Chapter 5 Galvanic and Stray Current Corrosion 1

























- Slides: 36

Chapter 5 Galvanic and Stray Current Corrosion 1

Overview • Galvanic Corrosion § Understanding Galvanic Corrosion § Controlling Galvanic Corrosion • Stray Current Corrosion § Understanding Stray Current Corrosion § Preventing Stray Current Corrosion § Testing for Stray Current 2

Galvanic Corrosion Understanding Galvanic Corrosion • • Causes Results Galvanic Series of Metals Additional Notes 3

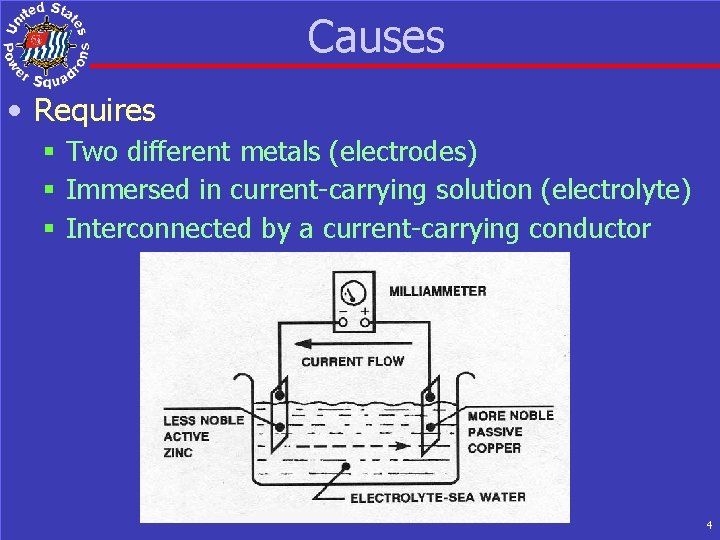
Causes • Requires § Two different metals (electrodes) § Immersed in current-carrying solution (electrolyte) § Interconnected by a current-carrying conductor 4

Results of Galvanic Corrosion New Zinc (for 1” diameter shaft) Old Zinc after 8 months (for 1” diameter shaft) 5

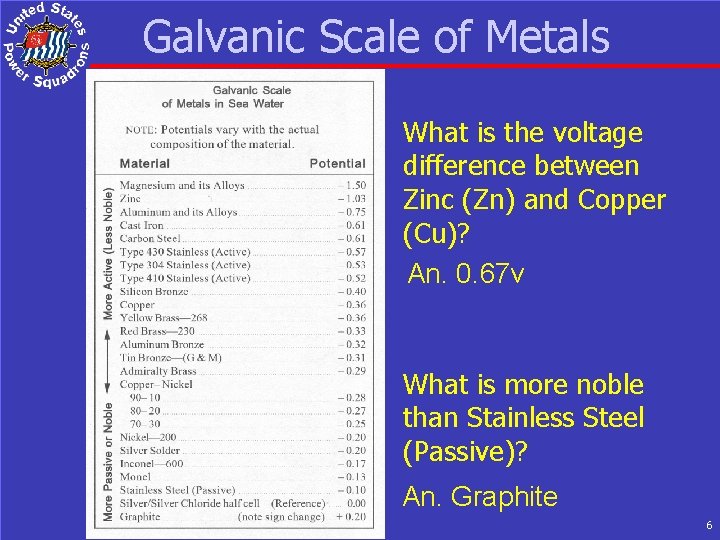
Galvanic Scale of Metals What is the voltage difference between Zinc (Zn) and Copper (Cu)? An. 0. 67 v What is more noble than Stainless Steel (Passive)? An. Graphite 6

Additional Notes • Expect corrosion with 0. 25 V difference • Most negative electrodes will decompose § Magnesium @ - 1. 50 V for freshwater § Zinc @ - 1. 03 V for saltwater § Aluminum @ - 0. 75 V will decompose if neither magnesium or zinc are present • Zinc (or magnesium) will protect § Stainless steel shaft § Bronze propeller § Aluminum outdrive 7

Signs of Galvanic Corrosion • Blistering of paint § 1 st Warning Sign • Formation of powdery substance § 2 nd Warning Sign • Pitting of metal § Too late § Severe Galvanic Corrosion • Don’t treat the symptom, fix the problem 8

Galvanic Corrosion Controlling Galvanic Corrosion • • Types of Metal Area of Metals Self-Destroying Metals Use of Sacrificial Anodes Indirect Cathodic Protection Resistance of an Electrical Path Between boats 9

Types of Metal • Copper, bronze and copper-nickel are compatible • Avoid bronze propeller on plain steel shaft • Stainless steel shaft with bronze prop may be used § Need zinc washer and/or zinc prop nut § Avoid graphite grease 10

Area of Metal • Good – applying a less noble metal to a large area § Bronze through-hull on steel hull • Bad – applying a more noble metal to a larger area § Steel screws / bolts on large bronze or monel plate 11

Self-Destroying Metals • Brass (an alloy of copper and zinc) § Zinc will corrode away in sea water, leaving a copper sponge • Stainless steel hose clamps with different metal take-up screws • Stainless steel should be non-magnetic § If magnetic, it will corrode 12

Use of Sacrificial Anodes • Made from active metals § Magnesium, zinc or aluminum • Corrosive action occurs on the expendable metal anode • Bolted to the metal they are to protect • Never painted • Replaced when half-corroded or annually Shaft Prop Nut Rudder 13

Powerboat Zincs Trim Tab 6 Zincs 14

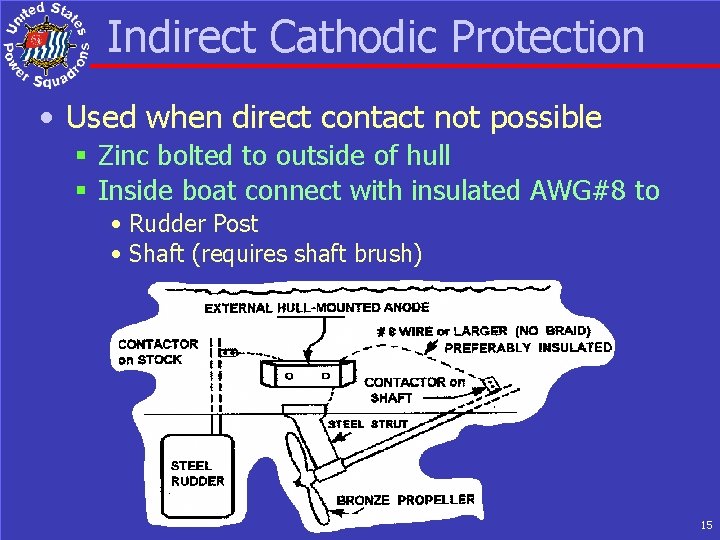
Indirect Cathodic Protection • Used when direct contact not possible § Zinc bolted to outside of hull § Inside boat connect with insulated AWG#8 to • Rudder Post • Shaft (requires shaft brush) 15

Resistance of Electrical Path • Fresh water is less conductive than salt water § Less galvanic current § Use magnesium sacrificial anodes • Salt water is more conductive than fresh water § More galvanic current § Use zinc sacrificial anodes • Magnesium sacrificial anodes will not last • Graphite grease is an excellent conductor, but is a cathode § Do NOT use in stuffing boxes § Do NOT use on shaft bearings 16

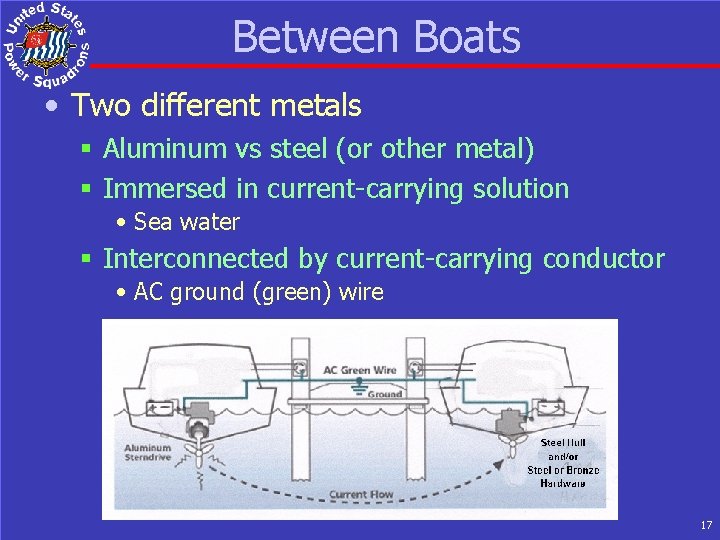
Between Boats • Two different metals § Aluminum vs steel (or other metal) § Immersed in current-carrying solution • Sea water § Interconnected by current-carrying conductor • AC ground (green) wire 17

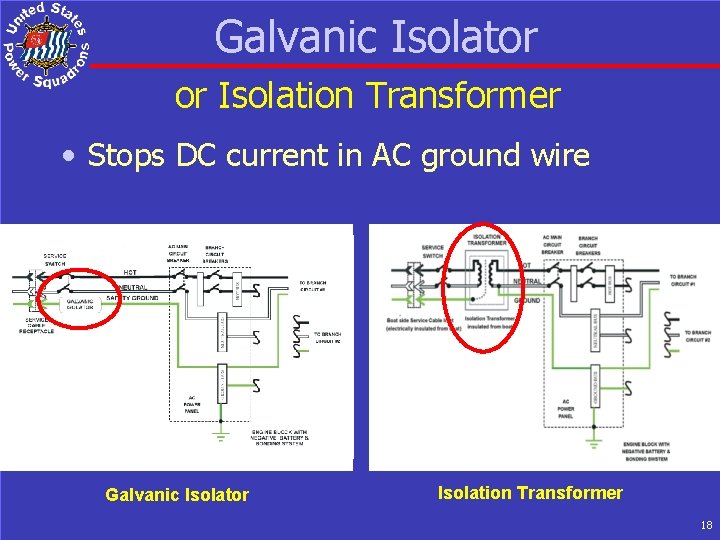
Galvanic Isolator or Isolation Transformer • Stops DC current in AC ground wire Galvanic Isolator Isolation Transformer 18

Stray Current Corrosion Understanding Stray Current Corrosion • Causes • Results • Additional Notes 19

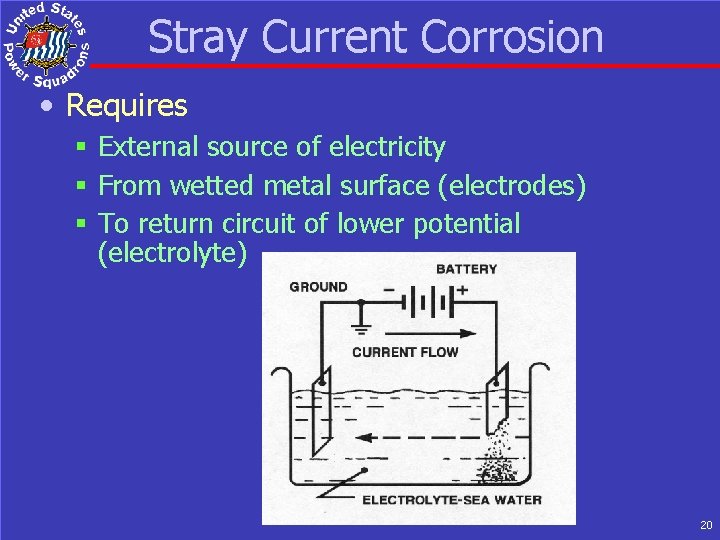
Stray Current Corrosion • Requires § External source of electricity § From wetted metal surface (electrodes) § To return circuit of lower potential (electrolyte) 20

Stray vs Galvanic Current • Stray current corrosion is more destructive § Hundreds of times stronger § Galvanic potential difference 0. 25 to 1. 5 volts § Stray current from 12 volt battery • Sources of stray current § Internal from boat’s 12 volt battery and defective wiring § External to boat from another source of DC 21

Results of Stray Current Corrosion 22

Additional Notes • Stronger than Galvanic current § 100 times more destructive • Metals can be similar or dissimilar § Current flow from positive through electrolyte § Positive DC terminal will corrode § Both AC terminals will corrode • Electrolyte is any moist surface § Bilge water § Wet wood § Wet or moist surface 23

Stray Current Corrosion Preventing Stray Current • • • Wiring Bonding Battery charger Galvanic isolators Isolation transformers 24

Wiring • Defective wiring is the most common cause § Deteriorated insulation on hot wire § Always use marine grade wires • Run wires above water line § Moist or wetted surfaces conduct current § Moisture in loose connections will cause corrosion • Wires in bilge § § Waterproof terminals and butt spices Heat shrink tubing is 2 nd choice Liquid electrical tape is also an option Electrical tape is inadequate 25

Bonding • Maintain adequate bonding system § All metallic bodies and surfaces at DC negative § Chapter 2 (Wiring) covered bonding • Propeller shaft bonding § Recommend by some authorities § Will also reduce propeller “hash” (Chapter 7) § Requires a shaft brush 26

AC Ground Isolation • If your boat has the better ground… and a nearby boat has stray current • Your boat will be damaged, unless… • Stop DC current in AC ground wire – Galvanic Isolators & Isolation Transformers but • Stray current may flow through your boat • In one underwater fitting • Through bonding system • Out another underwater fitting (remember corroded prop and shaft pictures) 27

Corrosion Facts • Not all corrosion is electrical § Seawater deteriorates all metals § Cavitation also erodes props • Stray current corrosion can be eliminated • Galvanic corrosion can be reduced and controlled • DC current is 100 times worse than AC current 28

Testing for Stray Current • Measuring Stray Current • Corrosion Source and Mitigation 29

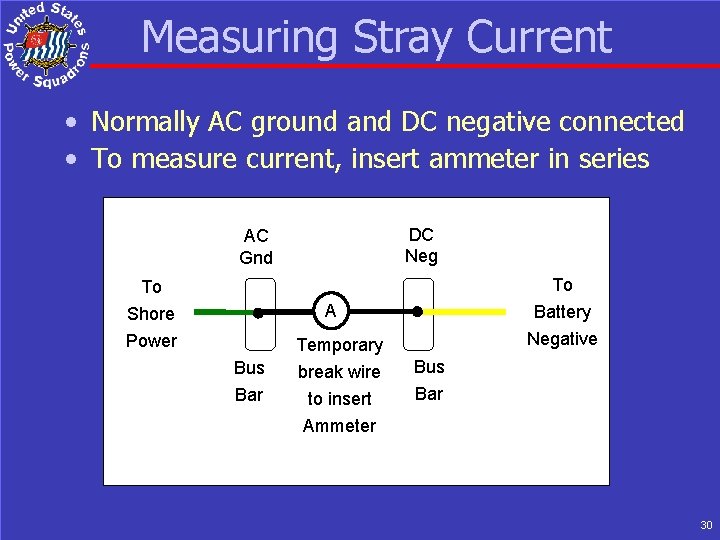
Measuring Stray Current • Normally AC ground and DC negative connected • To measure current, insert ammeter in series DC Neg AC Gnd To Shore Power To Battery Negative ABYC Req A Bus Bar Temporary break wire to insert Ammeter Bus Bar 30

AC Stray Current Testing • AC main circuit breaker “On” § All branch circuit breakers “Off” • Set multimeter to read AC current • Current should be less than 1 milliampere • Then selectively turn on each AC circuit • If AC current exceeds 1 m. A § You have stray current in that circuit • After testing § Reconnect AC ground & DC negative bus bars 31

DC Stray Current Testing • DC main circuit breaker “On” § All branch circuit breakers “Off” • Set multimeter to read DC current • Current should be less than 0. 01 milliampere • Then selectively turn on each DC circuit • If DC current exceeds 0. 01 m. A § You have stray current in that circuit • After testing § Reconnect AC ground and DC negative bus bars 32

Testing with Mitigation • Galvanic Isolators & Isolation Transformers § Stop DC current • To check for stray current with isolator § Place ammeter between DC negative bus and green shore power wire to isolator • To check for stray current with transformer § Place ammeter between DC negative bus and green shore power wire to transformer 33

Internal DC Current Testing • Turn off DC main and all branch breakers • Insert ammeter in battery negative cable • Hold down bilge pump float switch § So pump will not turn on • Turn on DC main and bilge pump breaker • Measure stray current, if any § Defective wiring or pump switch • Test other wiring with DC devices turned off 34

Summary 1 • Types of electronic corrosion § Galvanic caused by dissimilar metals § Stray current requires external current • Galvanic current § Requires • Different metals • Immersed in current carrying solution • Connect together by current carrying conductor § Brass will disintegrate in sea water § Zincs are used to protect other metal components 35

Summary 2 • Stray current § Requires an external source of current § Normally is caused by defective wiring • Especially in / through bilge – Make sure any connections are waterproof § DC is 100 times more destructive than AC • Over 1 m. A AC • Over 0. 01 m. A DC 36