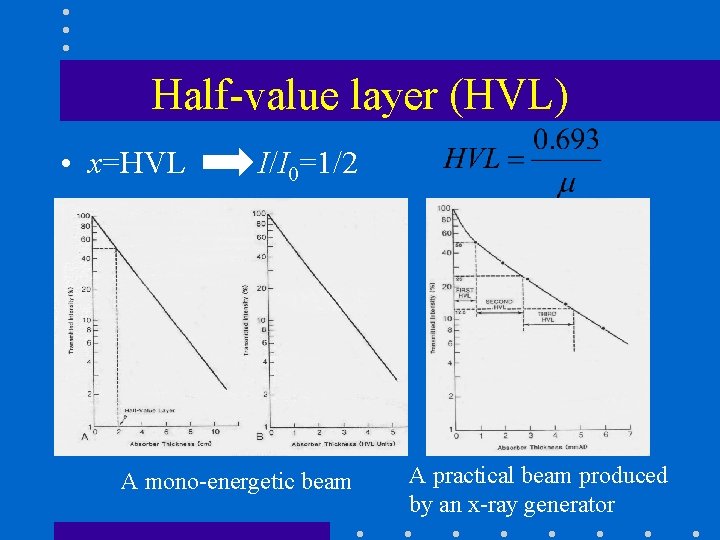
Half-value layer (HVL) • x=HVL I/I 0=1/2 A mono-energetic beam A practical beam produced by an x-ray generator

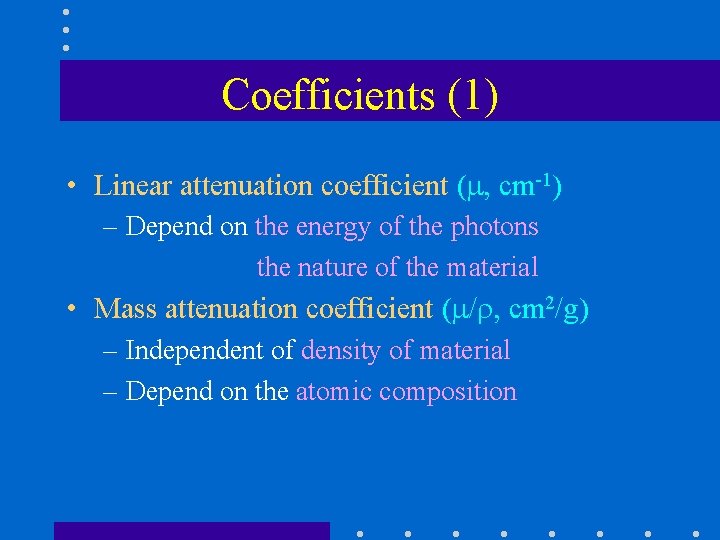
Coefficients (1) • Linear attenuation coefficient ( , cm-1) – Depend on the energy of the photons the nature of the material • Mass attenuation coefficient ( / , cm 2/g) – Independent of density of material – Depend on the atomic composition

Coefficients (2) • Electronic attenuation coefficient (e , cm 2/electron) • Atomic attenuation coefficient (a , cm 2/atom) Z the atomic number N 0 the number of electrons per gram NA Avogradro’s number AW the atomic weight

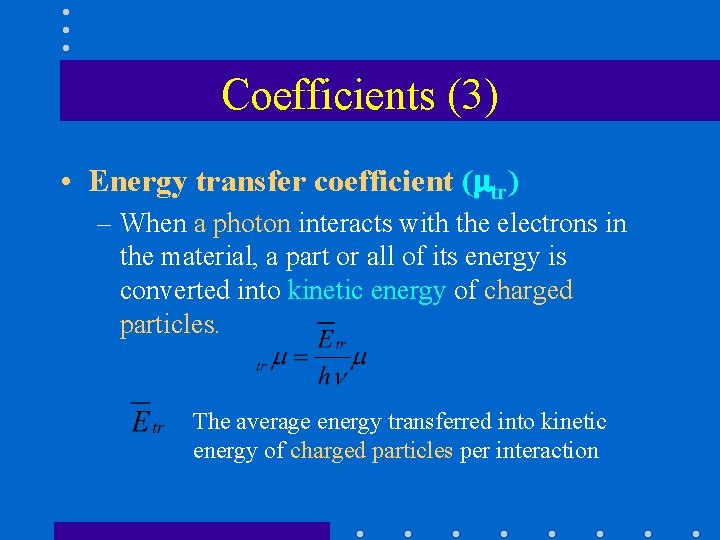
Coefficients (3) • Energy transfer coefficient ( tr) – When a photon interacts with the electrons in the material, a part or all of its energy is converted into kinetic energy of charged particles. The average energy transferred into kinetic energy of charged particles per interaction

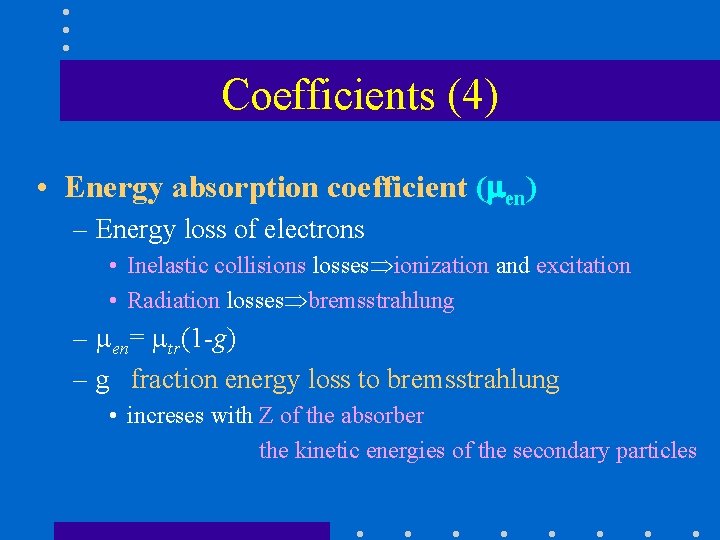
Coefficients (4) • Energy absorption coefficient ( en) – Energy loss of electrons • Inelastic collisions losses ionization and excitation • Radiation losses bremsstrahlung – en= tr(1 -g) – g fraction energy loss to bremsstrahlung • increses with Z of the absorber the kinetic energies of the secondary particles

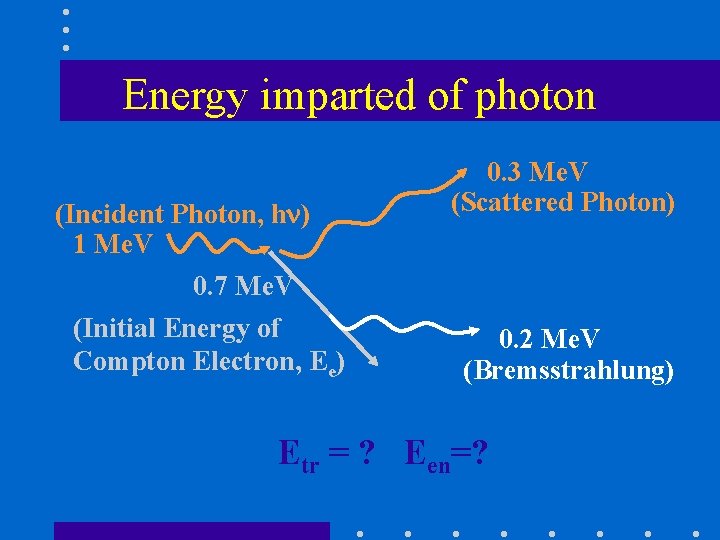
Energy imparted of photon (Incident Photon, h ) 1 Me. V 0. 7 Me. V (Initial Energy of Compton Electron, Ee) 0. 3 Me. V (Scattered Photon) 0. 2 Me. V (Bremsstrahlung) Etr = ? Een=?

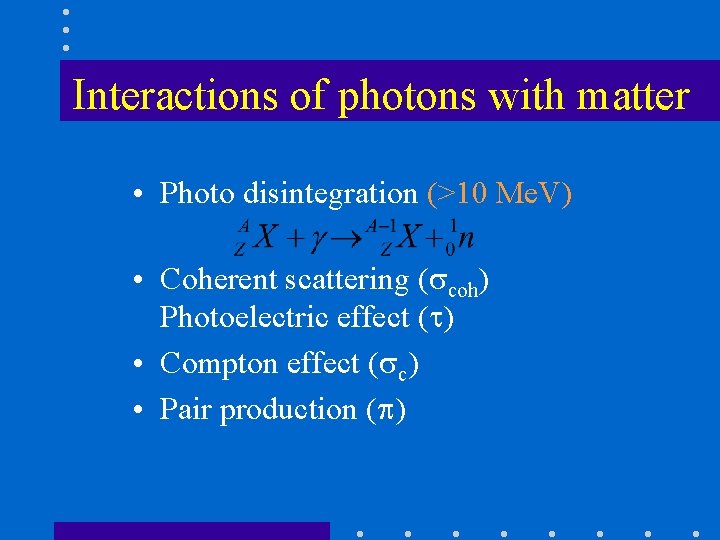
Interactions of photons with matter • Photo disintegration (>10 Me. V) • Coherent scattering ( coh) Photoelectric effect ( ) • Compton effect ( c) • Pair production ( )

Coherent scattering K L M • Classical scattering or Rayleigh scattering – No energy is changed into electronic motion – No energy is absorbed in the medium – The only effect is the scattering of the photon at small angles. • In high Z materials and with photons of low energy

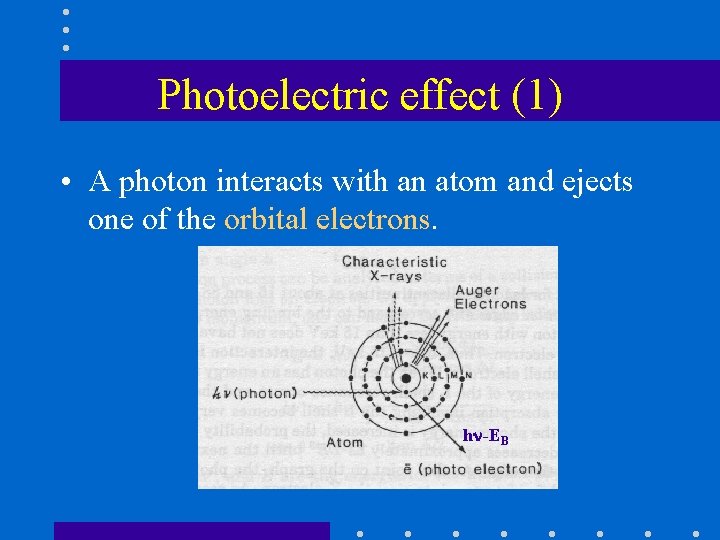
Photoelectric effect (1) • A photon interacts with an atom and ejects one of the orbital electrons. h -EB

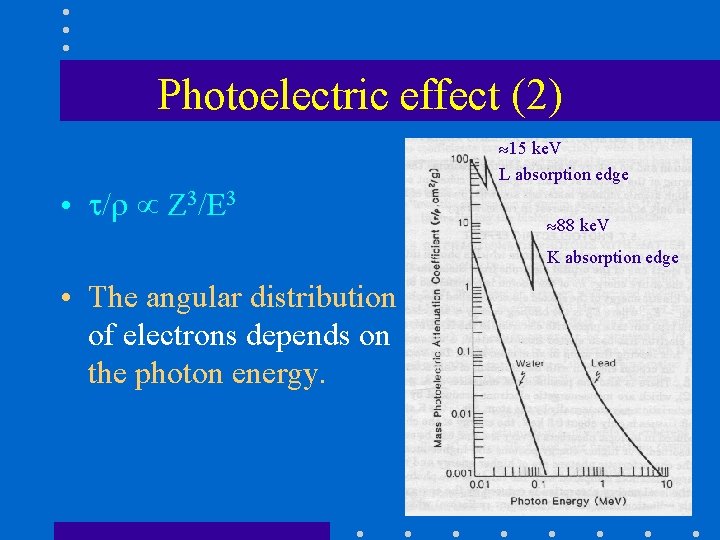
Photoelectric effect (2) • / Z 3/E 3 15 ke. V L absorption edge 88 ke. V K absorption edge • The angular distribution of electrons depends on the photon energy.

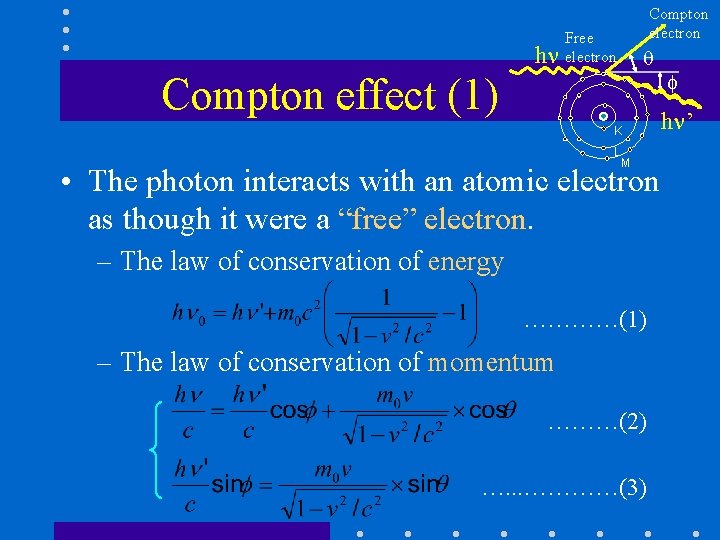
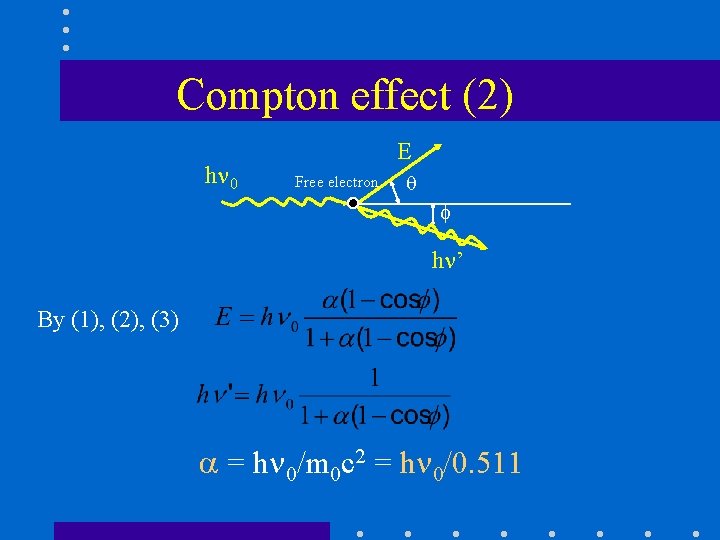
h Compton electron Free electron Compton effect (1) K L M • The photon interacts with an atomic electron as though it were a “free” electron. – The law of conservation of energy …………(1) – The law of conservation of momentum ………(2) …. . . …………(3) h ’

Compton effect (2) h 0 E Free electron h ’ By (1), (2), (3) = h 0/m 0 c 2 = h 0/0. 511

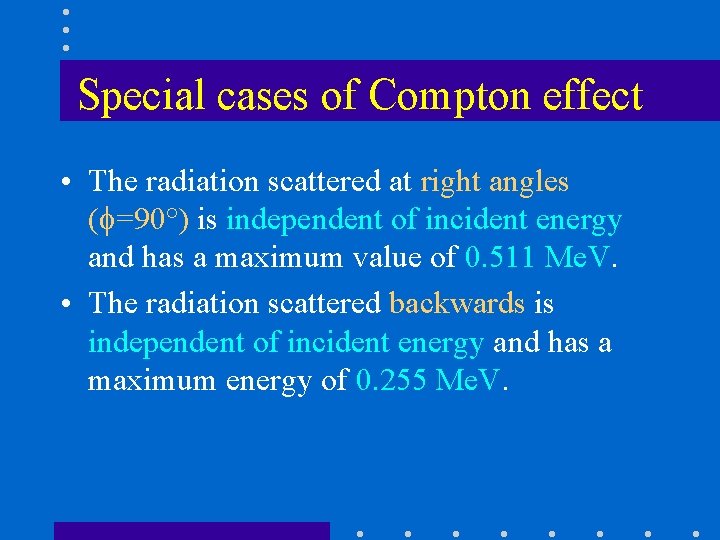
Special cases of Compton effect • The radiation scattered at right angles ( =90°) is independent of incident energy and has a maximum value of 0. 511 Me. V. • The radiation scattered backwards is independent of incident energy and has a maximum energy of 0. 255 Me. V.

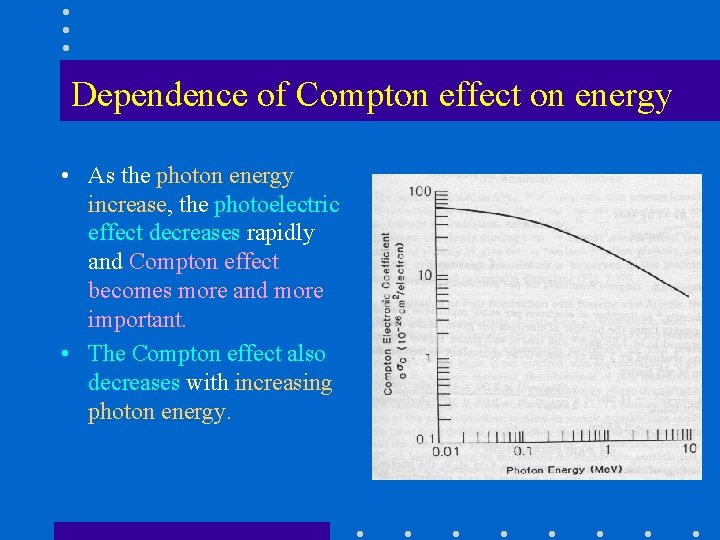
Dependence of Compton effect on energy • As the photon energy increase, the photoelectric effect decreases rapidly and Compton effect becomes more and more important. • The Compton effect also decreases with increasing photon energy.

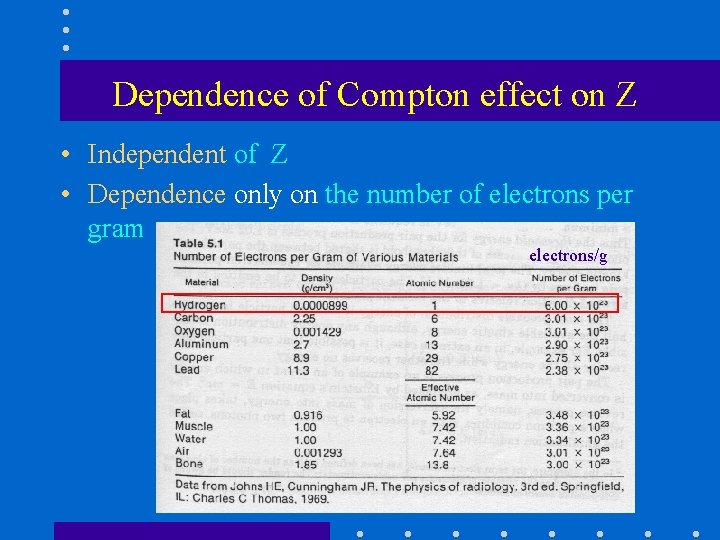
Dependence of Compton effect on Z • Independent of Z • Dependence only on the number of electrons per gram electrons/g

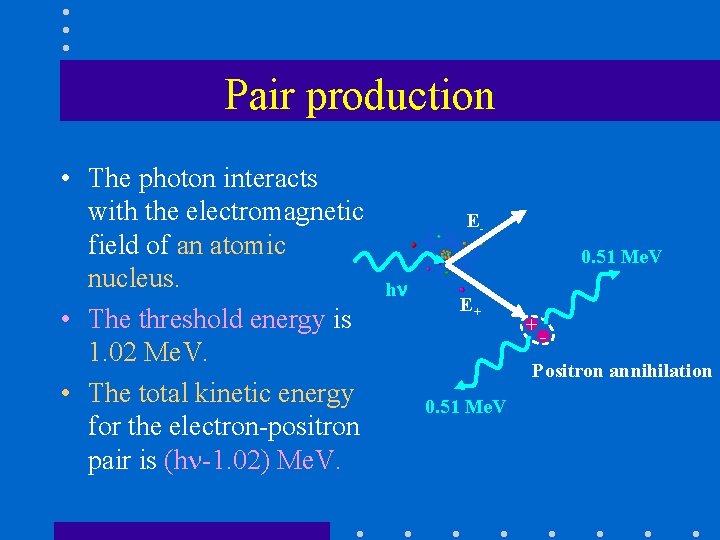
Pair production • The photon interacts with the electromagnetic field of an atomic nucleus. • The threshold energy is 1. 02 Me. V. • The total kinetic energy for the electron-positron pair is (h -1. 02) Me. V. E 0. 51 Me. V h E+ + - Positron annihilation 0. 51 Me. V

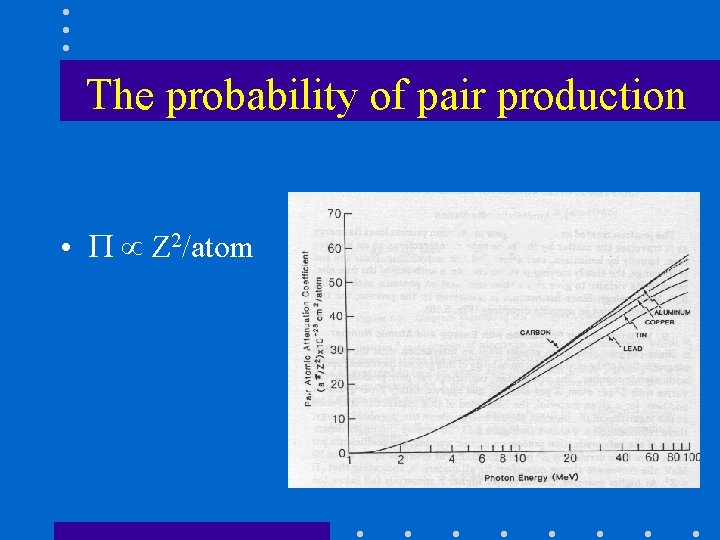
The probability of pair production • Z 2/atom

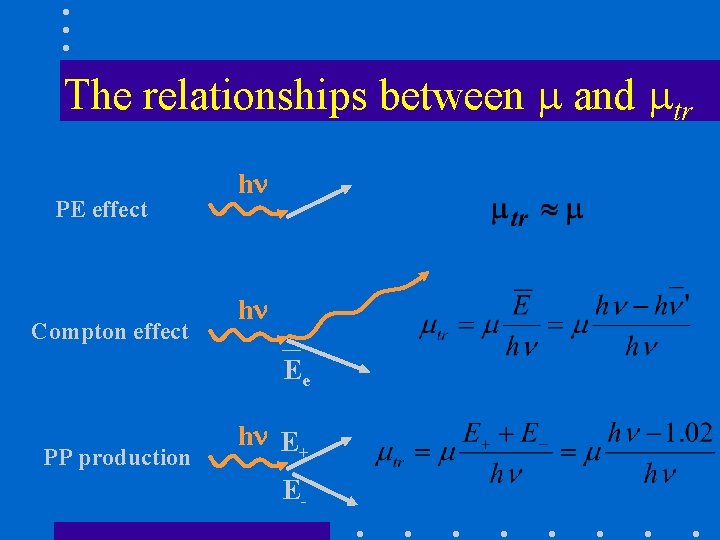
The relationships between and tr PE effect Compton effect h h Ee PP production h E+ E-

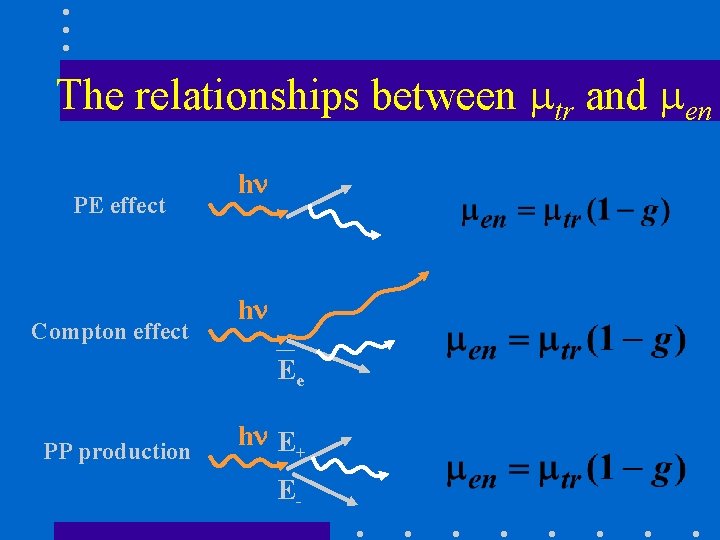
The relationships between tr and en PE effect Compton effect h h Ee PP production h E+ E-

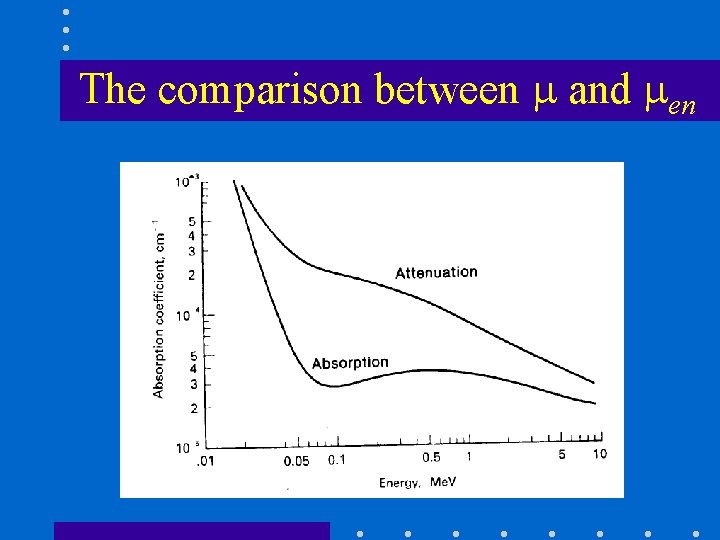
The comparison between and en

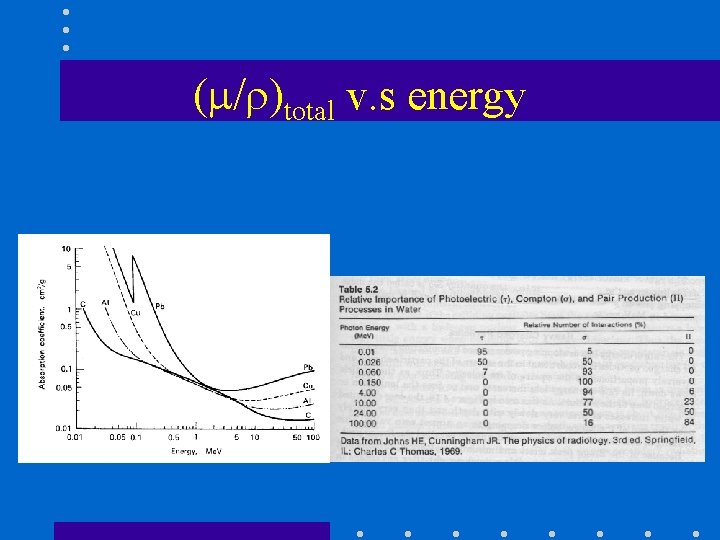
( / )total v. s energy

Interactions of charged particles • Coulomb force – Collisions between the particle and the atomic electrons result in ionization and excitation. – Collisions between the particle and the nucleus result in radiative loss of energy or bremsstrahlung. • Nuclear reactions • Stopping power (S) = • Mass stopping power (S/ , Me. V cm 2/g)

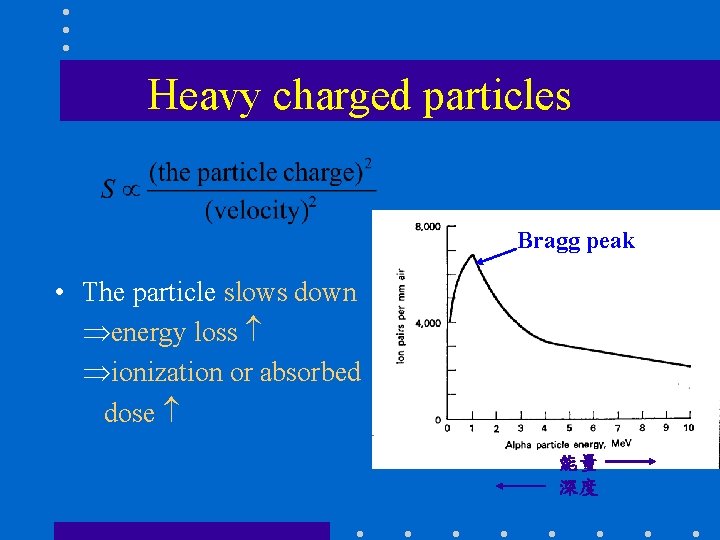
Heavy charged particles Bragg peak • The particle slows down energy loss ionization or absorbed dose 能量 深度

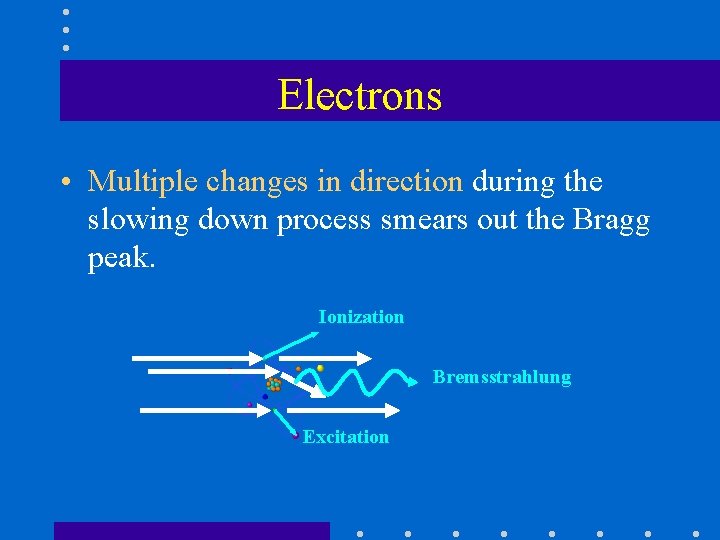
Electrons • Multiple changes in direction during the slowing down process smears out the Bragg peak. Ionization Bremsstrahlung Excitation

Interactions of neutrons • Recoiling protons from hydrogen and recoiling heavy nuclei from other elements – A billiard-ball collision – The most efficient absorbers of a neutron beam are the hydrogenous materials. • Nuclear disintegrations – The emission of heavy charged particles, neutrons, and rays – About 30% of the tissue dose

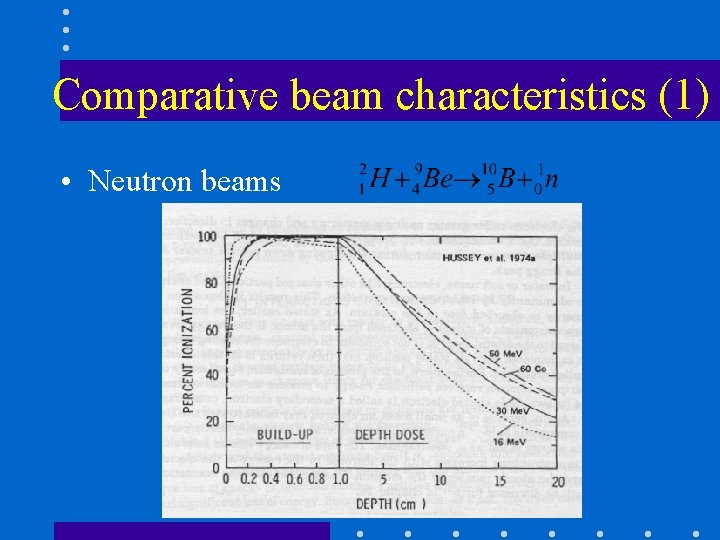
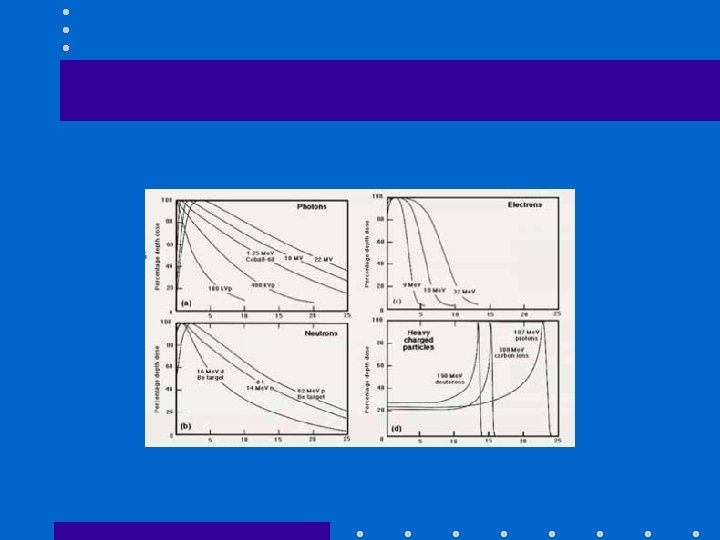
Comparative beam characteristics (1) • Neutron beams

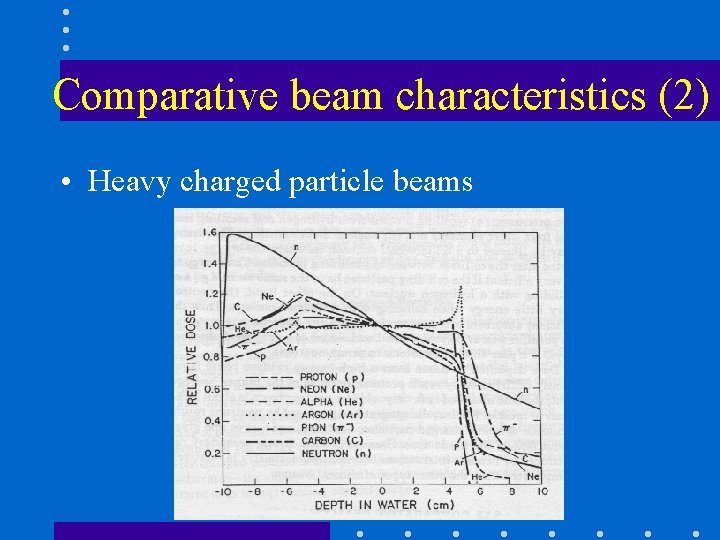
Comparative beam characteristics (2) • Heavy charged particle beams

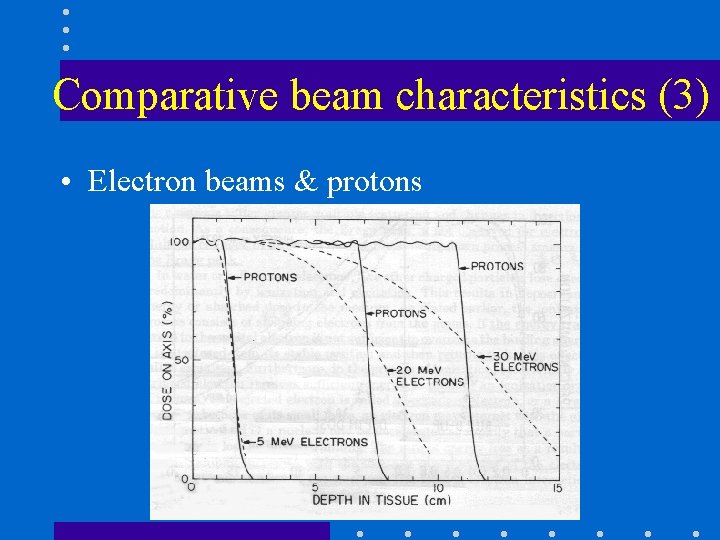
Comparative beam characteristics (3) • Electron beams & protons


Advantages of neutron, proton and heavy charged particle beams over the standard x ray and electron modalities: • Lower oxygen enhancement ratio (OER) for neutrons • Improved dose-volume histograms (DVHs) for protons and heavy charged particles. �� Disadvantage of neutron, proton and heavy charge particle beams in comparison with standard x ray and electron modalities: considerably higher capital, maintenance and servicing cost.

Thank you…