Chapter 5 Electrons in Atoms The chemical properties






![Element Lithium Configuration notation Orbital notation 1 s 22 s 1 [He]2 s 1 Element Lithium Configuration notation Orbital notation 1 s 22 s 1 [He]2 s 1](https://slidetodoc.com/presentation_image/fd630c37f44eb760db9fc67facfd0701/image-31.jpg)



![Exceptions �Chromium: [Ar] 4 s 13 d 5 �Copper: [Ar] 4 s 13 d Exceptions �Chromium: [Ar] 4 s 13 d 5 �Copper: [Ar] 4 s 13 d](https://slidetodoc.com/presentation_image/fd630c37f44eb760db9fc67facfd0701/image-49.jpg)
- Slides: 49

Chapter 5: Electrons in Atoms �The chemical properties of atoms, ions, and molecules are related to the arrangement of the electrons within them

Periodic Table Video �http: //www. learner. org/resources/series 61. html#

John Dalton (1803) �Atom was considered a solid indivisible mass

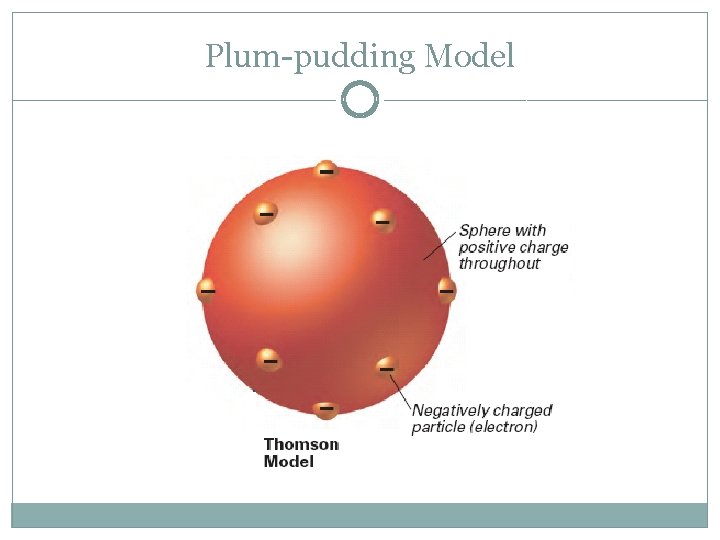
J. J. Thomson (1897) �Discovered the electron �Plum-pudding model: negatively charged electrons stuck into a lump of positively charged material

Plum-pudding Model

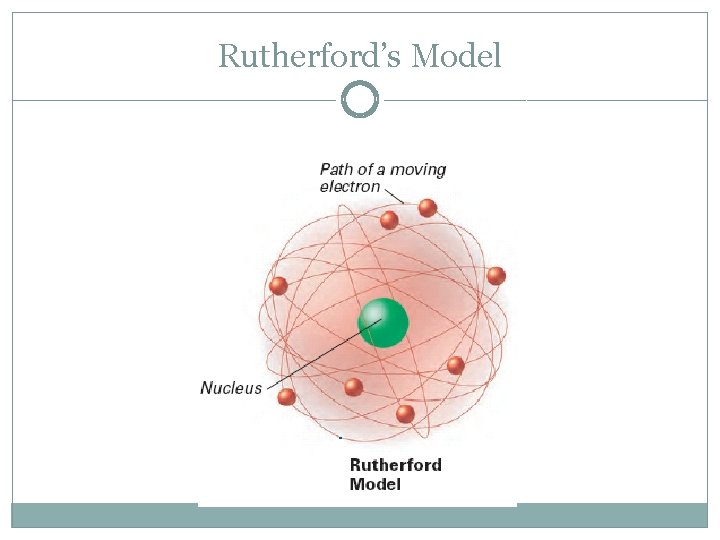
Ernest Rutherford (1911) �Discovered the nucleus (+ charge) �Proposed electrons surround a dense nucleus

Rutherford’s Model

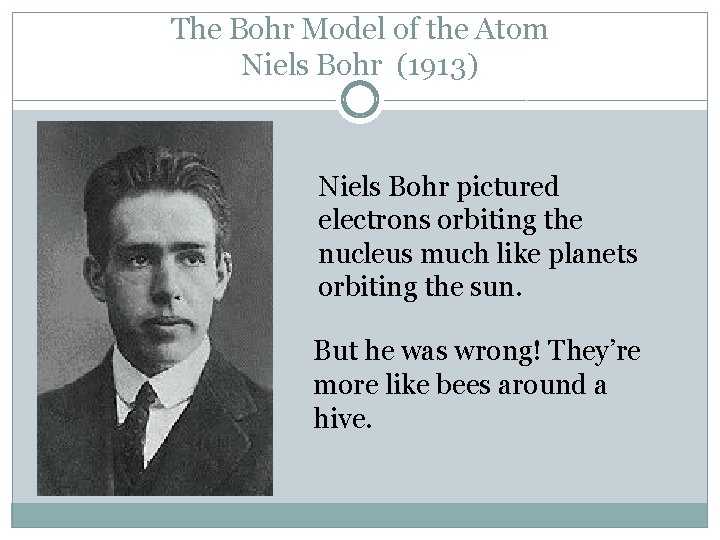
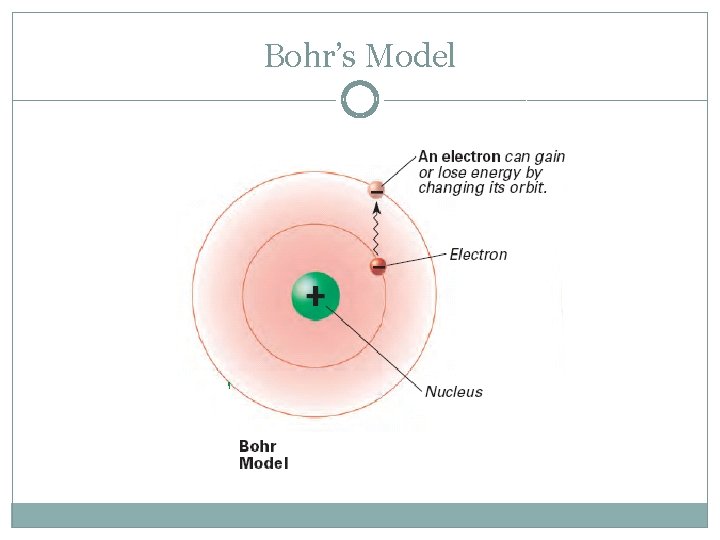
The Bohr Model of the Atom Niels Bohr (1913) Niels Bohr pictured electrons orbiting the nucleus much like planets orbiting the sun. But he was wrong! They’re more like bees around a hive.

Bohr’s Model

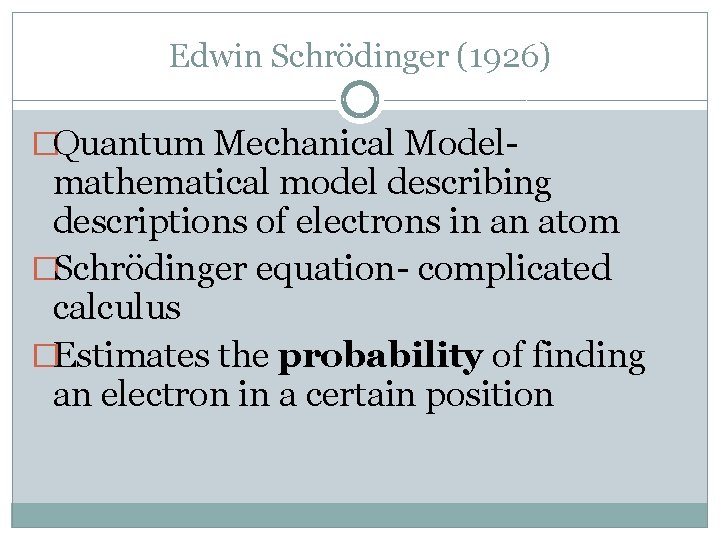
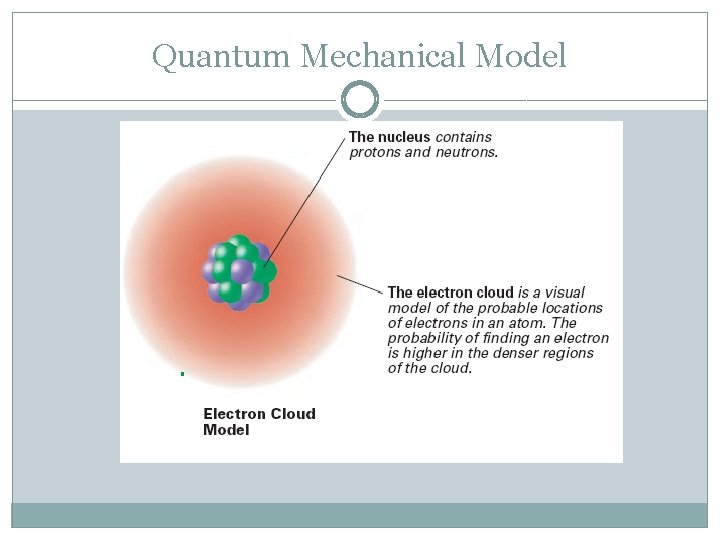
Edwin Schrödinger (1926) �Quantum Mechanical Model- mathematical model describing descriptions of electrons in an atom �Schrödinger equation- complicated calculus �Estimates the probability of finding an electron in a certain position

Quantum Mechanical Model

Heisenberg Uncertainty Principal Werner Heisenberg: Can find out where the electron is, but not where it is going– or vice versa. Cannot determine both position and momentum


Energy Level �Region around the nucleus where an electron is likely to be moving �Analogy: rungs of a ladder �Quantum of energy: amount of energy required to move an electron from its present energy level to the next one

Energy Level Diagram Nucleus

Energy Levels �Represented by principle quantum number (n) �n = 1, 2, 3, 4, … �Within each energy level, electrons can occupy sub-levels

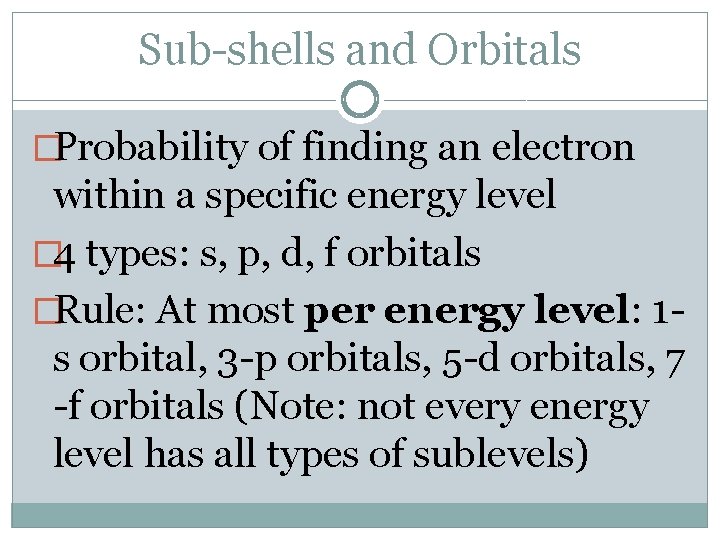
Sub-shells and Orbitals �Probability of finding an electron within a specific energy level � 4 types: s, p, d, f orbitals �Rule: At most per energy level: 1 s orbital, 3 -p orbitals, 5 -d orbitals, 7 -f orbitals (Note: not every energy level has all types of sublevels)

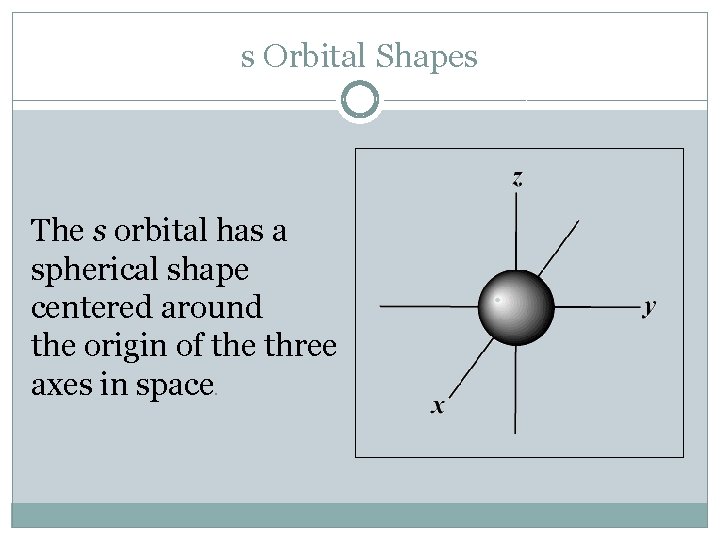
s Orbital Shapes The s orbital has a spherical shape centered around the origin of the three axes in space.

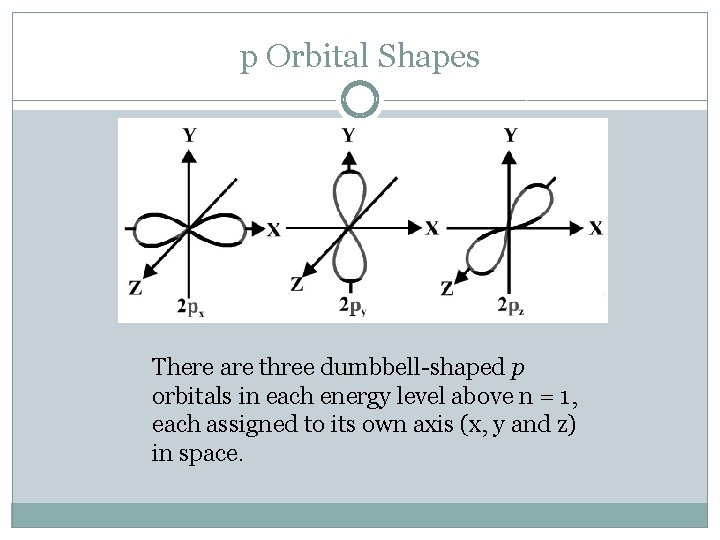
p Orbital Shapes There are three dumbbell-shaped p orbitals in each energy level above n = 1, each assigned to its own axis (x, y and z) in space.

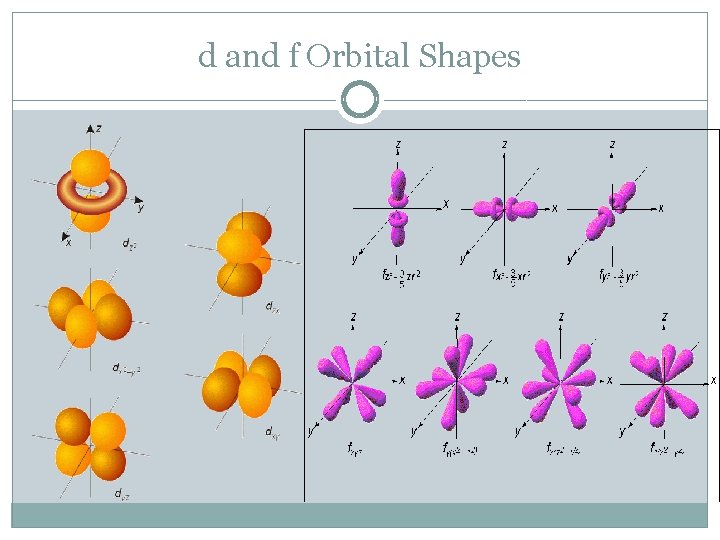
d and f Orbital Shapes

Electron Configuration �Way in which the electrons are arranged in an atom � 3 rules to follow: Aufbau principle- electrons fill in order of increasing energy Hund’s rule- when electrons occupy orbitals of equal energy, 1 electron must go in each before pairing up Pauli-Exclusion principle- 2 electrons max. per orbital

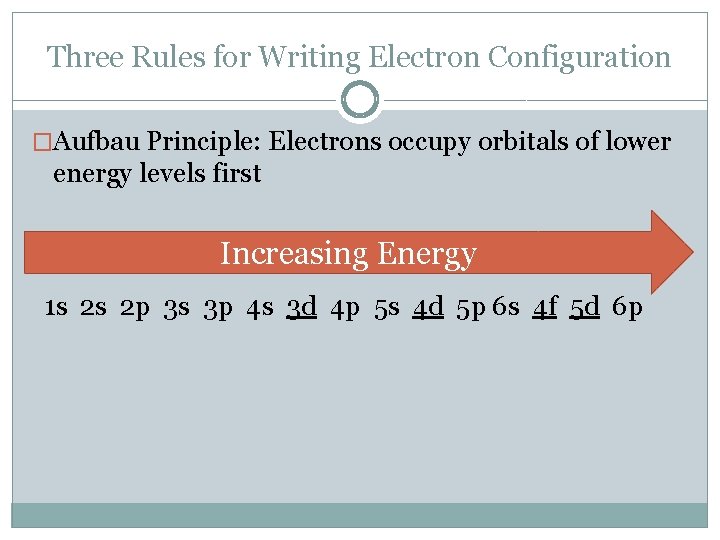
Three Rules for Writing Electron Configuration �Aufbau Principle: Electrons occupy orbitals of lower energy levels first Increasing Energy 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d 5 p 6 s 4 f 5 d 6 p

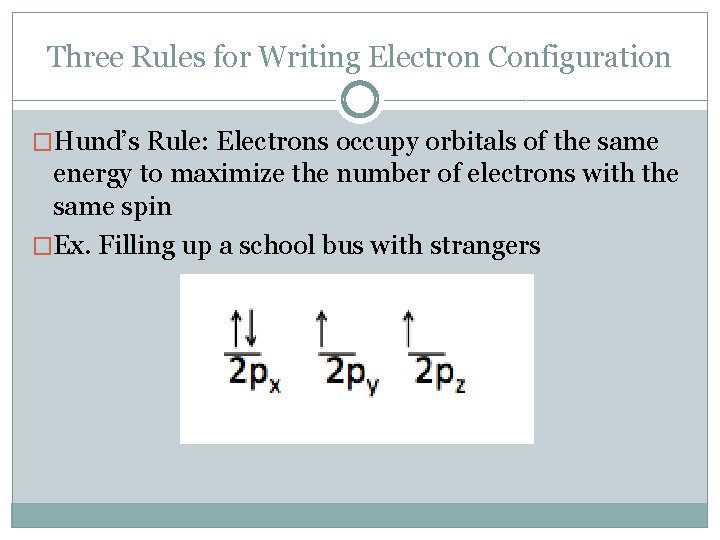
Three Rules for Writing Electron Configuration �Hund’s Rule: Electrons occupy orbitals of the same energy to maximize the number of electrons with the same spin �Ex. Filling up a school bus with strangers

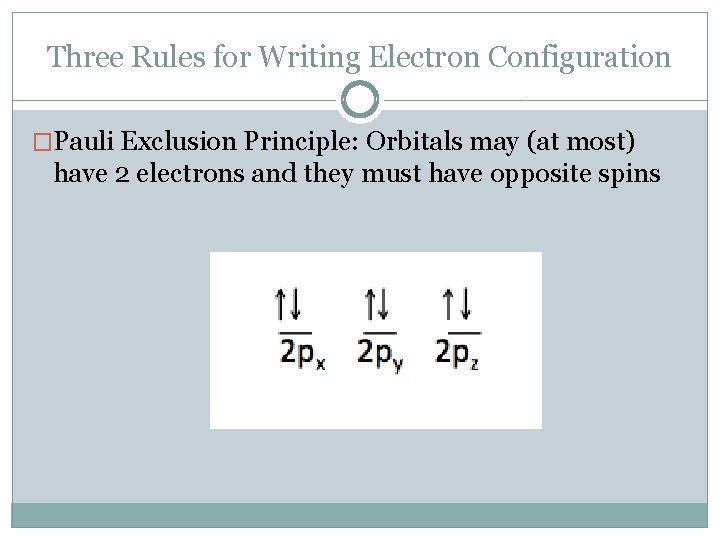
Three Rules for Writing Electron Configuration �Pauli Exclusion Principle: Orbitals may (at most) have 2 electrons and they must have opposite spins

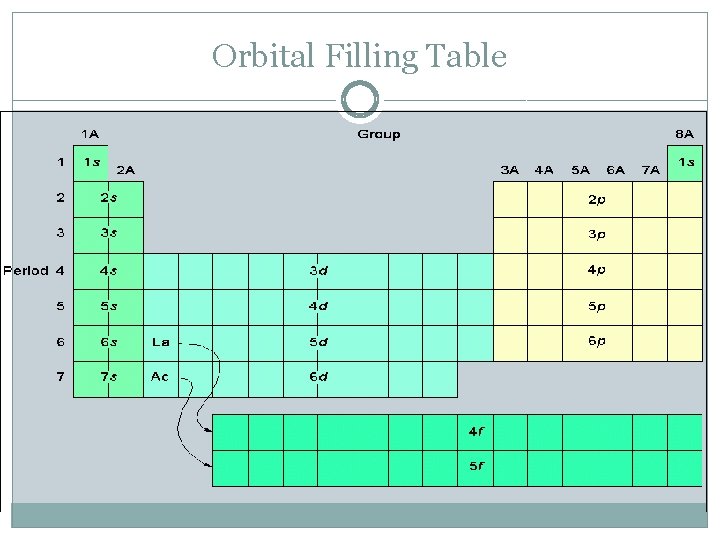
Orbital Filling Table

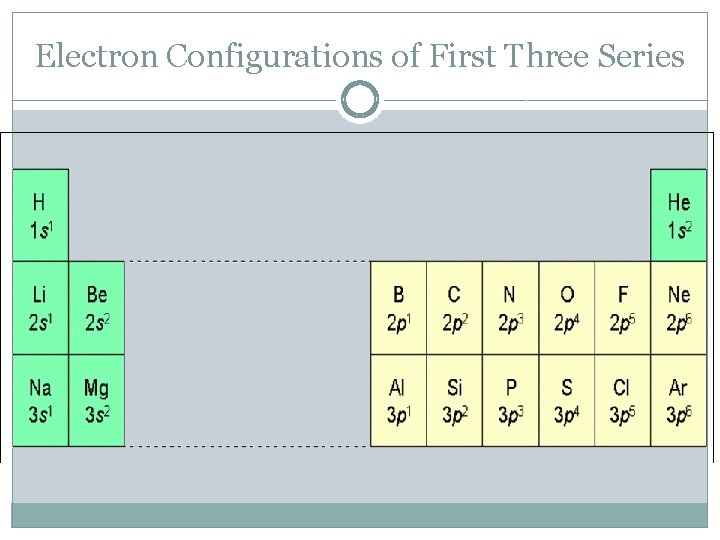
Electron Configurations of First Three Series

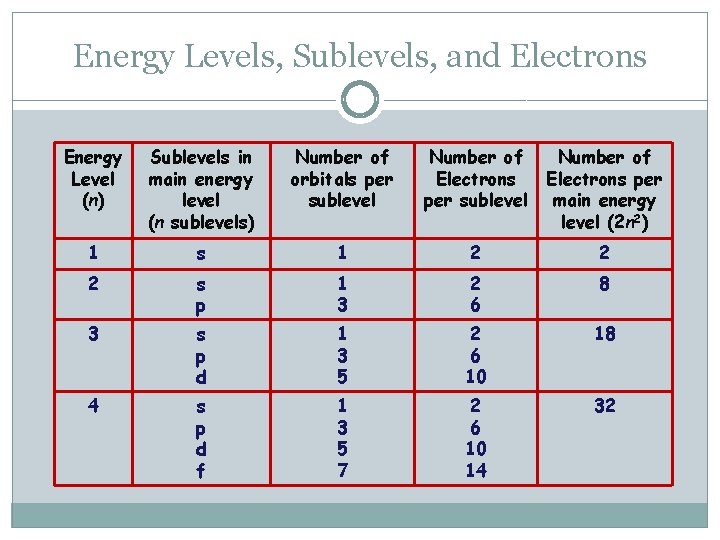
Energy Levels, Sublevels, and Electrons Energy Level (n) Sublevels in main energy level (n sublevels) Number of orbitals per sublevel Number of Electrons per main energy level (2 n 2) 1 s 1 2 2 2 s p 1 3 2 6 8 3 s p d 1 3 5 2 6 10 18 4 s p d f 1 3 5 7 2 6 10 14 32

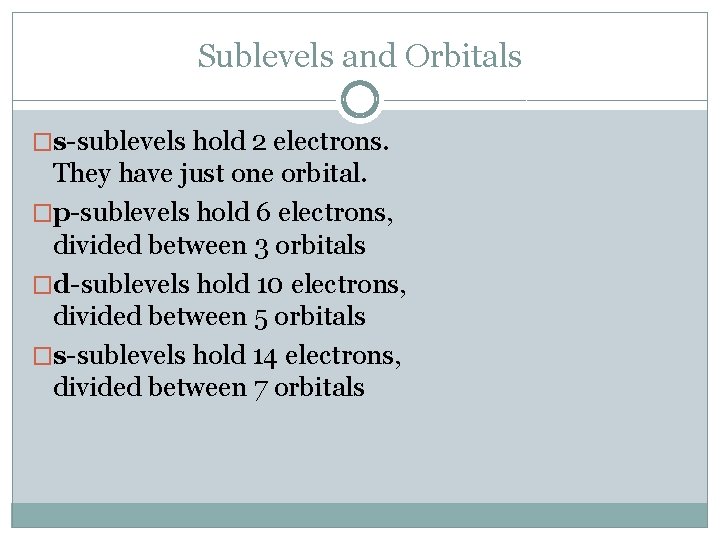
Sublevels and Orbitals �s-sublevels hold 2 electrons. They have just one orbital. �p-sublevels hold 6 electrons, divided between 3 orbitals �d-sublevels hold 10 electrons, divided between 5 orbitals �s-sublevels hold 14 electrons, divided between 7 orbitals

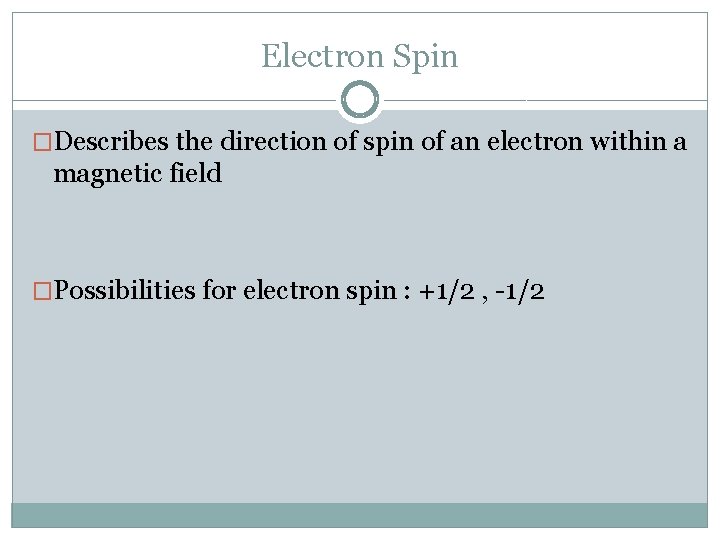
Electron Spin �Describes the direction of spin of an electron within a magnetic field �Possibilities for electron spin : +1/2 , -1/2

Sub-levels in each Energy Level �The energy level number is equal to the number of sub-levels �Question: How many orbitals are in the third energy level? How many electrons are in the fourth energy level?
![Element Lithium Configuration notation Orbital notation 1 s 22 s 1 He2 s 1 Element Lithium Configuration notation Orbital notation 1 s 22 s 1 [He]2 s 1](https://slidetodoc.com/presentation_image/fd630c37f44eb760db9fc67facfd0701/image-31.jpg)
Element Lithium Configuration notation Orbital notation 1 s 22 s 1 [He]2 s 1 ____ 1 s Beryllium ____ 2 p ____ 2 s ____ 2 p ____ [He]2 s 2 p 2 ____ 2 s ____ 2 p ____ 1 s 22 s 2 p 3 [He]2 s 2 p 3 ____ 2 s ____ 2 p ____ 1 s 22 s 2 p 4 [He]2 s 2 p 4 ____ 2 s ____ 2 p ____ 1 s 22 s 2 p 5 [He]2 s 2 p 5 ____ 1 s Neon ____ 2 s 1 s 22 s 2 p 2 ____ 1 s Fluorine ____ [He]2 s 2 p 1 ____ 1 s Oxygen ____ 2 p 1 s 22 s 2 p 1 ____ 1 s Nitrogen ____ [He]2 s 2 ____ 1 s Carbon ____ 2 s 1 s 22 s 2 ____ 1 s Boron Noble gas notation ____ 2 s ____ 2 p ____ 1 s 22 s 2 p 6 [He]2 s 2 p 6 ____ 1 s ____ 2 s ____ 2 p ____

Forming Ion’s �Ion’s are either negatively or positively charged �An atom gaining electrons is an anion (-) Nonmetals �An atom losing electrons is a cation (+) Metals � Transition metals have a roman numeral that indicates the charge

Ion Examples �Sodium (Na): 1 s 22 p 63 s 1 �Sodium ion (Na 1+): 1 s 22 p 6 �Fluorine (F): 1 s 22 p 5 �Fluorine ion (F 1 -): 1 s 22 p 6

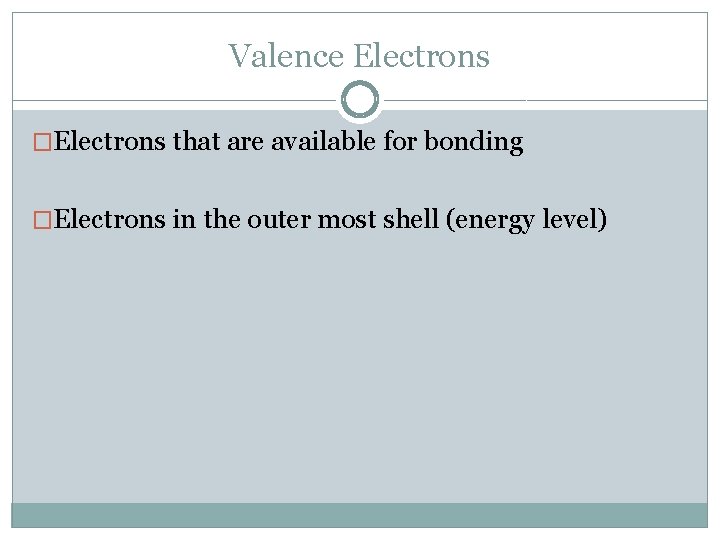
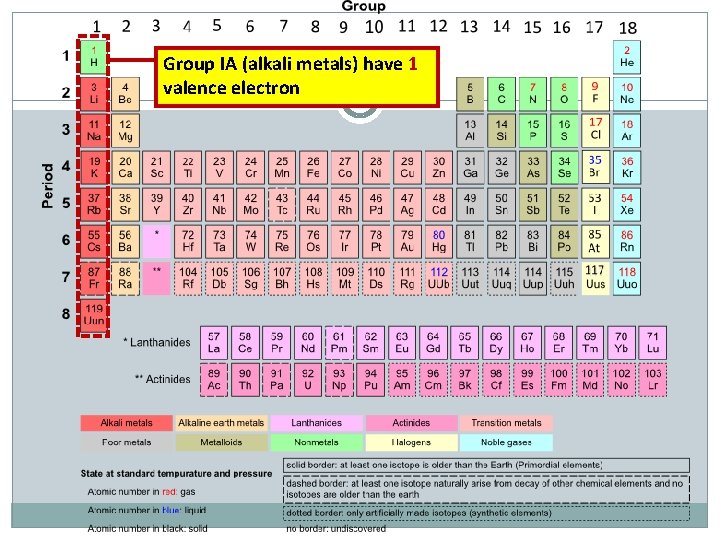
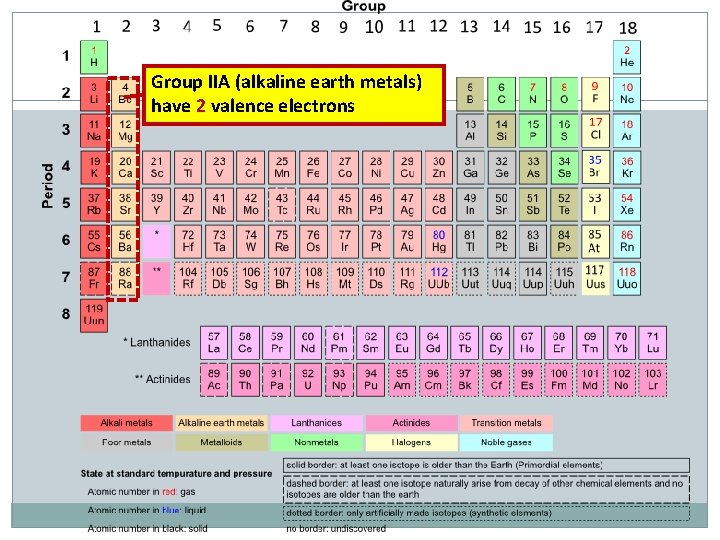
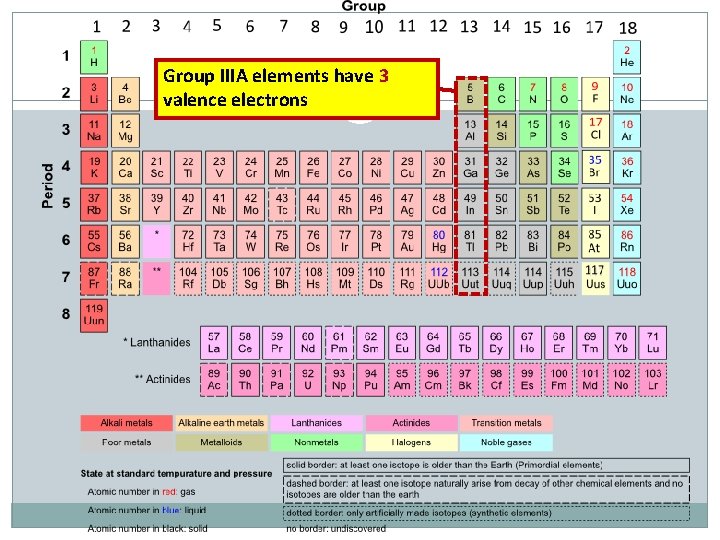
Valence Electrons �Electrons that are available for bonding �Electrons in the outer most shell (energy level)

Group IA (alkali metals) have 1 valence electron

Group IIA (alkaline earth metals) have 2 valence electrons

Group IIIA elements have 3 valence electrons

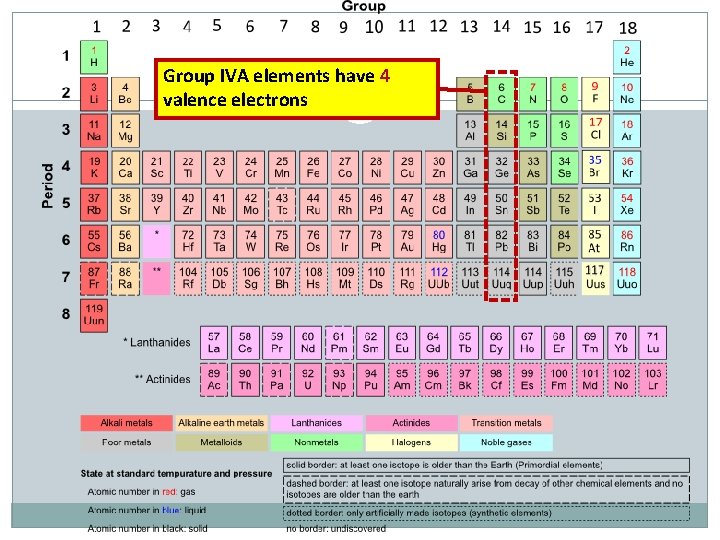
Group IVA elements have 4 valence electrons

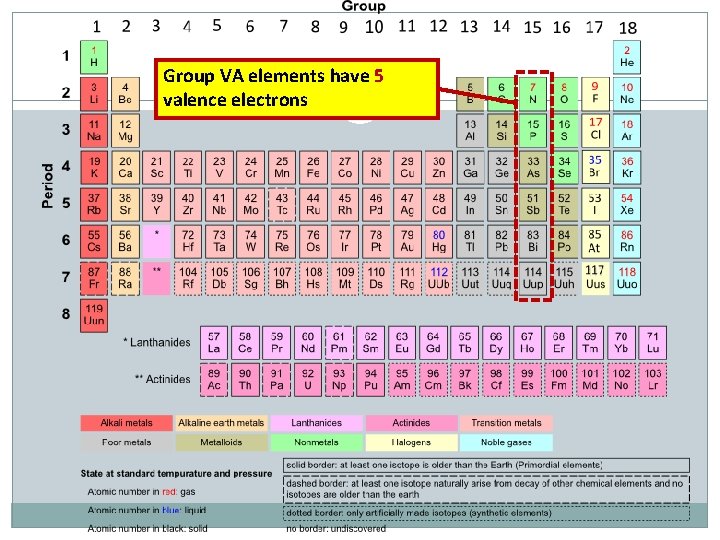
Group VA elements have 5 valence electrons

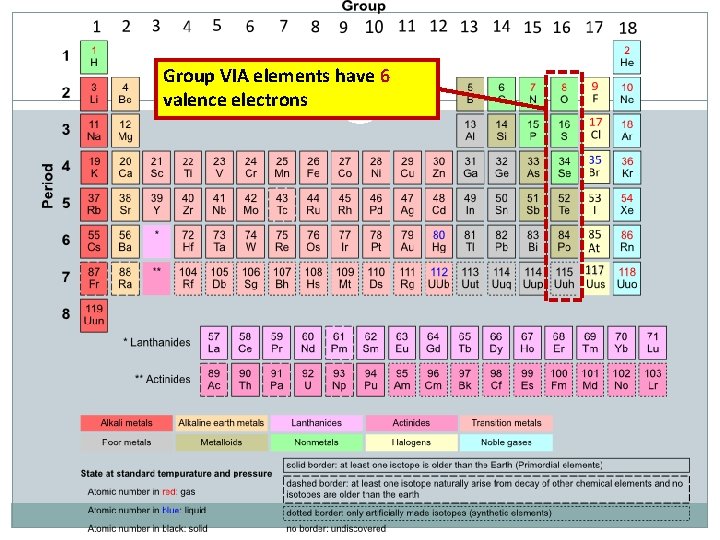
Group VIA elements have 6 valence electrons

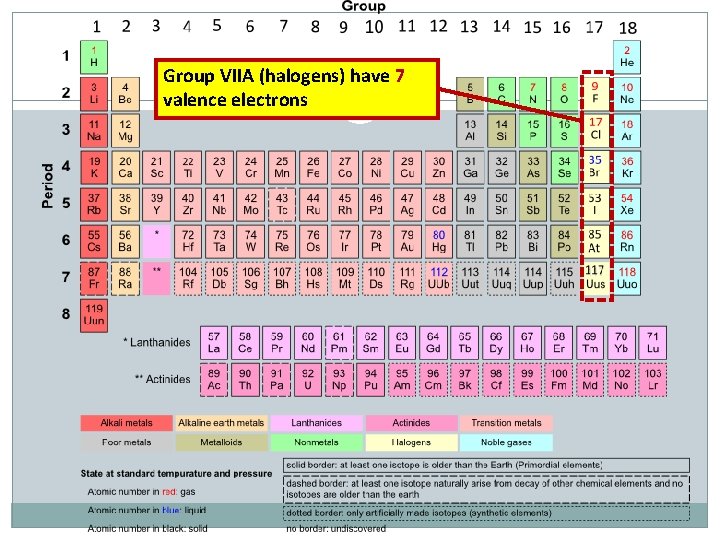
Group VIIA (halogens) have 7 valence electrons

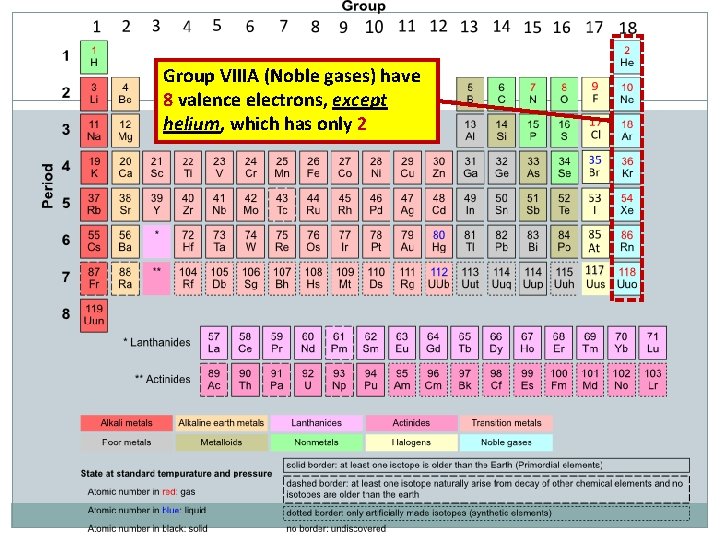
Group VIIIA (Noble gases) have 8 valence electrons, except helium, which has only 2

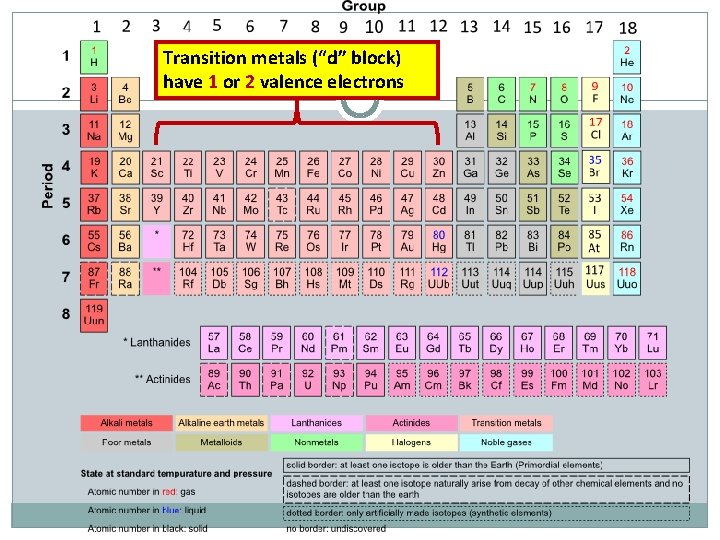
Transition metals (“d” block) have 1 or 2 valence electrons

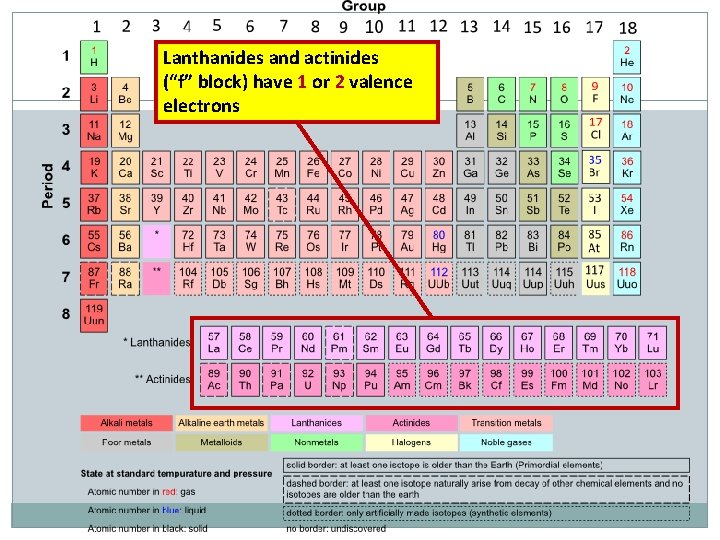
Lanthanides and actinides (“f” block) have 1 or 2 valence electrons

Lewis Dot Structures �Diagrams that show the number of valence electrons in an atom or ion �Octet Rule Atoms try to achieve stability by gaining/losing e- to have a filled outer energy level Filled s & p sublevels (2 + 6 = 8)

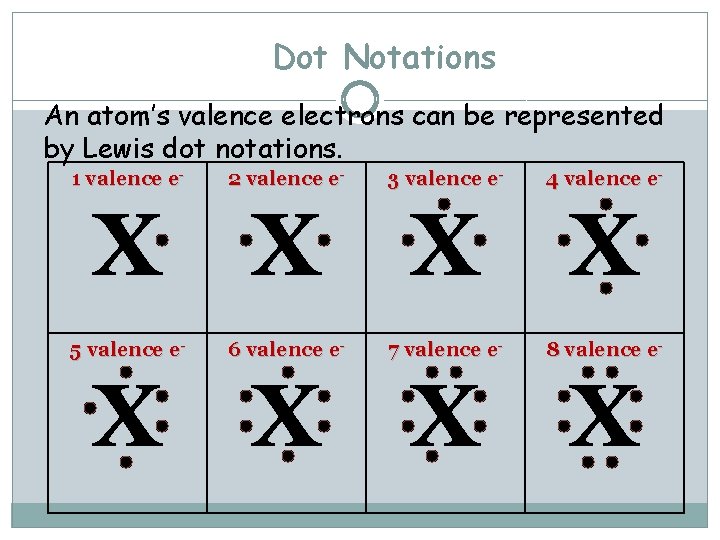
Dot Notations An atom’s valence electrons can be represented by Lewis dot notations. 1 valence e- 2 valence e- 3 valence e- 4 valence e- X X 5 valence e- 6 valence e- 7 valence e- 8 valence e- X X

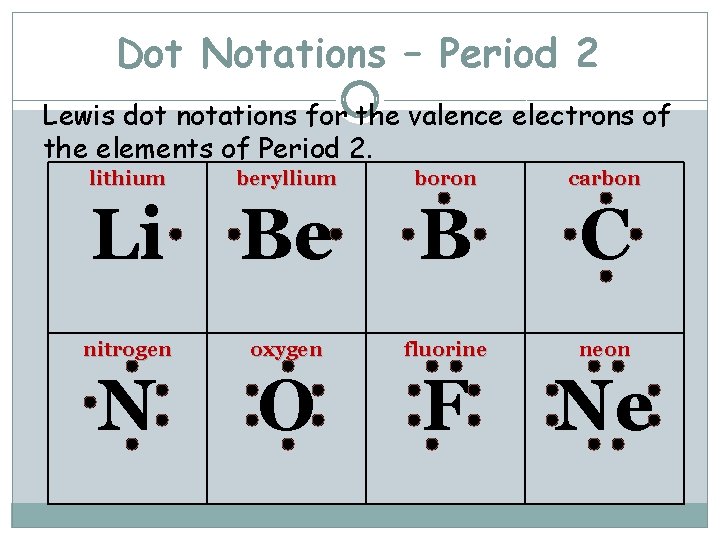
Dot Notations – Period 2 Lewis dot notations for the valence electrons of the elements of Period 2. lithium beryllium boron carbon Li Be B C nitrogen oxygen fluorine neon N O F Ne

Lewis Dot Structures of Ion’s �Make sure to include brackets and the charge!
![Exceptions Chromium Ar 4 s 13 d 5 Copper Ar 4 s 13 d Exceptions �Chromium: [Ar] 4 s 13 d 5 �Copper: [Ar] 4 s 13 d](https://slidetodoc.com/presentation_image/fd630c37f44eb760db9fc67facfd0701/image-49.jpg)
Exceptions �Chromium: [Ar] 4 s 13 d 5 �Copper: [Ar] 4 s 13 d 10 �Most stable: Completely filled > Half filled > All others