Chapter 5 Heat and Temperature TEMPERATURE Is the












- Slides: 18

Chapter 5 Heat and Temperature

TEMPERATURE �Is the average KINETIC ENERGY of particles in a sample! �Faster movement = More KE = Higher temperature �Three scales: degrees Fahrenheit (U. S. ) degrees Celsius (lab) Kelvin (SI) – not degrees!

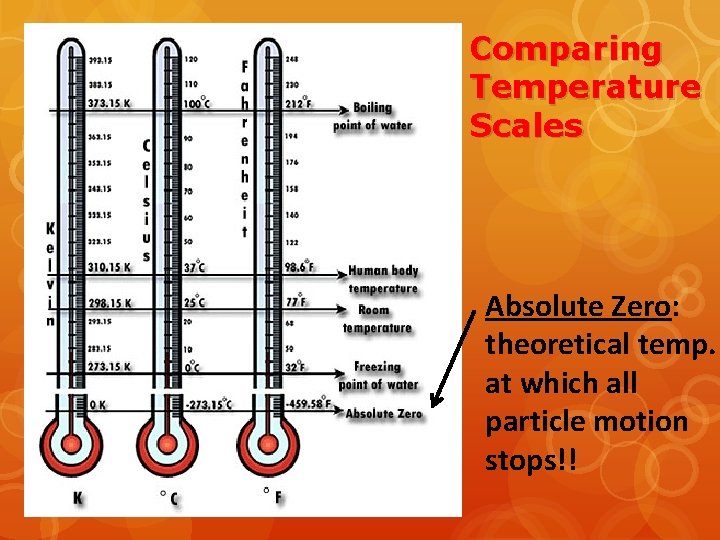
Comparing Temperature Scales Absolute Zero: theoretical temp. at which all particle motion stops!!

Converting Celsius to Fahrenheit (Celsius x 1. 8)+32 = Fahrenheit EXAMPLE: 20 degrees Celsius Conversion: (20 C x 1. 8)+32 = 68 F Converting Fahrenheit to Celsius (Fahrenheit – 32)/ 1. 8 = Celsius EXAMPLE: 95 degrees Fahrenheit Conversion: (95 F – 32)/ 1. 8 = 35 C

Converting Kelvin to Celsius K to C: K-273=C �Example: convert 375 K to degrees Celsius. �Answer: 375 − 273 = 102 °C C to K: C + 273=K Example: convert 25. 0 °C to Kelvin. Answer: 25. 0 + 273 = 298. 0

HEAT Is THERMAL ENERGY that flows from high temperature to low temperature Different materials need different amounts of heat to produce the same change in temperature (called the SPECIFIC HEAT) example: wood vs metal

HEAT TRANSFER CONDUCTION: Heat transfer between particles or objects (at diff. temps. ) in contact with each other. Heat flows from warmer object to cooler one. Ex: spoon in hot liquid becomes hot. CONVECTION: Heat transfer by movement of fluids (liquids gases). Hot fluids rise (less dense), cold fluids fall (more dense). Ex: water in a hot water tank, “radiators” that heat rooms RADIATION: Heat transfer by electromagnetic waves (infrared, visible light, UV). Can go in any direction, even through a vacuum.

CONDUCTORS Materials (usually solids) through which energy is easily transferred as heat. Ex: metals, glass

INSULATORS Poor energy (heat) conductors. Ex: wood, plastic, fiberglass, air, vacuum

THERMOS Insulation minimizes undesirable energy transfers! Ex: thermos (Keeps hot things hot, & cold things cold!) Vacuum or foam limits heat transfer Silver mirror inside and out limits infrared radiation

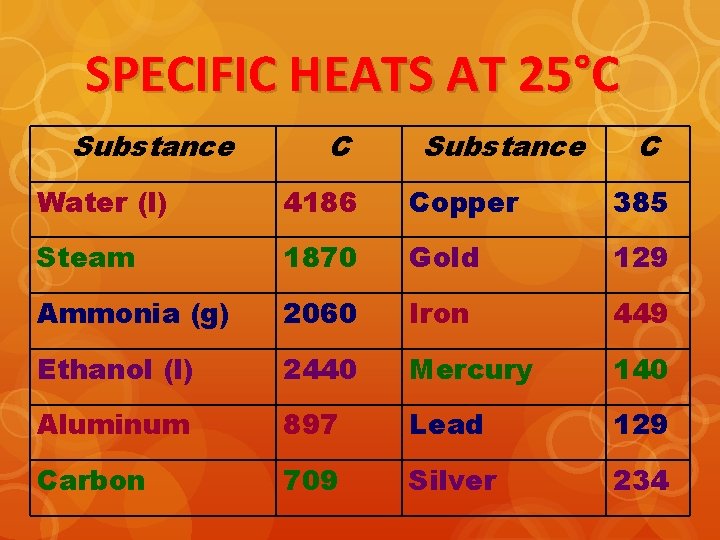
SPECIFIC HEATS AT 25°C Substance C Water (l) 4186 Copper 385 Steam 1870 Gold 129 Ammonia (g) 2060 Iron 449 Ethanol (l) 2440 Mercury 140 Aluminum 897 Lead 129 Carbon 709 Silver 234

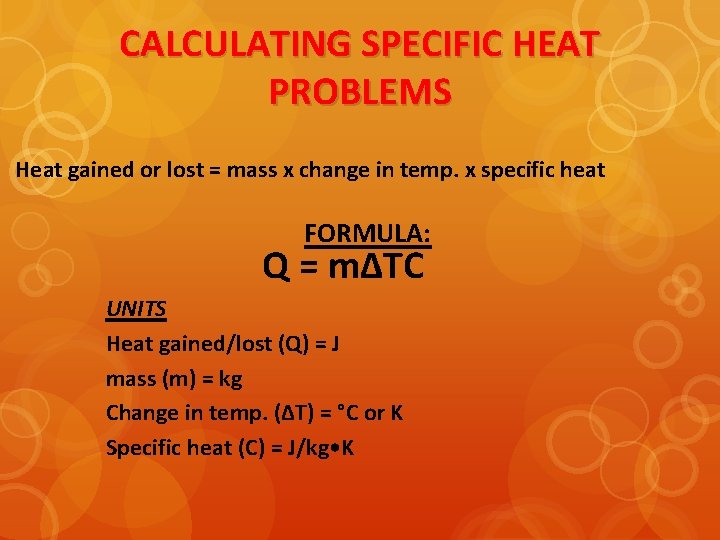
CALCULATING SPECIFIC HEAT PROBLEMS Heat gained or lost = mass x change in temp. x specific heat FORMULA: Q = mΔTC UNITS Heat gained/lost (Q) = J mass (m) = kg Change in temp. (ΔT) = °C or K Specific heat (C) = J/kg • K

SAMPLE PROBLEM How much energy must be transferred as heat to the 420 kg of water in a bathtub in order to raise the water’s temp. from 25°C to 37°C? Formula Data Q = mΔTC Q=x m = 420 kg c = 4186 J (water) ΔT = 37 -25 = 12 Work Q = (420 kg)(12°C)(4186 J/kg • K) Q = 21, 000 J or 2. 1 x 107 J

SOLVE THE PROBLEM How much energy is needed to raise the temperature of a silver necklace chain with a mass of 0. 225 kg from 25°C to 37°C? Data Formula Q = m x ∆T x Cp Complete Calculating Specific Heat Work

HEATING AND COOLING SYSTEMS DISADVANTAGES: Usable energy decreases when energy is transferred or transformed! HEATING SYSTEMS �Using work (mechanical) rubbing hands/sticks together �Using food (chemical) human body breaks down food & releases 60% energy as heat

�Using light (solar) cold-blooded animals, solar panels �Using water or air vhot water heater: electricity/burning vnatural gas heats water vcar heater: heat off engine warms air vcentral heating: furnace burns coal or natural gas, and heat is transferred to water, steam or air

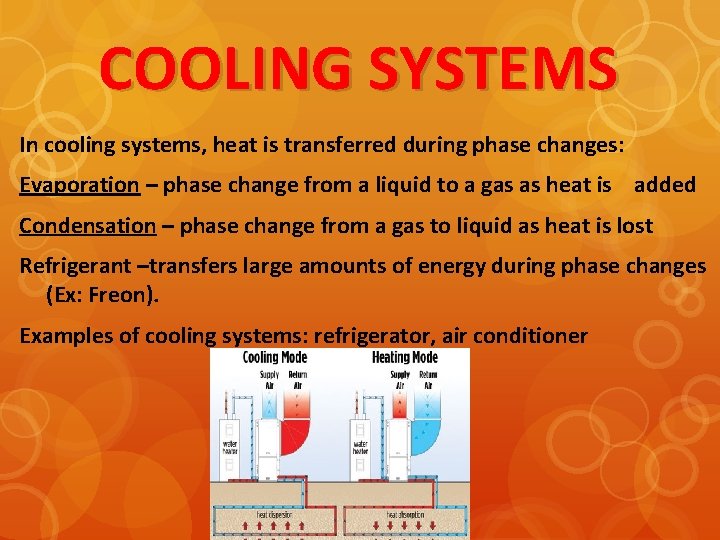
COOLING SYSTEMS In cooling systems, heat is transferred during phase changes: Evaporation – phase change from a liquid to a gas as heat is added Condensation – phase change from a gas to liquid as heat is lost Refrigerant –transfers large amounts of energy during phase changes (Ex: Freon). Examples of cooling systems: refrigerator, air conditioner

Heat Transfer in an Air Conditioner 1. A gas (refrigerant) is turned into a liquid by a compressor. 2. The cold, liquid refrigerant absorbs heat energy from air and turns back into a gas. 3. A blower blows the cooler, dryer air back through the house. 4. The gaseous refrigerant carries the heat energy pulled from the air to the outdoor unit. 5. Here the refrigerant passes through the condenser and an exhaust fan blows the heat energy into the surrounding air.