Chapter 5 Ground Rules of Metabolism Cengage Learning

Chapter 5 Ground Rules of Metabolism © Cengage Learning 2016

5. 1 A Toast to Alcohol Dehydrogenase • In the liver, the enzyme alcohol dehydrogenase breaks down ethanol • Ethanol breakdown interferes with normal processes of metabolism – Harms liver cells • Fats tend to accumulate as large globules in the tissues of heavy drinkers – Can lead to alcoholic hepatitis and cirrhosis of the liver © Cengage Learning 2016
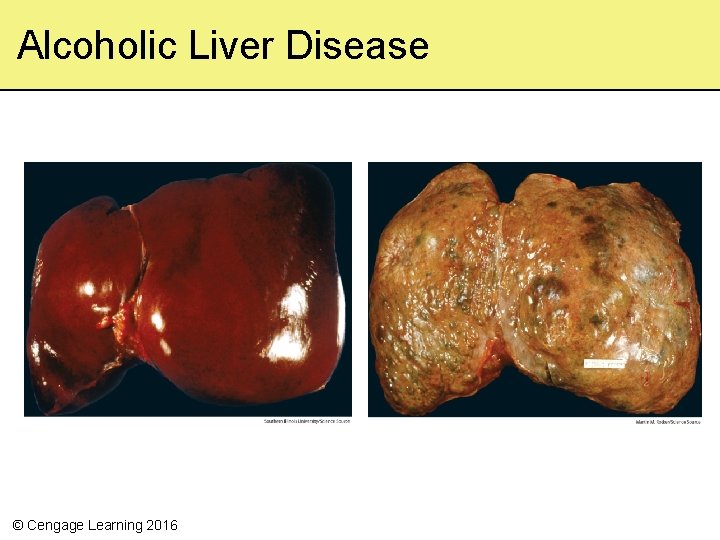
Alcoholic Liver Disease © Cengage Learning 2016

5. 2 Energy in the World of Life • Sustaining life’s organization requires ongoing energy inputs • Energy is the capacity to do work – One form of energy can be converted to another – Familiar forms of energy include light, heat, electricity, and motion (kinetic energy) – Energy in chemical bonds is a type of potential energy, because it can be stored © Cengage Learning 2016
Energy Disperses • First law of thermodynamics – Energy is neither created nor destroyed, but can be transferred from one form to another • Second law of thermodynamics – Entropy (a measure of dispersal of energy in a system) increases spontaneously – The entropy of two atoms decreases when a bond forms between them – Every time energy transfers, bits of it disperse © Cengage Learning 2016
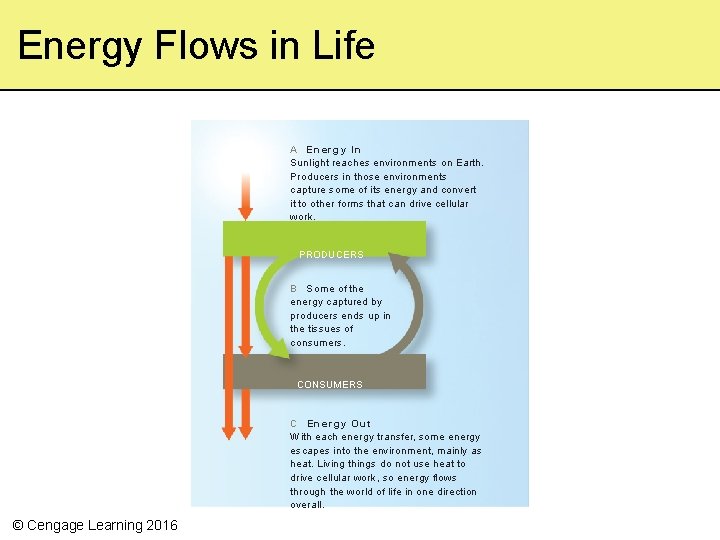
Energy Flows in Life A E n e r g y In Sunlight reaches environments on Earth. Producers in those environments capture some of its energy and convert it to other forms that can drive cellular work. PRODUCERS B Some of the energy captured by producers ends up in the tissues of consumers. CONSUMERS C Energy Out With each energy transfer, some energy escapes into the environment, mainly as heat. Living things do not use heat to drive cellular work, so energy flows through the world of life in one direction overall. © Cengage Learning 2016
5. 3 Energy in the Molecules of Life • Reaction – A chemical change that occurs when atoms, ions, or molecules interact • Reactant – Atoms, ions, or molecules that enter a reaction • Product – Atoms, ions, or molecules remaining at the end of a reaction © Cengage Learning 2016
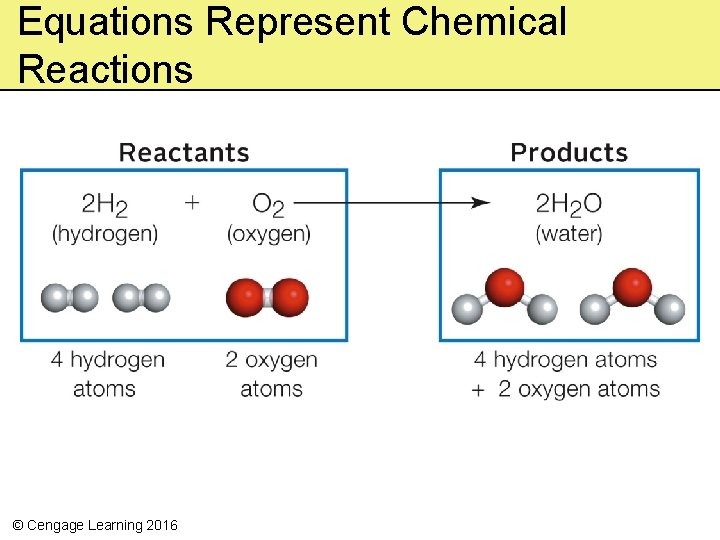
Equations Represent Chemical Reactions © Cengage Learning 2016
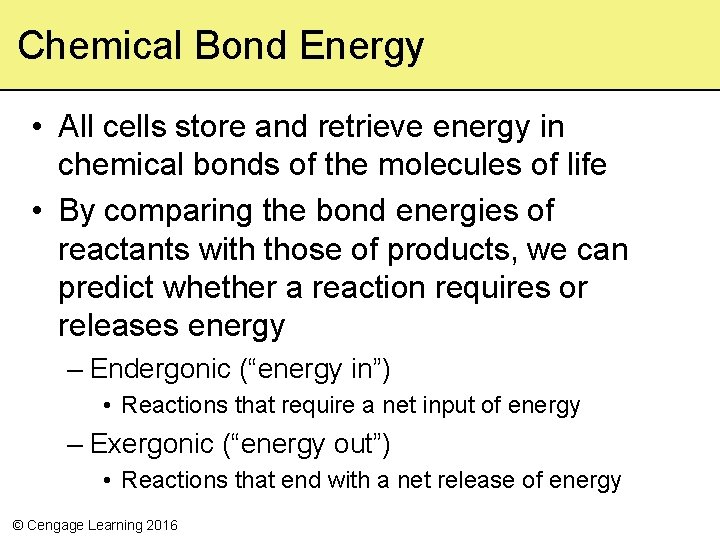
Chemical Bond Energy • All cells store and retrieve energy in chemical bonds of the molecules of life • By comparing the bond energies of reactants with those of products, we can predict whether a reaction requires or releases energy – Endergonic (“energy in”) • Reactions that require a net input of energy – Exergonic (“energy out”) • Reactions that end with a net release of energy © Cengage Learning 2016
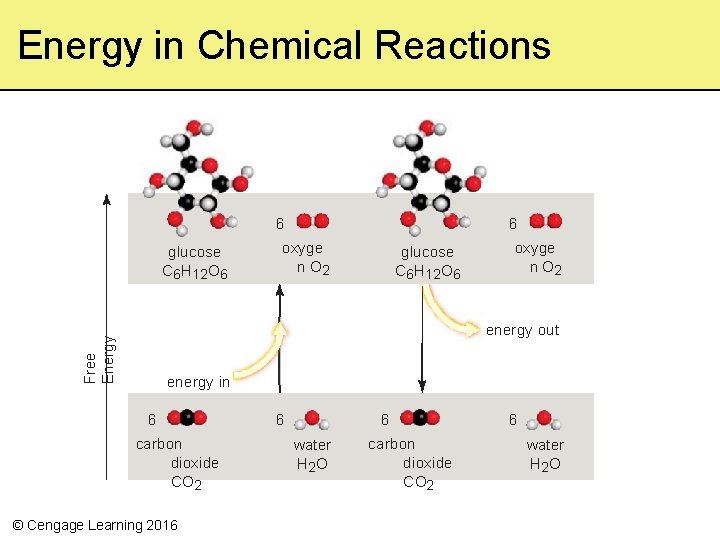
Energy in Chemical Reactions 6 glucose C 6 H 12 O 6 6 oxyge n O 2 glucose C 6 H 12 O 6 oxyge n O 2 Free Energy energy out energy in 6 carbon dioxide CO 2 © Cengage Learning 2016 6 6 water H 2 O carbon dioxide CO 2 6 water H 2 O

Why the Earth Doesn’t Go Up in Flames • Activation energy – The minimum amount of energy needed to get a reaction started – Some reactions require a lot of activation energy; others do not © Cengage Learning 2016
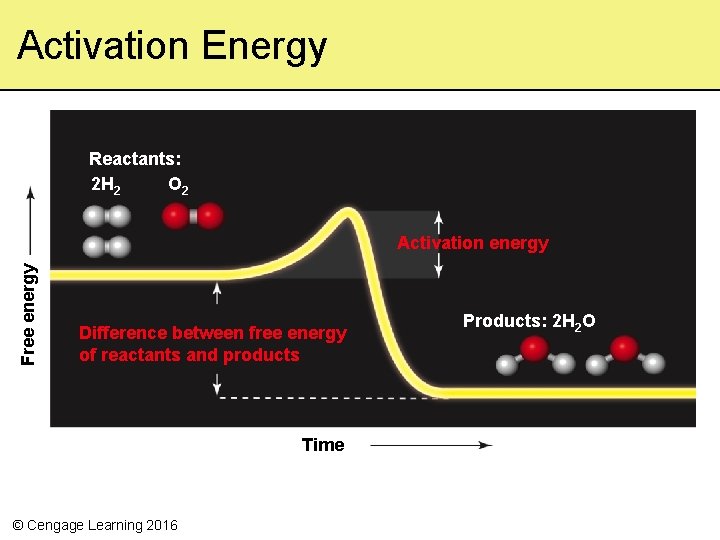
Activation Energy Reactants: 2 H 2 O 2 Free energy Activation energy Difference between free energy of reactants and products Time © Cengage Learning 2016 Products: 2 H 2 O
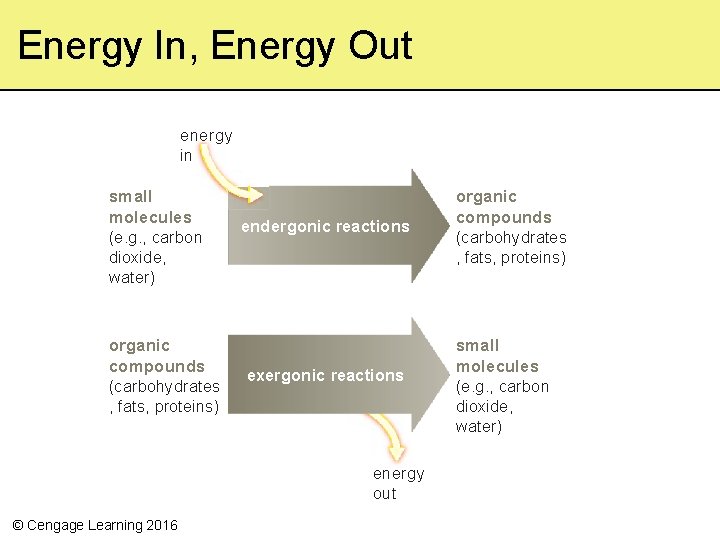
Energy In, Energy Out energy in small molecules (e. g. , carbon dioxide, water) organic compounds (carbohydrates , fats, proteins) endergonic reactions exergonic reactions energy out © Cengage Learning 2016 organic compounds (carbohydrates , fats, proteins) small molecules (e. g. , carbon dioxide, water)
5. 4 How Enzymes Work • In a process called catalysis, an enzyme makes a specific reaction occur much faster than it would on its own – Enzymes are not consumed or changed by participating in a reaction – Most are proteins; some are RNA • Substrate – The specific reactant acted upon by an enzyme © Cengage Learning 2016
The Transition State • Catalysis lowers activation energy – Brings on the transition state, where substrate bonds break and reactions run spontaneously • Active sites – Locations on the enzyme where substrates bind and reactions proceed – Complementary in shape, size, polarity and charge to the substrate © Cengage Learning 2016
Mechanisms of Enzyme-Mediated Reactions • Binding at enzyme active sites may bring on the transition state by four mechanisms – Bringing substrates physically together – Orienting substrates in positions that favor reaction – Inducing a fit between enzyme and substrate (induced-fit model) – Excluding water molecules © Cengage Learning 2016
Enzyme Activity • Raising the temperature boosts reaction rates by increasing free energy – But very high temperatures denature enzymes • Each enzyme has an optimum p. H range – In humans, most enzymes work at p. H 6 to 8 • Salt levels affect the hydrogen bonds that hold enzymes in their three-dimensional shape © Cengage Learning 2016
5. 5 Metabolism—Organized, Enzyme. Mediated Reactions • Molecules interact in organized pathways of metabolism (activities by which cells acquire and use energy) • A metabolic pathway is any series of enzyme-mediated reactions – Cells build, rearrange, or break down an organic substance – Linear pathways run from reactant to product – Cyclic pathways regenerate a molecule from the first step © Cengage Learning 2016
Controls Over Metabolism • Concentrations of reactants or products can make reactions proceed forward or backward • Mechanisms can adjust enzyme production, or activate or inhibit them – Feedback inhibition decreases or stops the activity – Regulatory molecules can activate or inhibit • Bind directly to active site • Bind outside active site in allosteric regulation © Cengage Learning 2016
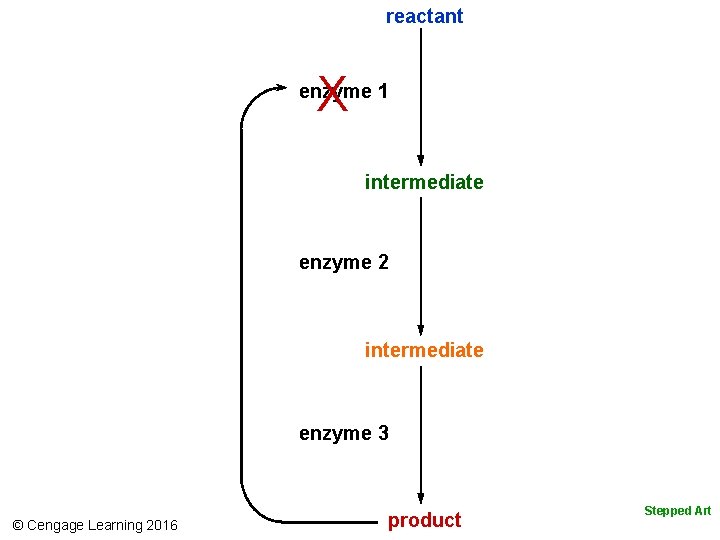
reactant X enzyme 1 intermediate enzyme 2 intermediate enzyme 3 © Cengage Learning 2016 product Stepped Art
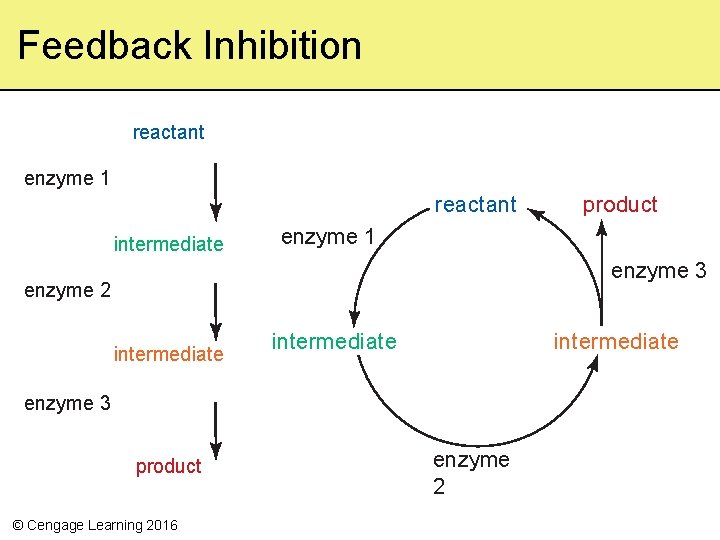
Feedback Inhibition reactant enzyme 1 reactant intermediate product enzyme 1 enzyme 3 enzyme 2 intermediate enzyme 3 product © Cengage Learning 2016 enzyme 2
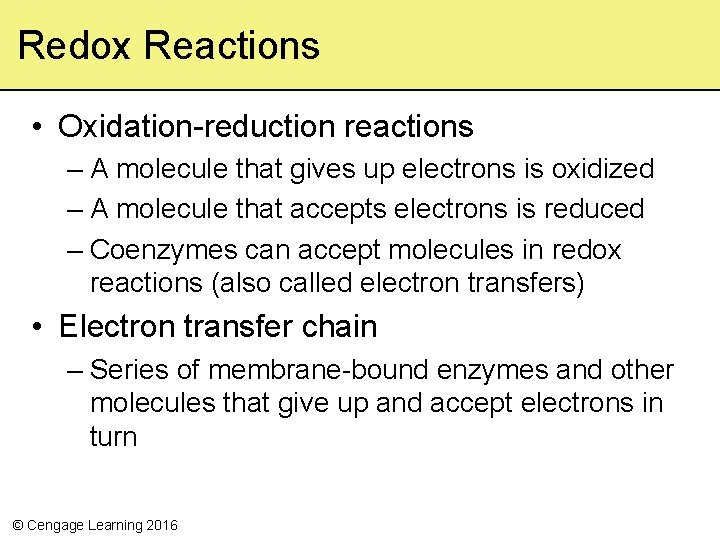
Redox Reactions • Oxidation-reduction reactions – A molecule that gives up electrons is oxidized – A molecule that accepts electrons is reduced – Coenzymes can accept molecules in redox reactions (also called electron transfers) • Electron transfer chain – Series of membrane-bound enzymes and other molecules that give up and accept electrons in turn © Cengage Learning 2016
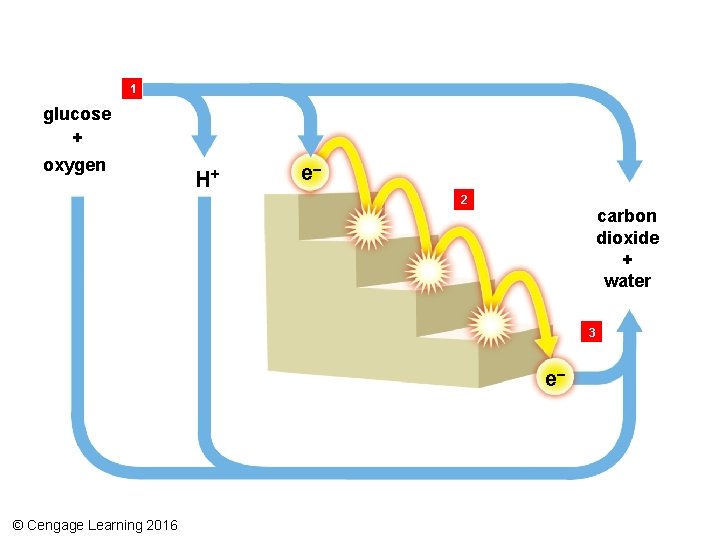
1 glucose + oxygen H+ e– 2 carbon dioxide + water 3 e– © Cengage Learning 2016
5. 6 Cofactors in Metabolic Pathways • Most enzymes require helper molecules – Cofactors • Atoms or molecules (other than proteins) that are necessary for enzyme function – Coenzymes • Organic cofactors such as vitamins • May become modified during a reaction © Cengage Learning 2016
ATP—A Special Coenzyme • Energy in ATP drives many endergonic reactions • ATP (adenosine triphosphate) – A nucleotide with three phosphate groups – Transfers a phosphate group and energy to other molecules • Phosphorylation – A phosphate-group transfer – ADP binds phosphate in an endergonic reaction to replenish ATP (ATP/ADP cycle) © Cengage Learning 2016
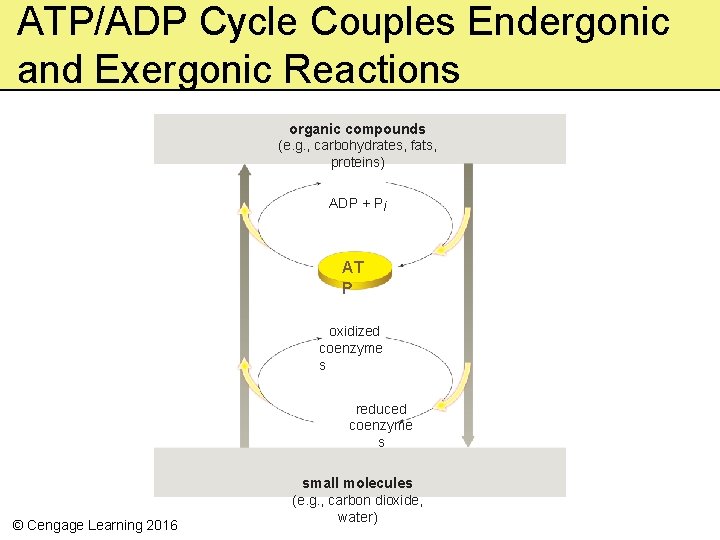
ATP/ADP Cycle Couples Endergonic and Exergonic Reactions organic compounds (e. g. , carbohydrates, fats, proteins) ADP + Pi AT P oxidized coenzyme s reduced coenzyme s © Cengage Learning 2016 small molecules (e. g. , carbon dioxide, water)
5. 7 A Closer Look at Cell Membranes • A cell membrane is organized as a lipid bilayer with many proteins embedded in it and attached to its surfaces • Phospholipid molecules in the plasma membrane have two parts – Hydrophilic heads interact with water molecules – Hydrophobic tails interact with each other, forming a barrier to hydrophilic molecules © Cengage Learning 2016
The Fluid Mosaic Model • Describes the organization of cell membranes – Phospholipids drift and move like a fluid – The bilayer is a mosaic mixture of phospholipids, steroids, proteins, and other molecules © Cengage Learning 2016
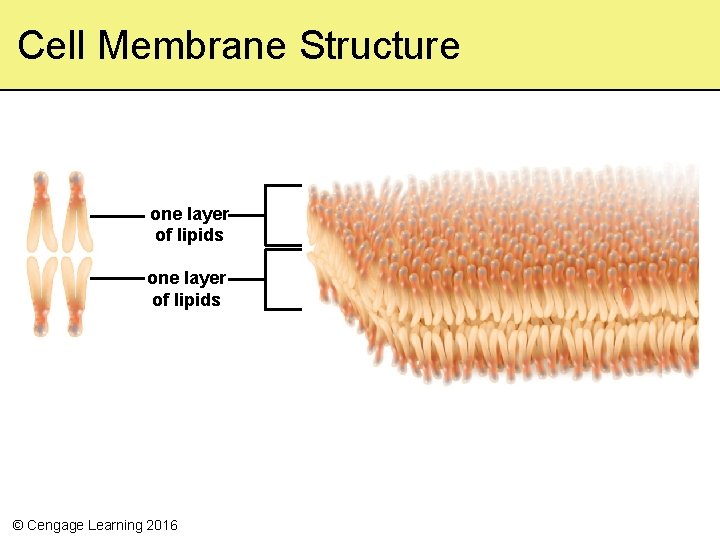
Cell Membrane Structure one layer of lipids © Cengage Learning 2016
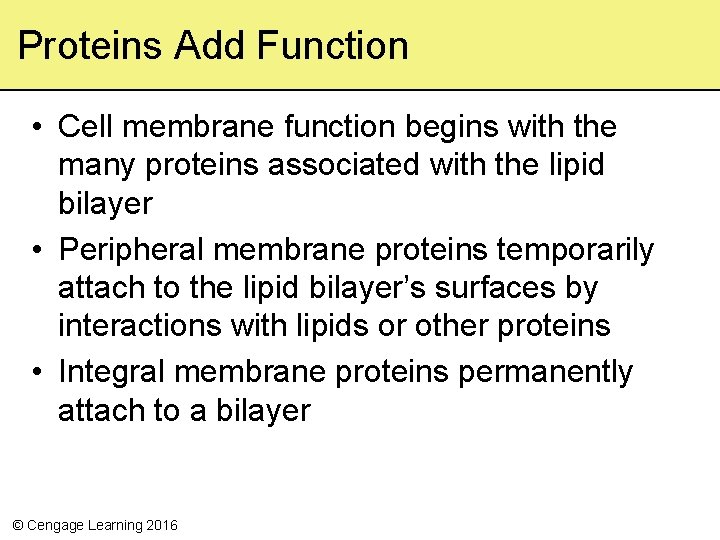
Proteins Add Function • Cell membrane function begins with the many proteins associated with the lipid bilayer • Peripheral membrane proteins temporarily attach to the lipid bilayer’s surfaces by interactions with lipids or other proteins • Integral membrane proteins permanently attach to a bilayer © Cengage Learning 2016
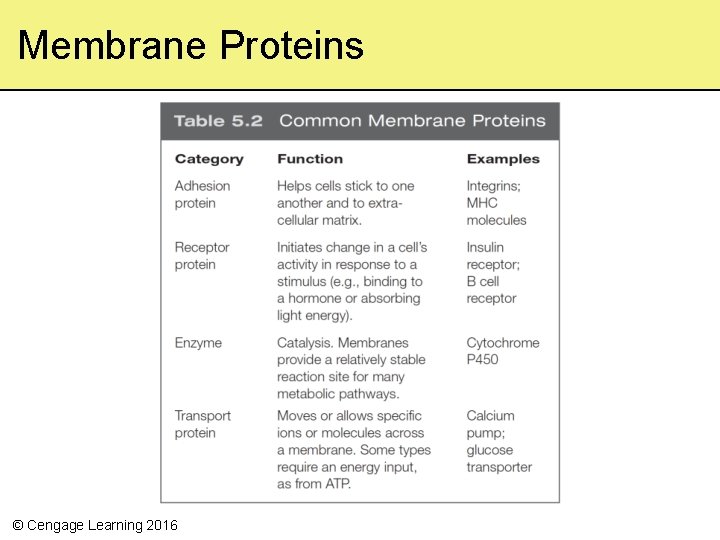
Membrane Proteins © Cengage Learning 2016
5. 8 Diffusion and Membranes • Diffusion – The net movement of molecules down a concentration gradient – Moves substances into, through, and out of cells – A substance diffuses in a direction set by its own concentration gradient © Cengage Learning 2016
The Rate of Diffusion • Depends on five factors – Size – Temperature – Steepness of the concentration gradient – Charge – Pressure © Cengage Learning 2016
Semipermeable Membranes • Selective permeability – The ability of a cell membrane to control which substances and how much of them enter or leave the cell – Allows the cell to maintain a difference between its internal environment and extracellular fluid – Supplies the cell with nutrients, removes wastes, and maintains volume and p. H – Water diffuses across membranes by osmosis © Cengage Learning 2016
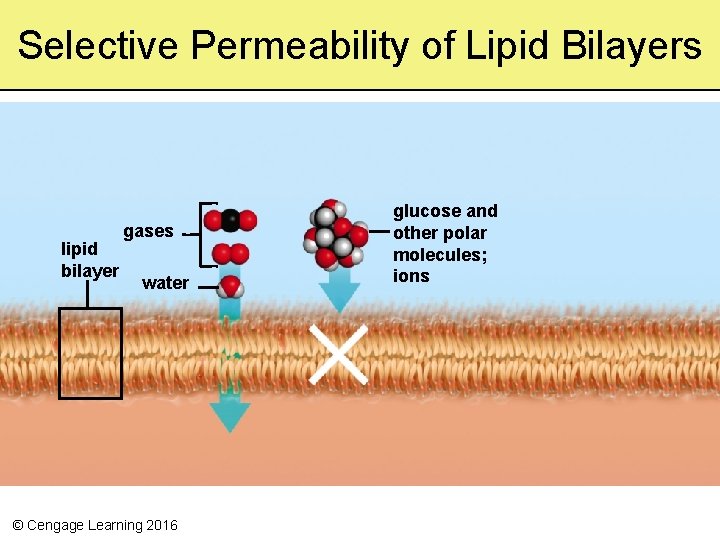
Selective Permeability of Lipid Bilayers lipid bilayer gases water © Cengage Learning 2016 glucose and other polar molecules; ions
Tonicity • Relative concentrations of solutes in two fluids separated by a selectively permeable membrane can differ (tonicity) • When separated by a membrane, solutions are either: – Isotonic with the same solute concentration – Hypotonic solution with a lower solute concentration – Hypertonic solution with a higher solute concentration © Cengage Learning 2016
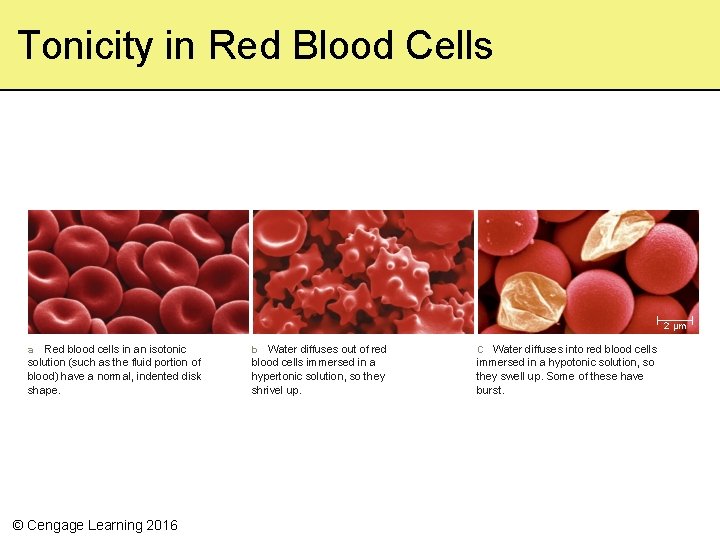
Tonicity in Red Blood Cells 2 µm a Red blood cells in an isotonic solution (such as the fluid portion of blood) have a normal, indented disk shape. © Cengage Learning 2016 b Water diffuses out of red blood cells immersed in a hypertonic solution, so they shrivel up. C Water diffuses into red blood cells immersed in a hypotonic solution, so they swell up. Some of these have burst.

Turgor • The pressure exerted by a volume of fluid against a surrounding structure (membrane, tube, or cell wall), which resists volume change • Osmotic pressure – The amount of turgor that can stop water from diffusing into cytoplasmic fluid or other hypertonic solutions – Keeps walled cells plump © Cengage Learning 2016
5. 9 Membrane Transport Mechanisms • Many types of molecules and ions can cross a lipid bilayer only with the help of transport proteins – Transport proteins allow a specific substance to cross • Ions and large polar molecules require other mechanisms to cross the cell membrane – Passive transport – Active transport © Cengage Learning 2016
Passive Transport • Requires no energy input – Driven entirely by concentration gradient • Facilitated diffusion is a specific type of passive transport – A gated passive transporter changes shape when a specific molecule binds to it © Cengage Learning 2016
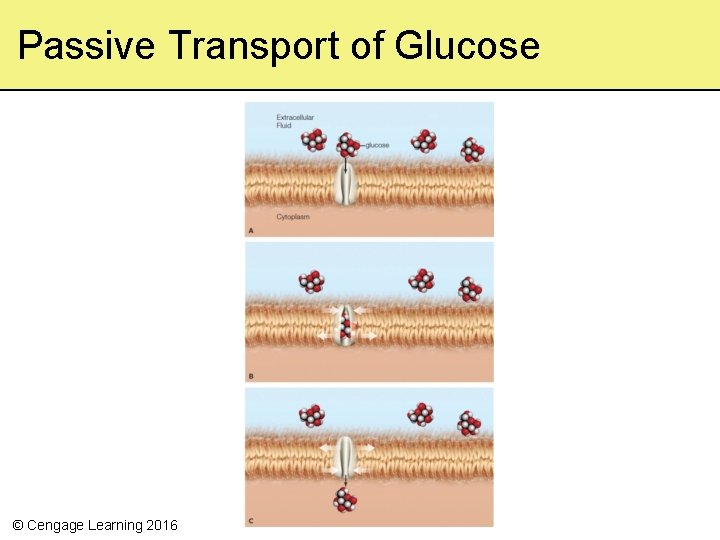
Passive Transport of Glucose © Cengage Learning 2016
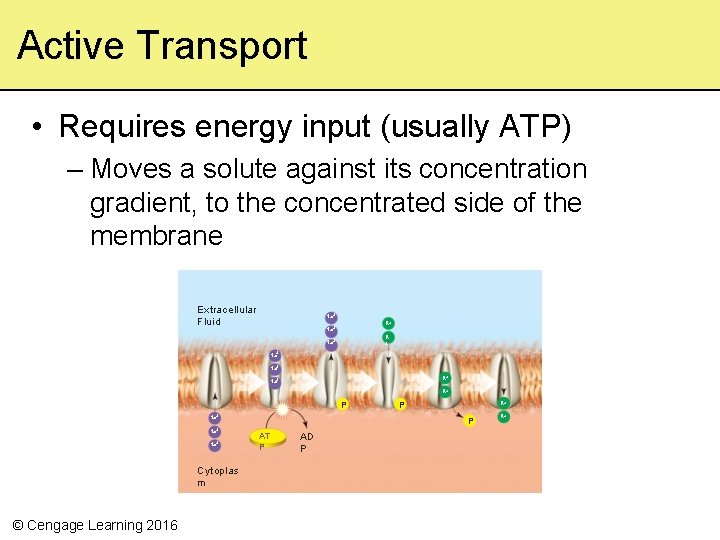
Active Transport • Requires energy input (usually ATP) – Moves a solute against its concentration gradient, to the concentrated side of the membrane Extracellular Fluid Na+ K+ Na+ K + Na+ Na+ K+ P Na+ Na+ Cytoplas m © Cengage Learning 2016 K+ P P AT P AD P K+
5. 10 Membrane Trafficking • By processes of endocytosis and exocytosis, cells take in and expel particles that are too big for transport proteins, as well as substances in bulk • Requires formation and movement of vesicles formed from membranes • Involves motor proteins and ATP © Cengage Learning 2016
Exocytosis and Endocytosis • Exocytosis – The fusion of a vesicle with the cell membrane, releasing its contents to the surroundings • Endocytosis – The formation of a vesicle from the cell membrane, enclosing materials near the cell surface and bringing them into the cell © Cengage Learning 2016
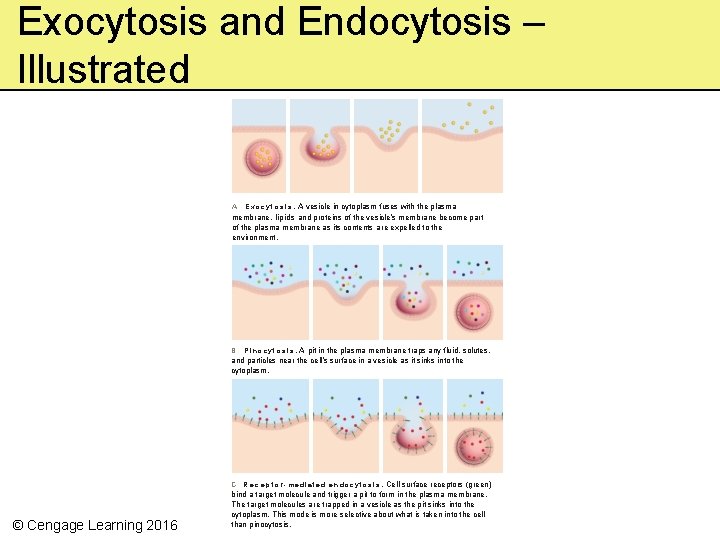
Exocytosis and Endocytosis – Illustrated E x o c y t o s i s. A vesicle in cytoplasm fuses with the plasma membrane. lipids and proteins of the vesicle’s membrane become part of the plasma membrane as its contents a re expelled to the environment. A P i n o c y t o s i s. A pit in the plasma membrane traps any fluid, solutes, and particles near the cell’s surface in a vesicle as it sinks into the cytoplasm. B C R e c e p t o r- m e d i a t e d e n d o c y t o s i s. Cell surface receptors (green) © Cengage Learning 2016 bind a target molecule and trigger a pit to form in the plasma membrane. The target molecules are trapped in a vesicle as the pit sinks into the cytoplasm. This mode is more selective about what is taken into the cell than pinocytosis.
Three Pathways of Endocytosis • Pinocytosis • A pathway that brings a drop of extracellular fluid along with suspended particles into cells • Receptor-mediated endocytosis • Specific molecules bind to surface receptors, which are then enclosed in an endocytic vesicle • Phagocytosis • Larger target particles such as microbes or cellular debris are engulfed by pseudopods, which merge as a vesicle © Cengage Learning 2016
Recycling Membrane • Exocytosis and endocytosis continually replace and withdraw patches of the plasma membrane • New membrane proteins and lipids are made in the ER, modified in Golgi bodies, and form vesicles that fuse with plasma membrane © Cengage Learning 2016
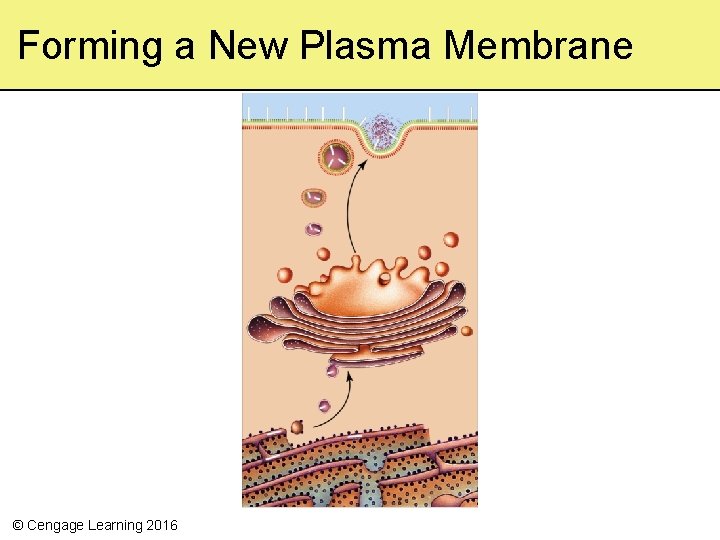
Forming a New Plasma Membrane © Cengage Learning 2016

Points to Ponder • What factors are involved in how much alcohol each individual can process? • Why is heat is the most common form of entropy? • What would happen if our body p. H rose to 9. 5? © Cengage Learning 2016
- Slides: 49